Abstract
Background
Gastrointestinal and abdominal bleeding can lead to life-threatening situations. Embolization is considered a feasible and safe treatment option. The relevance of surgery has thus diminished in the past. The aim of the present study was to evaluate the role of surgery in the management of patients after embolization.
Methods
We performed a retrospective single-center analysis of outcomes after transarterial embolization of acute abdominal and gastrointestinal hemorrhage between January 2009 and December 2012 at the Sisters of Charity Hospital, Linz. Patients were divided into three groups, as follows: upper gastrointestinal bleeding (UGIB), lower gastrointestinal bleeding (LGIB), and abdominal hemorrhage.
Results
Fifty-four patients with 55 bleeding events were included. The bleeding source could be localized angiographically in 80 %, and the primary clinical success rate of embolization was 81.8 % (45/55 cases). Early recurrent bleeding (<30 days) occurred in 18.2 % (10/55) of the patients, and delayed recurrent hemorrhage (>30 days) developed in 3.6 % (2/55). The mean follow-up was 8.4 months, and data were available for 85.2 % (46/54) of the patients. Surgery after embolization was required in 20.4 % of these patients (11/54). Failure to localize the bleeding site was identified as predictive of recurrent bleeding (p = 0.009). More than one embolization effort increased the risk of complications (p = 0.02) and rebleeding (p = 0.07).
Conclusions
Surgery still has an important role after embolization in patients with gastrointestinal and abdominal hemorrhage. One of five patients required surgery in cases of early and delayed rebleeding or because of ischemic complications (2/55 both had ischemic damage of the gallbladder) and bleeding consequences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal bleeding is a common medical condition that results in significant morbidity and medical care costs. Nowadays the affected patient population tends to be older, has more comorbidities, and is prescribed antiplatelet or antithrombotic medications more frequently [1]. Because of the associated risks, the gastroenterological diagnosis might be masked, affecting the choice of therapy [2, 3]. In recent decades the regional annual incidence of gastrointestinal bleeding in Europe has been described as affecting between 37 and 172/100,000 population [4, 5]. Upper gastrointestinal bleeding (UGIB), lower gastrointestinal bleeding (LGIB), and some kinds of intra-abdominal and retroperitoneal bleeding can lead to life-threatening situations. Mortality from UGIB is estimated to be as high as 15 % [6]; that for LGIB, up to 5 % [7].
The most common reason for UGIB (above the ligament of Treitz) is peptic ulcers (in 30 %) [8]. Endoscopy is the most important tool in the diagnosis and therapy of UGIB. The development of angiography and therapeutic embolization has provided an encouraging additional treatment option if endoscopy fails or cannot be performed [9, 10]. Surgery still remains a treatment option in case of a failure of endoscopic or angiographic intervention. Negative selection in these cases, in combination with prolonged hemorrhagic shock, leads to high mortality (up to 40 %) in the operative group [11–13].
Hospitalization is frequently required for patients with LGIB (below the ligament of Treitz), particularly the elderly. The bleeding source is in the colon in 90 % and in the small bowel in only 10 %. Older patients most often have colonic sources of bleeding, caused by diverticula, colitis, angiodysplasia, or cancer [14–16]. Although haemorrhage stops spontaneously in most patients (80 %) without intervention, a small number of LGIB can present as acute and life-threatening events. Different diagnostic studies are available for evaluation and treatment, including colonoscopy, angiography, radionuclide scintigraphy, and multi-detector computed tomography [14, 16–18]. Angiography is used if colonoscopy fails or cannot be performed. Surgery is the final approach and is used in cases of severe bleeding [19].
An important cause of intra-abdominal or retroperitoneal bleeds is the inflammatory enzymatic erosion of visceral arteries conditioned by hemosuccus pancreaticus [20, 21]. If surgical intervention cannot be avoided, there is a clear trend away from radical approaches that involve extensive resections toward tailored surgery [22]. The increased implementation of percutaneus liver puncture and transhepatic drains has led to an increase in hemobilia (1 and 4 %) [23, 24]. The surgical method in case of bleeding or bile obstruction depends on intrahepatic or extrahepatic localization [25]. The mainstay of diagnosis and initial treatment is angiography and embolization [20, 21, 23].
