Abstract
Background
Proximal gastrectomy with esophagogastrostomy (PGEG) has been widely applied as a comparatively simple method. In this study, we used a questionnaire survey to evaluate the influence of various surgical factors on post-operative quality of life (QOL) after PGEG.
Methods
In this post-gastrectomy syndrome assessment study, we analyzed QOL in 2,368 cases. Among these, 193 had undergone proximal gastrectomy and 115 had undergone PGEG. The Post-Gastrectomy Syndrome Assessment Scale (PGSAS)-45 is a questionnaire consisting of 45 items, including the SF-8, the Gastrointestinal Symptom Rating Scale (GSRS), and other symptom items seemed to be specific to post-gastrectomy. The 23 symptom items were composed of seven symptom subscales (SS), including esophageal reflux, abdominal pain, and meal-related distress. These seven SS, total symptom score, ingested amount of food per meal, necessity for additional meals, quality of ingestion SS, ability to work, dissatisfaction with symptoms, dissatisfaction with the meal, dissatisfaction with working, dissatisfaction with daily life SS and change in body weight were evaluated as main outcome measures. In PGEG cases, we evaluated the influence on QOL of various surgical factors, such as procedures to prevent gastroesophageal regurgitation and size of the remnant stomach.
Results
The scores for esophageal reflux and dissatisfaction with the meal were higher in patients who had not undergone an anti-reflux procedure. In most cases, the preserved remnant stomach was more than two-thirds the size of the pre-operative stomach. When comparing patients with a remnant stomach two-thirds the pre-operative size and those with more than three-quarters, the diarrhea SS and necessity for additional meals scores were lower in the group with more than three-quarters. The indigestion, constipation, and abdominal pain subscales, and the total symptom score, were higher in patients who had not undergone pyloric bougie than in those who had.
Conclusion
These results indicated that QOL was better in patients with a large remnant stomach. Procedures to prevent gastroesophageal reflux, and the use of pyloric bougie as a complementary drainage procedure, were considered effective ways to reduce the deterioration of QOL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of gastric cancer in the upper third of the stomach, and the ratio of early cases, has been increasing continuously in the past decade [1, 2]. The Japanese Gastric Cancer Association guidelines for the treatment of gastric cancer [3] recommend modified procedures as the standard treatment for early gastric cancer. Therefore, proximal gastrectomy has been widely performed as the function-preserving procedure for proximal early gastric cancer, with the aim of improving post-operative quality of life (QOL). It has been reported that QOL after proximal gastrectomy is better than that after total gastrectomy, but previous reports have shown that proximal gastrectomy is more likely to produce complications such as heartburn, poor appetite, and poorer nutritional status than other types of gastrectomy [4–8]. Therefore, there are many problems to resolve in proximal gastrectomy, such as post-operative reflux esophagitis, gastric emptying, and reservoir capacity. Reported reconstruction methods after proximal gastrectomy have included esophagogastrostomy, jejunal interposition, and jejunal pouch interposition. Among these reconstruction methods, esophagogastrostomy has been widely applied as a comparatively simple method [9].
The Post-Gastrectomy Syndrome Assessment Scale (PGSAS)-45 is an integrated questionnaire that has been developed by a voluntary group, the Japan Postgastrectomy Syndrome Working Party, in order to investigate symptoms and lifestyle changes among patients who have undergone gastrectomy [10]. Using this PGSAS-45, a nationwide, multi-institution surveillance study was performed in 2,368 cases. Among these cases, 193 patients had undergone proximal gastrectomy, and 115 of these 193 cases underwent reconstruction by esophagogastrostomy.
The aim of the present study was to use the data from the PGSAS study to evaluate the influence of various surgical factors (size of remnant stomach, anti-reflux procedures, pyloric bougie, and reservation of pyloric vagal nerve) on post-operative complaints and QOL after proximal gastrectomy with esophagogastrostomy.
Materials and methods
Patients
A total of 52 institutions participated in this study. Eligibility criteria for patients were as follows: (1) pathologically proven stage IA or IB gastric cancer; (2) underwent gastrectomy for the first time; (3) aged ≥20 and ≤75 years; (4) no history of chemotherapy; (5) no obvious recurrence or metastasis; (6) an interval of 1 year or more after gastrectomy; (7) Eastern Cooperative Oncology Group performance status ≤1; (8) fully capable of understanding and responding to the questionnaire; (9) no history of other disease or surgeries that might influence responses to the questionnaire; (10) no organ failure or mental illness; (11) written informed consent. Patients with dual malignancy and concomitant resection of other organs for another disease (co-resection equivalent to cholecystectomy being the exception) were excluded.
