Abstract
Background
This study was performed as a substudy analysis of a randomized trial comparing conventional open esophagectomy [open surgical technique (OE)] by thoracotomy and laparotomy with minimally invasive esophagectomy [minimally invasive procedure (MIE)] by thoracoscopy and laparoscopy. This additional analysis focuses on the immunological changes and surgical stress response in these two randomized groups of a single center.
Methods
Patients with a resectable esophageal cancer were randomized to OE (n = 13) or MIE (n = 14). All patients received neoadjuvant chemoradiotherapy. The immunological response was measured by means of leukocyte counts, HLA-DR expression on monocytes, the acute-phase response by means of C-reactive protein (CRP), interleukin-6 (IL-6), and interleukin-8 (IL-8), and the stress response was measured by cortisol, growth hormone, and prolactin. All parameters were determined at baseline (preoperatively) and 24, 72, 96, and 168 h postoperatively.
Results
Significant differences between the two groups were seen in favor of the MIE group with regard to leukocyte counts, IL-8, and prolactin at 168 h (1 week) postoperatively. For HLA-DR expression, IL-6, and CRP levels, there were no significant differences between the two groups, although there was a clear rise in levels upon operation in both groups.
Conclusion
In this substudy of a randomized trial comparing minimally invasive and conventional open esophagectomies for cancer, significantly better preserved leukocyte counts and IL-8 levels were observed in the MIE group compared to the open group. Both findings can be related to fewer respiratory infections found postoperatively in the MIE group. Moreover, significant differences in the prolactin levels at 168 h after surgery imply that the stress response is better preserved in the MIE group. These findings indicate that less surgical trauma could lead to better preserved acute-phase and stress responses and fewer clinical manifestations of respiratory infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies have shown that trauma, surgery, and anesthesia induce a state of immunosuppression [1, 2]. A downward regulation of the immune response and host defense against tumor cells may occur in the perioperative period. As a consequence, patients are more susceptible to infections and sepsis [1]. The immunological response to surgery has been studied more since the introduction of minimally invasive techniques. Laparoscopic surgery reduces the magnitude of operative trauma. If alterations of the systemic immune response are proportional to the extent of injury, then the response to minimally invasive techniques will be reduced when compared with open surgery. A key factor in postoperative morbidity is the surgical stress response with ensuing increased demand on the patient’s reserves and the immunological competence. Increased demands on organ functions are thought to be mediated by trauma-induced endocrine and metabolic changes. The stress response has been studied by monitoring levels of hormones such as cortisol, prolactin, and growth hormones, whereas the acute-phase response has been measured by means of various cytokine and acute-phase response protein levels. Of these, interleukin-6 (IL-6), interleukin-8 (IL-8), and C-reactive protein (CRP) are among the most frequently studied after surgical trauma [3–9]. Each of the two cytokines represents a different component of the inflammatory response: IL-6 is a mediator of the acute phase, inducing synthesis of proteins by the liver, whereas IL-8 has chemoattractant activity and is able to activate and degranulate neutrophils [10]. Immune status, inflammatory response, and stress response have been analyzed in different studies comparing conventional open surgery to minimally invasive surgery, and the minimally invasive approach has been favored [3–5].
Currently, for patients with advanced esophageal cancer, the best curative treatment is surgical resection. This extensive surgical procedure involves a thoracotomy and laparotomy with gastric tube reconstruction and an esophagogastric anastomosis. A high morbidity rate, mostly due to respiratory complications, and a long intensive care unit and hospital stay are consequences of this procedure.
In recent years, many surgical techniques have been developed to reduce morbidity rates after esophageal resection. Minimally invasive approaches are likely to be favorable over conventional open procedures, as observed in previous studies [11]. This study was performed as a substudy of a randomized trial (TIME trial: traditionally invasive versus minimally invasive esophagectomy) that compares conventional open esophagectomy by thoracotomy and laparotomy [open surgical technique (OE)] with minimally invasive esophagectomy [minimally invasive procedure (MIE)] by thoracoscopy and laparoscopy [12]. Different parameters, i.e., stress response hormone levels, acute-phase response protein levels, and immunological status, were studied during different postoperative stages in both groups.
Methods
Study design
Data were collected of patients who had been included in a randomized, multicenter trial comparing conventional esophagectomy with minimally invasive esophagectomy (TIME trial, NTR TC 2452). Details of the trial design have been published elsewhere [12, 13]. From 2009 to 2010, a total of 27 consecutive patients who participated in the TIME trial in the VU University Medical Center were included into this substudy.