Embolization is now accepted as feasible and safe, with durable bleeding control and low complication rates in UGIB and LGIB, but also in different kinds of intra-abdominal and retroperitoneal hemorrhage [25–27]. Nevertheless, postembolic complications can occur. These complications are typically characterized by ischemic small or large bowel damage in LGIB, whereas ischemia rarely occurs in UGIB because of better vascular collateralization. Another characteristic complication is rebleeding in consequence of embolic agent migration, insufficient vessel occlusion, or formation of collateral circulation [26–28].
It has been advocated that surgery be seen as no longer relevant, because of the development of modern endoscopy and radiology with many techniques and tools for hemostasis [27–29]. The aim of the present study was to evaluate the role of surgery in the management of failed embolization of abdominal sources of bleeding or treatment of complications following angiographic embolization and to identify predictive factors of treatment success.
Methods
A retrospective single-center analysis of patient outcomes after severe gastrointestinal or abdominal bleeds, reported from the Department of General and Visceral Surgery at the Sisters of Charity Hospital Linz, Austria, between January 2009 and December 2012 was performed. Virtually all embolization procedures were documented in an electronic database. We searched systematically for gastrointestinal and abdominal (retroperitoneal) bleeds and excluded all patients from other specialist disciplines (urological, gynecological). There were no further exclusion criteria, and all consecutive patients who underwent radiological transarterial catheter embolization via a percutaneous transfemoral approach were included. Rebleeding was defined as a bleeding event that occurred after the embolization session, including removal of the transarterial catheter (early rebleeding <30 days and delayed rebleeding >30 days). Technical and clinical success rates of embolization related to the frequency of direct proof of bleeding sources were evaluated. Clinical treatment success of radiological intervention was defined as embolization without rebleeding within 30 days after withdrawal of the transarterial catheter. If the catheter was left in place up to 24 h for re-angiography, repeat embolizations were recorded as efforts to correct ongoing bleeds and not as treatment for rebleeding.
Patients were cathgorized into three groups, as follows: of upper gastrointestinal bleeds (UGIB), lower gastrointestinal bleeds (LGIB), and intra-abdominal or retroperitoneal bleeds. Localization of the exact bleeding source, diagnosis, malignancy, intensity of bleeding, hemodynamic impact, extend of blood product substitution, coagulopathy, rate and type of pre-existing medical anticoagulation, and comorbidities were documented. Diagnosis before angiography in cases of gastrointestinal bleeding was achieved by gastroscopy and/or colonoscopy.
If patients had with hemodynamic instability, primary angiography was performed after endoscopy and resuscitation in the critical care unit. In stable patients, or in patients with abdominal bleeds, a contrast-enhanced computed tomography study was performed before angiography for localizing the bleeding source. One of three experienced interventional radiologists is on call around the clock.
Management in cases of technically or clinically unsuccessful embolization and in the event of postembolic ischemic complications was analyzed, and the need for surgical interventions was determined. Despite successful embolization, we recorded the following surgical procedures performed to manage further bleeding from intra-abdominal, retroperitoneal, intraluminal, or parenchymal hematoma.
Furthermore, delayed surgery more than 30 days after embolization was evaluated as it affected length of hospital stay and time of occurrence during the follow-up period. In addition, the 30 day and overall mortality were documented.
Routine follow-up included endoscopic re-evaluation after embolization of UGIB and LGIB before the patient was discharged from hospital and for 4–6 weeks after discharge. Patients with endoscopically inaccessible small bowel bleeds and abdominal or retroperitoneal hemorrhage were followed up by anamnesis, physical examination, and blood test 4 and 6 weeks after diagnosis. All patients were advised to call an emergency doctor and to seek immediate hospital admission in case of clinical signs of recurrent bleeding. Data were collected from the institution’s medical records (SAP) and medical files.
The protocol for this research project was approved by the ethics committee of the Sisters of Charity Hospital, Linz, Austria, and it conformed to the provisions of the Declaration of Helsinki (as revised in Seoul 2008).