QOL assessment
The PGSAS-45 questionnaire consists of 45 questions, with eight items from the Short-Form Health Survey (SF-8) [11], 15 items from the Gastrointestinal Symptom Rating Scale (GSRS) [12], and 22 clinically important items selected by the Japan Postgastrectomy Syndrome Working Party (Table 1). The PGSAS-45 questionnaire includes 23 items pertaining to post-operative symptoms (items 9–33), including 15 items from the GSRS and eight newly selected items. In addition, 12 questionnaire items pertaining to dietary intake, work, and level of satisfaction with daily life were selected. Dietary intake items include five questions about the amount of food ingested (items 34–37, 41) and three about the quality of ingestion (items 38–40). One questionnaire item pertains to work (item 41), while three items address the level of satisfaction with daily life (items 43–45). A seven-point (1–7) Likert scale was used for the 23 symptom items, and a five-point (1–5) Likert scale was used for all other items, except items 1, 4, 29, 32, and 34–37. For items 1–8, 34, 35, and 38–40, higher scores indicate a better condition. For items 9–28, 30, 31, 33, 36, 37, and 41–45, higher scores indicate a worse condition.
The 23 items were consolidated into seven symptom subscales. Each subscale score was calculated as the mean of included items, except the physical component summary (PCS) and the mental component summary (MCS) of the SF-8; the total symptom score was calculated as the mean of the seven symptom subscales. Assessment data included total symptom score, quality of ingestion, level of satisfaction with daily life, the PCS and MCS of the SF-8, and seven symptom subscales as main outcome measures. In addition, changes in body weight, amount of food ingested per meal, necessity of additional meals, ability to work, dissatisfaction with symptoms, dissatisfaction with the meal, and dissatisfaction with working were selected as main outcome measures.
Surgical procedure
From the operation record, five surgical procedure variables were investigated: (1) whether or not procedures such as fundoplication or creation of an angle of His were undertaken to prevent gastroesophageal regurgitation; (2) the length of resected abdominal esophagus; (3) the size of the remnant stomach (more than three-quarters, two-thirds, and less than one-half);(4) whether or not drainage procedures, such as pyloroplasty or pyloric bougie, were performed; and (5) whether or not preservation of the vagal nerve was performed.
Study methods
This study utilized continuous sampling from a central registration system to enroll participants. The questionnaire was distributed to all eligible patients. Patients were instructed to complete the questionnaire and return it to the data center. All QOL data from the questionnaire were matched with individual patient data collected via case report forms. This study was registered with the University Hospital Medical Information Network’s Clinical Trials Registry (UMIN-CTR; registration number 000002116). This study was approved by local ethics committees at each institution.
Statistics
The unpaired t test was used to compare between two groups. Analysis of variance (ANOVA) was used to compare among more than three groups. A p value <0.05 was considered statistically significant in both tests. When the p value in ANOVA was <0.1, a Bonferroni–Dunn multiple comparisons test was used for analysis of quantitative differences among the groups; in these, p values <0.05 divided by the number of combinations among the groups were considered statistically significant. When the p values were <0.1 in the t test or were less than double the significant level in the Bonferroni–Dunn multiple comparisons test, the effect size (Cohen’s d) was calculated. The value of Cohen’s d reflects the impact of each causal variable: values between 0.2 and <0.5 denote a small but clinically meaningful difference between groups; values between 0.5 and <0.8 denote a medium effect; and values ≥0.8 indicate a large effect.
All statistical analyses were conducted with StatView software for Windows version 5.0 (SAS Institute Inc., Cary, NC, USA).
Results
Retrieving the questionnaire
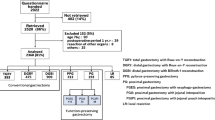
The questionnaire was distributed to 2,922 patients between July 2009 and December 2010 (Fig. 1). Of these distributed questionnaires, 2,520 (86 %) were retrieved; 152 questionnaires met exclusion criteria and were therefore excluded. A total of 2,368 questionnaires were analyzed. Proximal gastrectomy was performed in 193 of these 2,368 patients, and 115 cases were reconstructed by esophagogastrostomy. Questionnaires of these 115 cases were selected for examination in this study.
Patient characteristics
Patient characteristics for the 115 cases are listed in Table 2. The mean post-operative period was more than 3 years. The pyloric branch of the vagal nerve was preserved in most cases, and the size of the remnant stomach was more than two-thirds the pre-operative size in most cases.