Patient selection
Patients were eligible for this trial if they had a histologically proven squamous cell carcinoma, adenocarcinoma, or undifferentiated carcinoma of the intrathoracic esophagus and gastroesophageal junction tumors that were considered surgically resectable (T1-3, N0-1, M0). Moreover, the eligible patients must have had an eastern cooperative oncology group (ECOG) performance status of 0, 1, or 2. All patients in this trial received neoadjuvant chemoradiotherapy, as defined in the CROSS protocol [14].
Surgical technique
The OE involves a right thoracotomy with lung blockade and laparotomy with either a cervical or a thoracic anastomosis. The MIE involves a right thoracoscopy with the patient in the prone position with a single lumen tube and laparoscopy with either a cervical or thoracic anastomosis.
End points
A choice was made of the most standardized markers to study the end points. End points were stress response measurements of cortisol, prolactin, and growth hormone levels; immune status as manifested by the preservation of HLA-DR on monocytes; white blood cell count; and acute inflammatory response measured by means of IL-6, IL-8, and CRP level determinations. All measurements were performed preoperatively and on postoperative day 1, 3, 4, and 7.
Material and methods
Peripheral blood and serum (BD Vacutainer Systems, Plymouth, UK) were collected preoperatively (baseline) and 24, 72, 96, and 168 h after surgery. Serum IL-6 and IL-8 samples were obtained by centrifugation for 10 min at 3,000 rpm at 4 °C. All samples were stored in aliquots at −80 °C until tested.
Immunological response
White blood cell count and phenotype were determined from fresh heparinized venous blood (within 2 h after obtainment). Phenotyping was performed by using CD14-PE and HLA-DR-FITC moAbs (Becton Dickinson, Franklin Lakes, NJ, USA), lysis of erythrocytes, and fixation with paraformaldehyde. Evaluation of monocyte HLA-DR expression was performed by FACS analysis (FACS Calibur, Becton Dickinson), quantified by using calibration beads (Quantum™ 26, Flow Cytometry Standards Corp, Bangs Laboratories Inc, Fishers, IN, USA), and expressed as the ratio of the mean fluorescence intensity after surgery/before surgery.
Acute inflammatory response
Concentrations of IL-6 and IL-8 were measured using commercially available enzyme-linked immunosorbent assay kits (PeliKine compact ELISA kit, Sanquin, Amsterdam, The Netherlands). CRP was measured in blood serum by immunoturbidimetric testing using the BM/Hitachi 705 (Boehringer, Mannheim, Germany).
Stress response
Cortisol and growth hormone concentrations were measured by competitive immunoassay (Bayer Diagnostics, Mijdrecht, The Netherlands). Prolactin was measured by immunometric assays (DPC, Los Angeles, CA, USA).
Statistical analysis
Statistical analysis was performed using the SPSS software package 15 (SPSS Inc., Chicago, IL, USA). The results for the two different groups were compared by means of Mann–Whitney U test. Categorical parameters were analyzed with Fisher’s exact test. Significance was set at p < 0.05.
Results
A total of 27 patients were included in this substudy of the TIME trial, 14 patients in the minimally invasive group and 13 patients in the open group.
Clinical characteristics
Demographic parameters, surgical data, and pathological tumor indices are given in Table 1. No significant differences were observed between groups with respect to gender, age, tumor location, and operation time. There was significantly less blood loss in the MIE group (450 vs. 275 ml, p < 0.05). General outcome and postoperative morbidity are depicted in Table 2.
Immunological response
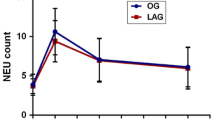
At baseline, white blood cell count was comparable in both groups (Table 3) At 24, 72, and 96 h after surgery, there were no significant differences between the open and minimally invasive groups. However, at 168 h (1 week) after surgery, there was a significant difference in white blood cell count due to an increase in the OE group as opposed to a sustained decrease in the MIE group (Fig. 1) The change in leukocyte count after 1 week is also significantly different between the groups (p = 0.011).
The expression of HLA-DR on monocytes showed a decrease of 60 % or more in both groups at all measured moments (p < 0.05). This was lowest for the open group at 72 h after surgery (23.8 %), although no statistical significance between the groups was reached (Fig. 2).
Acute inflammatory response
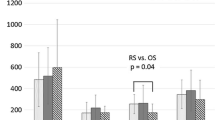
IL-6, IL-8, and CRP levels were comparable at baseline (Table 3). There were no significant differences between the groups in the postoperative IL-6 and CRP levels. However, CRP levels peaked at 72 h after surgery for both groups (Fig. 3), and both IL-6 and IL-8 levels showed an increase at 24 h compared to baseline measurements (Figs. 4, 5). Remarkably, at 168 h after surgery, the IL-8 level in the OE group was significantly higher than that in the MIE group (p = 0.047) (Fig. 5).