Statistical analysis was performed with SPSS Statistical Analysis Software, version 20. (SPSS Inc. Chicago, IL). Comparison of data was accomplished with the paired t test or the Wilcoxon signed rank test on a per subject basis. Population homogeneity was conducted using either the independent t-tests or the Mann–Whitney U test. If normally distributed, they were additionally presented as means. A multivariate analysis for predictors of complications and rebleeding was performed. For correlated proportions at the margins of a 2 × 2 contingency table, McNemar’s test and in some cases, descriptive statistics were used. Probability recorded as p < 0.05 was regarded as statistically significant.
Results
Over a period of four years (January 2009–December 2012), 54 consecutive in-patients hospitalized in our Department for Surgery or Gastroenterology with acute gastrointestinal or visceral and retroperitoneal bleeding were recruited for the study. An overview of those patients’ demographic and disease characteristics is shown in Table 1. One patient had two different bleeding sources and localizations (duodenum and ileum) which were embolized in two sessions. As a consequence, a total of 55 acute transarterial angiographic interventions were analyzed.
Angiography and transarterial catheter embolization were technically effective in all 55 cases (100 %), and the overall clinical success rate (no rebleeding within 30 days after intervention) was 81.8 % (45/55) with 18.2 % (10/55) recurrent bleeds.
Figures 1, 2, and 3 show the disease characteristics of the three groups of patients with bleeding classification, localization diagnosis, and rebleeding management. The statistically significant factors influencing embolization failure (rebleeding and complications) are shown in Table 2.
The impracticality of clear localization of the bleeding source by angiography before radiological intervention was a significant predictor of increased recurrence (p = 0.009; OR 6.5, CI 1.438–29.377).
Application of coils for embolization showed a significantly lower rate of recurrent bleeds than other agents (liquids/gelatin sponge/particles: 5/41,12.2 % coils vs 5/14, 35.7 % other agents, p = 0.049; OR 4.0, CI 0.949–16.862).
The occurrence of rebleeding after embolization efforts showed no significance, but there was a trend toward an increased rate of rebleeding after more than one embolization effort, recorded as follows: 5/40 (12.5 %) after one effort vs 5/15 (33.3 %) after two or more efforts (p = 0.07; OR 3.5, CI 0.842–14.552).
Repeated embolization efforts significantly increased the rate of postembolic complications; none of 40 (0 %) occurred after one embolization effort vs 2/15 (13.3 %) after two or more embolization efforts (p = 0.02; OR 1.154, CI 0.946–1.407). The parameters that did not have a statistically significant influence on treatment success are outlined in Table 3.
Follow-up, morbidity, and mortality
The mean follow-up was 8.4 months, ranging from 1 to 40 months. Routine follow-up was available for all surviving patients. Some patients required a longer follow-up period because of their underlying diseases or other medical reasons. Long-term follow-up was individually arranged, and medical follow-up data were available for 46 of the 54 patients (85.2 %). Four patients died within 30 days in hospital and four patients refused follow-up examination without stating complaints.
The overall mortality rate during follow-up was 14.8 % (8/54); 30 day mortality was 7.4 % (4/54). Three patients died as a consequence of progressive and metastatic malignant disease; one patient of multimorbidity and cardiac decompensation. No deaths were related to uncontrollable bleeding or complications of embolization. From the remaining 4 patients who died beyond 30 days, three had progressive and metastatic malignancies and one had biliary necrotizing pancreatitis.
Surgery
For both early and delayed surgery, one of five (11/54; 20.4 %) patients required surgical intervention for bleeding and embolization. Figure 4 shows an overview of surgical indications, and Table 4 provides a summary of the diagnosis and surgical treatment.