Anti-reflux procedure
Anti-reflux procedures (e.g. fundoplication or creation of an angle of His) were included in 73.9 % of the cases (Table 3). Among the main outcome measures, the scores for the esophageal reflux subscale (p = 0.054, Cohen’s d 0.44), amount of food per meal (p = 0.087, Cohen’s d 0.38), and dissatisfaction with the meal (p = 0.053, Cohen’s d 0.43) were lower (i.e. better condition) in the patients who had undergone an anti-reflux procedure than in those who had not, with marginal significance. Among the 23 abdominal symptom items, the scores for acid regurgitation (p = 0.020, Cohen’s d 0.51), and bile regurgitation (p = 0.062, Cohen’s d 0.42) were lower among patients who had undergone an anti-reflux procedure, and were significant and marginally significant, respectively.
Length of resected abdominal esophagus
The length of co-resected abdominal esophagus was classified into two categories: <1.5 and >2 cm (Table 4). The post-operative QOL of patients with a resected esophagus length of <1.5 cm was relatively better than the others in the aspects of meal-related distress (p = 0.055, Cohen’s d 0.47), diarrhea subscales (p = 0.052, Cohen’s d 0.45), and the PCS of the SF-8 (p = 0.071, Cohen’s d 0.40), with borderline significance. Among the other symptom outcomes measures, borborygmus (p = 0.008, Cohen’s d 0.65), increased passage of stools (p = 0.062, Cohen’s d 0.43), urgent need for defecation (p = 0.053, Cohen’s d 0.45), sense of food sticking (p = 0.076, Cohen’s d 0.46), and early satiation (p = 0.064, Cohen’s d 0.43) were better in the group in which the esophagus was co-resected <1.5 cm, and were either significant or marginally significant. In contrast, the score for necessity of additional meals was relatively worse in the <1.5 cm group than in the other (p = 0.091, Cohen’s d 0.44).
Size of remnant stomach
In most cases (84.3 %), the preserved remnant stomach was more than two-thirds of the pre-operative size (Table 5). Comparing cases with stomach size two-thirds of the original and cases with more than three-quarters the original, the scores for necessity of additional meals (p = 0.011, Cohen’s d 0.66) and the subscale of diarrhea (p = 0.022, Cohen’s d 0.57) were lower (i.e. better condition) in the ‘more than three-quarters’ group, and were either significant or marginally significant, and had medium effect in terms of Cohen’s d values.
Pyloric bougie
Neither pyloric bougie nor pyloroplasty were performed in 84 cases; pyloric bougie was performed in 26 cases (Table 6). In the pyloric bougie cases, the abdominal pain subscale (p = 0.037, Cohen’s d 0.53), indigestion subscale (p = 0.047, Cohen’s d 0.48), total symptom score (p = 0.021, Cohen’s d 0.61), ingested amount of food per meal (p = 0.028, Cohen’s d 0.49), and dissatisfaction with the meal (p = 0.044, Cohen’s d 0.44) were significantly better than those for cases without pyloric bougie. The constipation subscale (p = 0.068, Cohen’s d 0.40) as well as the MCS of the SF-8 (p = 0.059, Cohen’s d 0.44) were better in the cases with pyloric bougie, with borderline significance. Scores of the five abdominal symptoms (sucking sensations in the epigastrium (p = 0.016, Cohen’s d 0.66), nausea and vomiting (p = 0.049, Cohen’s d 0.45), feeling of incomplete evacuation (p = 0.038, Cohen’s d 0.50), eructation (p = 0.096, Cohen’s d 0.41), and increased passage of stools (p = 0.085, Cohen’s d 0.44) were better in cases with pyloric bougie and were either significant or marginally significant.
Preservation of the pyloric branch of the vagal nerve
Preservation of the pyloric branch of the vagal nerve was performed in 91 (79.1 %) cases (Table 7). The diarrhea subscale score was lower (i.e. better condition) in nerve-preserving cases, with borderline significance (p = 0.080, Cohen’s d 0.40). Among the abdominal symptoms scores, those for urgent need for defecation (p = 0.040, Cohen’s d 0.47) and increased passage of stools (p = 0.083, Cohen’s d 0.39) were lower in the nerve-preservation cases, and were significant and marginally significant, respectively.
Discussion
Proximal gastrectomy is adopted for early gastric cancer in the upper third of the stomach as a function-preserving operation [4–7]. Although the usefulness of proximal gastrectomy has been reported, there are some problems to consider after proximal gastrectomy in terms of reflux esophagitis, gastric emptying, reservoir capacity, and so on [8, 9]. The relatively common reconstructive methods after proximal gastrectomy are esophagogastrostomy, jejunal interposition, and jejunal pouch reconstruction. Our PGSAS study evaluated 193 cases of proximal gastrectomy, and the methods adopted most frequently were esophagogastrostomy [115 cases (59.6 %)], jejunal pouch reconstruction [44 (22.8 %)], and jejunal interposition [40 (20.7 %)]. It is assumed that reconstruction via esophagogastrostomy is most widely used as it is a comparatively simple and easy method. With the growing use of laparoscopic surgery, esophagogastrostomy has become increasingly common as a reconstructive method after proximal gastrectomy. However, a higher incidence of reflux esophagitis has been observed with this method compared with other procedures [9].
Many surgical options can influence QOL after proximal gastrectomy: size of the remnant stomach, anastomosis method, addition of procedures to preserve anti-reflux function (e.g. fundoplication or creation of an angle of His), length of resected abdominal esophagus, plasty or bougie of the pyloric ring, and preservation of the pyloric vagal nerve.
In this study, as part of the PGSAS study, the influence of these surgical procedures on the QOL of patients after proximal gastrectomy with esophagogastrostomy were examined using a questionnaire survey.
Procedures to prevent gastroesophageal reflux (e.g. fundoplication or creation of an angle of His [13, 14] ) were undertaken in 71.3 % of cases. These procedures were estimated to be effective for acid and bile regurgitation and led to relatively better QOL in the esophageal reflux, ingested amount of food per meal, and dissatisfaction at the meal subscales.
In terms of the length of resected abdominal esophagus, among the cases in which the resected esophagus was >2 cm, the function of the lower esophageal sphincter (LES) was assumed to be almost lost; among cases <1.5 cm, such function was likely to be maintained. The subscales of meal-related distress, diarrhea, and the PCS of the SF-8 were relatively worse in cases with a resected esophagus length >2.0 cm, while the necessity of additional meals was unexpectedly worse in cases <1.5 cm. Our results raised an apparent discrepancy concerning the implication of the length of resected esophagus that we are unable to explain.
Regarding the size of the remnant stomach, more than two-thirds of the stomach was preserved in 84.3 % of our patients. Comparing cases with two-thirds remnant stomach and those with more than three-quarters, patients with two-thirds remnant stomach required significantly more additional meals and had relatively higher scores (i.e. worse condition) in the diarrhea subscale than those with larger remnants. In this study, although a large remnant of the stomach was preserved in most cases, we have shown that QOL was improved with a larger reservoir capacity.
The necessity for pyloroplasty as a drainage procedure after proximal gastrectomy has been controversial for some time [15–17]. However, proximal gastrectomy is currently mostly applied for early gastric cancer in which resected proximal stomach is mostly less than half and, in many cases, the pyloric branch of the vagal nerve is preserved. In such cases, pyloroplasty is considered unnecessary as a drainage procedure, especially in cases where the pyloric branch of the vagal nerve is preserved. Therefore, only four cases (3.5 %) in the present study included the addition of pyloroplasty. Pyloric bougie has been reported to achieve results similar to those of conventional pyloroplasty in the early post-operative period [18–20]; 26 (23.6 %) of our cases employed the pyloric bougie as a complementary drainage procedure. In cases without pyloric bougie, several abdominal symptoms (e.g. sucking sensation in the epigastrium, nausea and vomiting, feeling of incomplete evacuation) were increased compared with cases with pyloric bougie. This may have resulted in decreased QOL, reflected in higher scores in the subscales of abdominal pain, indigestion, and total symptoms, and diminished ingested amount of food per meal. The pyloric bougie method is commonly considered effective in the early post-operative period. Interestingly, our results indicated that pyloric bougie was associated with improved QOL, even after a longer period such as more than 1 year after surgery. The pyloric bougie method might be effective as a complementary drainage procedure for years after surgery. In our study, preservation of the pyloric branch of the vagal nerve was undertaken in 79.1 % of patients. Patients in whom the pyloric branch was not preserved reported more instances of increased passage of stools, urgent need for defecation, and diarrhea than those in whom the nerve was preserved. Cooperative pyloric function was affected by the excision of the pyloric branch of the vagal nerve, and we considered that the above symptoms were caused by rapid emptying of food from the remnant stomach.