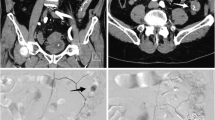
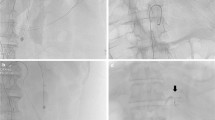
Postembolic complications occurred in 2/54 patients (3.7 %). In both patients surgical intervention was necessary because of ischemic damage and inflammation of the gallbladder leading to laparoscopic cholecystectomy. Two more patients (2/54; 3.7 %) required surgery despite successful embolization: One patient developed a large hematoma in the bursa omentalis (Fig. 5), because of inflammatory enzymatic blood vessel erosion in association with pancreatitis. The hematoma was evacuated and drained at laparotomy. The other patient developed an ileus of the small bowel after embolization of a jejunal angiodysplasia refractory to conservative treatment. Intraoperatively we found adhesions and a small bowel wherein blood clots blocked intestinal passage. The clots were evacuated by enterotomy. Because of localized intestinal wall ischemia, a segment of small bowel needed to be resected, and adhesiolysis was performed as well (Fig. 6).
Discussion
The present study shows that in a relevant proportion of patients after interventional radiology because of gastrointestinal and abdominal bleeding, surgery remains the cornerstone of successful treatment. Gastrointestinal hemorrhage is now an example of a condition being addressed by patient-oriented, interdisciplinary, and individualized therapy [19, 30]. Transarterial catheter embolization techniques using coils, sponges, particles, and liquids has become established as an attractive means for detecting, treating, and controlling abdominal and gastrointestinal bleeding, especially when endoscopy fails or cannot be performed [9, 10, 27]. However, a recent review from 2013 by Yap et al. [26] that included 95 patients—80 % UGIB and 20 % LGIB—reported 30 days rebleeding rates of 23 % and 30 days mortality of 18 %.
Another review, by Mirsadraee et al. [10], included 927 patients with non-variceal UGIB and demonstrated equivalent mortality and clinical success rates between embolization and surgery. In their retrospective analysis, Lee et al. reported that angiographic embolization helps to decrease the rate of emergency surgical interventions, which shows a greater 30 days mortality that non-embolization. Blind embolization was accompanied by higher rates of rebleeding and was defined as an independent predictor of death in patients without active extravasation [31]. The results of our study underline these findings, showing a significantly higher risk of rebleeding in cases of blind embolization.
In peptic ulcer bleeds, embolization may be effective for even the most gravely ill patients, because of blind but effective embolization of the gastroduodenal artery. For these patients, surgery is not a reliable option, even when extravasation is not visualized by angiography. This clinical event might be seen as an exception with mostly successful blind embolization. So the role of the surgeon in this clinical sphere is dramatically diminishing. Surgery is typically reserved for patients in whom the bleeding failed to respond to all previous treatments. Such a situation has become extremely rare [27, 28, 32, 33]. The results of our study show that surgery plays no substantial role in the management of patients with peptic ulcer bleeding.
Endoscopy was performed in all cases, but in cases of Forrest Ia/b bleeds, we did not take the risk of endoscopic hemostasis and patients underwent early coil embolization of the gastroduodenal artery with high rates of success. The available data from reported series comparing embolization with surgery for UGIB after failed endoscopic hemostasis suggest that transarterial embolization (TAE) is a good alternative to surgery and an approach that can be considered the treatment of choice. We agree with that conclusion, and therefore embolization is our mainstay of therapy after failed endoscopic localization of the bleeding site or successful endoscopic hemostasis in cases of severe UGIB. Nevertheless, prospective studies are needed to compare these management strategies [27, 32, 33]. From the radiological perspective, it is indispensable to know exactly the vascular anatomy and variations for successful embolization, information that is sometimes required bilaterally. Furthermore, interventional radiology and the chance for embolization is not available everywhere. To avoid time loss, we also recommend that prolonged endoscopy not be carried out in unstable patients with Forrest 1a back-wall duodenal ulcer bleeds. In such a delicate condition only emergency surgery can save the patient’s life. Therefore, if initial endoscopy is successful clinicians should consider transporting the patient to a specialized center for embolization because of high risk of rebleeding and increased mortality [11–13, 27, 28, 32, 33].
A concern of pancreaticoduodenal embolization is the risk of ischemic damage to the gallbladder, which has an inconsistent blood supply and which is difficult to assess. This was the only form of ischemic complication that occurred in our series.
In comparison to LGIB, embolization of UGIB may not lead to such excellent results (recurrent bleeding up to 27 %). The complex blood supply and collateral vessels might therefore be the reason. Nevertheless, ischemic complications are rare [34].