This study is a part of the PGSAS study, a nationwide, multi-institution surveillance study to investigate the QOL of patients following gastrectomy. The findings of this study may help to determine the appropriate surgical procedures to improve QOL in patients undergoing proximal gastrectomy with esophagogastrostomy. However, this study has limitations; namely, the large number of statistical comparisons that were necessary to examine in detail the implications of various surgical procedures on patient’s QOL. Since our study was an exploratory study, further study is needed to prove the differences found in the present study.
Conclusion
Our results indicate that in proximal gastrectomy with esophagogastrostomy, improved QOL could be associated with larger remnant stomach, shorter length of resected abdominal esophagus, procedures to prevent gastroesophageal reflux, pyloric bougie as a complementary drainage procedure, and preservation of the pyloric branch of the vagal nerve.
References
Salvon-Harman JC, Cady B, Nikulasson S et al (1994) Shifting proportion of gastric adenocarcinomas. Arch Surg 129:381–389
Okabayashi T, Gotoda T, Kondo H et al (2000) Early carcinoma of the gastric cardia in Japan: is it different from that in the west? Cancer 89:2555–2559
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123
Sun Y, Yang Y (2011) Study for the quality of life following total gastrectomy of gastric carcinoma. Hepatogastroenterology 58:669–673
Kunisaki S, Akiyama H, Nomura M et al (2005) Surgical outcomes for early gastric cancer in the upper third of the stomach. J Am Coll Surg 200:15–19
Shiraishi N, Adachi Y, Kitano S et al (2002) Clinical outcome proximal versus total gastrectomy for proximal gastric cancer. World J Surg 26:1150–1154. doi:10.1007/s00268-002-6369-6
Katai H, Morita S, Saka M et al (2010) Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg 97:558–562
Kumagai K, Shimizu K, Yokoyama N et al (2012) Questionnaire survey regarding the current status and conventional issues concerning reconstruction after gastrectomy in Japan. Surg Today 42:411–418
Lei W, Xin-Zu C, Bin W et al (2012) Total vs. proximal gastrectomy for proximal gastric cancer: a systemic preview and meta-analysis. Hepatogastroenterology 59(114):633–640
Nakada K, Ikeda M, Takahashi M et al (2013) Development and validation of PGSAS-45, an Integrated Questionnaire to Assess Postgastrectomy Syndrome. Gastroenterology 144(5 Supple 1):S–1111
Turner-Bowker DM, Bayliss MS, Ware JE Jr et al (2003) Usefulness of the SF-8 Health Survey for comparing the impact of migraine and other conditions. Qual Life Res 12:1003–1012
Svedlund J, Sjodin I, Dotevall G (1988) GSRS: a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 33:129–134
Ichikawa D, Komatsu S, Okamoto K et al (2013) Evaluation of symptoms related to reflux esophagitis in patients with esophagogastrostomy after proximal gastrectomy. Langenbecks Arch Surg 398:697–701
Matsushiro T, Hariu T, Nagashima H et al (1986) Valvuloplasty plus fundoplasty to prevent esophageal regurgitation in esophagogastrostomy after proximal gastrectomy. Am J Surg 152:314–319
Nakane Y, Michiura T, Inoue K et al (2004) Role of pyloroplasty after proximal gastrectomy for cancer. Hepatogastroenterology 51:1867–1871
Velanovich V (2003) Esophagogastrostomy without pyloroplasty. Dis Esophagus 16:243–245
Tokunaga M, Hiki N, Ohyama S et al (2009) Effects of reconstruction methods on a patient’s quality of life after a proximal gastrectomy: subjective symptoms evaluation using questionnaire survey. Langenbecks Arch Surg 396:637–641
Yamashita Y, Hirai T, Mukaida H et al (1999) Finger bougie method compared with pyloroplasty in the gastric replacement of the esophagus. Surg Today 29:107–110
Kuwabara K, Matsuda S, Fushimi K et al (2012) Comparative study on the difference in functional outcomes at discharge between proximal and total gastrectomy. Case Rep Gastroenterol 6:400–409
Hai AA, Singh A, Mittal VK (1986) Closed pyloroduodenal digital dilatation as a complementary drainage procedure to truncal vagotomy. Int Surg 71(2):87–90
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inada, T., Yoshida, M., Ikeda, M. et al. Evaluation of QOL After Proximal Gastrectomy Using a Newly Developed Assessment Scale (PGSAS-45). World J Surg 38, 3152–3162 (2014). https://doi.org/10.1007/s00268-014-2712-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2712-y