The results of our study do not confirm previous findings. In our series, early rebleeding after embolization of UGIB tended to be less frequent than in cases of LGIB. A potential explanation for this fact might be that bleeds from duodenal peptic ulcers show excellent results following gastroduodenal artery embolization. However, we observed one case of embolization failure because of vast collaterals after previous chemoembolization.
Concerning LGIB, postembolic ischemic complications are also decreasing, because of technical advancement. Superselective probing with pinpoint occlusion of small blood vessels could be performed in LGIB [35]. We did not see any ischemic damage in LGIB. This was unexpected. The risk is described at about 4 % in a recent report [26]. It is remarkable that even in repeat endoscopy by knowing the embolization area no mucosal discolorations could be detected. From the radiological point of view it is very important to perform superselective probing with embolization on the level of the vasa recta, and maximally two vessels next to each other have to be closed. However, the surgical case of blood clotting ileus showed local ischemia of the intestinal wall after two embolization efforts. The clinical relevance of this finding was not clear, and therefore it was not counted as a postembolic complication but rather as bleeding consequence requiring surgery despite successful embolization. For good measure, a segmental bowel resection was performed.
Recent data regarding outcomes after embolization of LGIB support TAE as being feasible, safe, and effective in primary treatment [36–40], but also as a durable and definitive treatment in the long-term follow-up of 72 months [41, 42].
In the series cited, the rate of technical success ranged from 84 to 100 %; the clinical success, from 63 to 90 %. Postembolic complications occurred in 6 to 8 %, rebleeding occurred in 10 to 26 %, and surgery was required in 10 to 28 %.
One study, by Gillespie et al. [36], analyzed 83 cases of LGIB and documented the finding that repeated embolization was associated with a higher rate of complications. In our series, two postembolic complications, in the form of ischemic gallbladder lesions, occurred after repeated embolization, and one-third of patients after two or more sessions of embolization suffered from early recurrent bleeding. These results suggest that repeated embolization might be associated with a higher risk of complications and failure. Embolization is sometimes performed without extravasation, but in some cases vascular irregularities may hint at subsequent bleeding. When there is doubt, it is advisable to leave the femoral transarterial approach for immediately re-angiography in cases of re-bleedings.
Although indications for surgery in LGIB have been widely described, current practice varies. The findings of a retrospective review suggest that even patients with approved indications for surgery can be safely managed nonoperatively, even when the bleeding source has not been unlocalized [43]. Whatever surgery is necessary in LGIB, the indication and method depend on severity, cause, and localization, but also on patient age, general health condition, and comorbidities. The mortality increases up to 10 to 57 % when surgery must be done because all other treatments have failed [44–46]. We recorded no surgically associated mortality after embolization failure in LGIB, but the surgical effort was high. Five of 8 surgical resections were required in these cases. In one case, specific resection was possible only after a loop stoma was created as a means of limiting the bleeding source.
From the radiological point of view, in cases of large angiodysplasias, arteriovenous malformations, and extensive tumor bleeds, embolization should be restrictively applied because of vascular collateralization, an irregular and entangled blood supply, and a high failure rate.
Regarding the higher embolization success with the use of coils as embolic agents, the radiological explanation is as follows: liquids and particles do not provide the permanent vascular obliteration coils do, and they tend to be used more frequently in inconclusive situations according to the investigators’ preferences.
A limitation of our study was its retrospective single-arm observational design. A strength of the study was the inclusion of all consecutive patients undergoing gastrointestinal and abdominovisceral or retroperitoneal embolization during the study period. The interventional procedures were performed by only three experienced radiologists using the same techniques. The follow-up period was adequate and recruitment was high.
In conclusion, surgery is still an important part of the management of patients who undergo embolization because of severe gastrointestinal and abdominal bleeding. In our series, overall, one of five patients required surgery. Surgery has lost its importance for initial therapy and for avoiding recurrent bleeding. In cases of early rebleeding (<30 days after the initial procedure), surgery is often required (5/10; 50 %). Furthermore, surgery must be performed in cases of ischemic complications after embolization therapy (2/54; 3.7 %). Even if embolization is performed successfully, bleeding consequences can require surgery (2/54; 3.7 %). Surgery is also playing a part in the follow-up later than 30 days. In our series this involved cases of delayed recurrent hemorrhage (2/54; 3.7 %).
References
Sam C, Massaro JM, D’Agostino RB Sr et al (2004) Warfarin and aspirin use and the predictors of major bleeding complications in atrial fibrillation (the Framingham heart study). Am J Cardiol 94:947–951
Higham J, Kang JYMA (2002) Recent trends in admission and mortality due to peptic ulcer in England. Increasing frequency of haemorrhage among older subjects. Gut 50:460–464
Derry S, Loke JK (2000) Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ 321:1183–1187
van Leerdam ME, Vreeburg EM, Rauws EA et al (2003) Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenetrol 98:1494–1499
Blatchford O, Davidson LA, Murray WR et al (1997) Acute upper gastrointestinal hemorrhage in west of Scotland: case ascertainment study. BMJ 315(7107):510–514
van Leerdam ME (2008) Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 22:209–224
Longstreth GF (1997) Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 92:419–424
Di Fiore F, Lecleire S, Merle V et al (2005) Changes in characteristics and outcome of acute upper gastrointestinal haemorrhage: a comparison of epidemiology and practices between 1996 and 2000 in a multicenter French study. Eur J Gastroenterol Hepatol 17:641–647
Walker TG, Salazar GM, Waltman AC (2012) Angiographic evaluation and management of acute gastrointestinal hemorrhage. World J Gastroenterol 18:1191–1201
Mirsadraee S, Tirukonda P, Nicholson A et al (2011) Embolization for non variceal upper gastrointestinal tract haemorrhage: a systematic review. Clin Radiol 66:500–509
Bulut OB, Rasmussen C, Fischer A (1996) Acute surgical treatment of complicated peptic ulcers with special reference to the elderly. World J Surg 20:574–577. doi:10.1007/s002689900089
Hermansson M, Stael von Holstein C, Zilling T (1997) Peptic ulcer perforation before and after the introduction of H2-receptor blockers and proton pump inhibitors. Scand J Gastroenterol 32:523–529
Becker HD, Schriefers KH (1987) Operative treatment of gastric and duodenal hemorrhage. Surgery of the stomach. Indications, methods, complications. Springer, New York, pp 275–281
Farrell JJ, Friedmann LS (2005) The management of lower gastrointestinal bleeding. Aliment Pharamcol Ther 21:1281–1298
Foitzik T, Klar E (1996) Vaskuläre Notfälle des Darmes. In Winkeltau GJ, Lerch MM, (Hrsg) Gastroenterologische Notfalltherapie, Stuttgart, Wiss Verlag Ges, S, pp 239–247
Jensen DM, Machiado GA, Juthaba R et al (2000) Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med 342:78–82
Lhewa DY, Strate LL (2012) Pros and cons of colonoscopy in management of lower gastrointestinal bleeding. World J Gastroenterol 18:1185–1190
Geffroy Y, Rodallec MH, Boulay-Coletta I et al (2011) Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. M Radiographics 31:E35–E46
Barnert J, Messmann H (2009) Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol 6:637–646
Boudghene F, L’Hermine C, Bigot JM (1993) Arterial complications of pancreatitis: diagnosis and therapeutic aspects in 104 cases. J Vasc Interv Radiol 4:551–558
Jaideep U, Barge MD, Jorge E et al (2012) Vascular complications of pancreatitis: role of interventional therapy. Korean J Radiol 13:45–55
Knoefel WT, Hosch SB, Peiper M (2004) Chronic pancreatitis—from losing heart to acting smart! Eur J Med Res 9:563–564
Dousset B, Sauvanet A, Bardou M (1997) Selective surgical indications for iatrogenic hemobilia. Surgery 121:37–41
Pongchairerks P (1993) Ultrasound-guided liver biopsy: accuracy, safety and sonographic findings. J Med Assoc Thai 76:597–600
Rudich SM, Kinkhabwal MM, Murray NG (1998) Successful treatment of mycotic hepatic artery pseudoaneurysms with arterial reconstruction and liposomal amphotericin B. Liver Transpl Surg 4:91–93
Yap FY, Omene BO, Patel MN (2013) Transcatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomes. Dis Sci 58:1976–1984
Loffroy R, Estivalet L, Cherblanc V et al (2012) Transcatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomes. World J Gastrointest Surg 4:223–227
Loffroy R (2013) Transcatheter embolization as the new reference standard for endoscopically unmanageable upper gastrointestinal bleeding. Management of duodenal ulcer bleeding resistant to endoscopy: surgery is dead! World J Gastroenterol 19:1150–1151
Koh DC, Luchtefeld MA, Kim DG (2009) Efficacy of transarterial embolization as definitive treatment in lower gastrointestinal bleeding. BR Colorectal Dis 11:53–59
Bardou M, Benhaberou-Brun D, Le Ray I (2012) Diagnosis and management of nonvariceal upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol 9:97–104
Lee L, Iqbal S, Najmeh S et al (2012) Mesenteric angiography for acute gastrointestinal bleed; predictors of active extravasation and outcomes. Can J Surg 55:382–388
Eriksson LG, Ljungdahl M, Sundbom M et al (2008) Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol 19:1413–1418
Venclauskas L, Bratlie SO, Zachrisson K et al (2010) Is transcatheter arterial embolization a safer alternative than surgery when endoscopic therapy fails in bleeding duodenal ulcer? Scand J Gastroenterol 45:299–304
Defreyne L, Vanlangenhove P, De Vos M et al (2001) Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology 218:739–748
Funaki B, Kostelic JK, Lorenz J et al (2001) Superselective microcoil embolization of colonic hemorrhage. AJR 177:829
Gillespie CJ, Sutherland AD, Mossop PJ et al (2010) Mesenteric embolization for lower gastrointestinal bleeding. Dis Colon Rectum 53:1258–1264
Tan KK, Wong D, Sim R (2008) Superselective embolization for lower gastrointestinal hemorrhage: an institutional review over 7 years. World J Surg 32:2707–2715. doi:10.1007/s00268-008-9759-6
Lipof T, Sardella WV, Bartus CM et al (2008) The efficacy and durability of super-selective embolization in the treatment of lower gastrointestinal bleeding. Dis Colon Rectum 51:301–305
Kickuth R, Rattunde H, Gschossmann J et al (2008) Acute lower gastrointestinal hemorrhage: minimally invasive management with microcatheter embolization. J Vasc Interv Radiol 19:1289–1296
Teng HC, Liang HL, Lin YH et al (2013) The efficacy and long-term outcome of microcoil embolotherapy for acute lower gastrointestinal bleeding. Korean J Radiol 14:259–268
Ahmed TM, Cowley JB, Robinson G et al (2010) Long term follow-up of transcatheter coil embolotherapy for major colonic haemorrhage. Colorectal Dis 12:1013–1017
Maleux G, Roeflaer F, Heye S et al (2009) Long-term outcome of transcatheter embolotherapy for acute lower gastrointestinal hemorrhage. Am J Gastroenterol 104:2042–2046
Yi WS, Vegeler R, Hoang K et al (2012) Watch and wait: conservative management of lower gastrointestinal bleeding. J Surg Res 177:315–319
Garcia-Osogobio S, Remes-Troche JM, Takahaschi T et al (2002) Surgical treatment of lower digestive tract hemorrhage. Rev Invest Clin 54:119–124
Farner R, Lichliter W, Kuhn J et al (1999) Total colectomy versus limited colonic resection or acute lower gastrointestinal bleeding. Am J Surg 178:587–591
Renzulli P, Maurer CA, Netzer P et al (2002) Subtotal colectomy with primary ileorectostomy is effective for unlocalized diverticular hemorrhage. Langenbecks Arch Surg 387:67–71
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Köhler, G., Koch, O.O., Antoniou, S.A. et al. Relevance of Surgery after Embolization of Gastrointestinal and Abdominal Hemorrhage. World J Surg 38, 2258–2266 (2014). https://doi.org/10.1007/s00268-014-2570-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2570-7