Stress response
Cortisol, prolactin, and growth hormone at baseline in both groups were comparable (Table 3). Cortisol levels upon surgery were elevated at all time measurements for both groups as growth hormone peaked at 24 h for the OE group but showed no significant differences between the groups (data not shown). Prolactin levels in the OE group, however, fluctuated in time, being significantly elevated at 168 h (1 week) after surgery compared to the MIE group (Fig. 6).
Discussion
Immunological reaction after surgery may contribute to infectious complications, sepsis, and tumor growth [1, 2]. Minimally invasive surgery has been shown to preserve immunological function better than conventional open surgery in different surgical procedures, including cholecystectomy, Nissen fundoplication, and laparoscopic colorectal surgery [3–5]. Scheepers et al. [8] described the immunological consequences of laparoscopic versus open transhiatal resection for malignancies of the esophagus. That nonrandomized study compared six patients in the laparoscopic group with 11 patients in the open group. They found an increase in all markers, with significantly higher levels of IL-6 for the open group, suggesting that the surgical trauma in the minimally invasive group was less extensive. This increase of IL-6, as an expression of the extent of trauma and predictor for postoperative complications, is also seen in this study. A peak is observed 24 h after surgery, as in other studies [3, 6, 8]. However, no significant differences were found in IL-6 and CRP levels between the OE group and the MIE group. Remarkably, the leukocyte count was significantly higher in the OE group at 7 days, whereas the expression of HLA-DR on monocytes was decreased postoperatively but without a significant difference between the groups. A possible explanation is that the extent of trauma was so predominant in both groups of transthoracic esophagectomy that differences, measured by IL-6 and CRP, were masked. However, it must be acknowledged that this study includes a small number of patients. Additional differences would probably be seen with a larger number of patients.
There was a significant increase in IL-8 and white blood cell counts in the OE group 1 week after surgery. Not only was the difference in white blood cell count at 1 week significant, but the change in leukocyte count was also significantly different in both groups, in favor of the minimally invasive group. IL-8 is thought to play an important role in the development of pneumonia [7, 10, 15, 16]. Fujimori et al. [15] investigated the role of IL-8 in interstitial pneumonia and found that an increased level is associated with fibrosis and injury of the lung. Yamada et al. [7] evaluated serum IL-6 and IL-8 in patients who underwent conventional thoracic surgery and found a significant increase in IL-8 levels until the postoperative day 7 in patients who developed postoperative pulmonary infections. The authors suggest that this increase in IL-8 may reflect the severe surgical stress due to reperfusion of ischemic lung tissue as a result of one-lung ventilation during thoracotomy. In our study we also found increased IL-8 levels in the early postoperative period for both groups, with a significant difference at postoperative day 7 in favor of the MIE group. This could explain the trend of fewer respiratory infections observed in the MIE group in comparison to the OE group (seven in the OE group and three in MIE group). This trend actually was confirmed in the main study, the TIME trial, which was recently published [12]. Other studies that compared open to minimally invasive techniques used in cholecystectomy, Nissen fundoplication, and colorectal surgery have shown better immunological outcomes in favor of the minimally invasive techniques [3–5]. However, an important difference between esophagectomy and the other interventions, e.g., cholecystectomy, is the amount of surgical trauma. It is probably more difficult to demonstrate significant differences in immunosuppression in interventions with a huge wound surface like the esophagectomy. For example, expression of HLA-DR was decreased at all times for both groups in our study, whereas after Nissen fundoplication and even after colorectal surgery the expression of HLA-DR on monocytes returned to preoperative levels within a week [4, 5].
Since the increase in IL-8 levels has been identified both during and after surgery, the possibility has risen to block this increase intraoperatively to minimize respiratory infections after esophagectomy [7, 9]. Kawahara et al. [9] introduced the administration of a neutrophil elastase inhibitor in patients undergoing esophageal resection by thoracoscopy. They showed that this inhibitor seems to partially suppress the postoperative increase in IL-8 levels and shorten the duration of systemic inflammatory response in a randomized trial. As a result, acute lung injury and respiratory infections could be prevented.
The increase of prolactin, cortisol, and growth hormone levels seems to be less intense and of shorter duration after laparoscopic gynecologic pelvic surgery [17, 18]. An increase in these levels with open surgery might not be due to surgery alone, but also to the administration of anesthetic drugs such as morphine [19]. All patients in our study received epidural anesthesia for at least 3 days. At 1 week after surgery a significant difference in prolactin levels between the two groups was observed. This might be attributable to the use of analgesics. At 1 week after surgery, the use of morphine was increased in the open group (11 vs. 5 patients).
In conclusion, in this substudy of a randomized trial comparing minimally invasive with conventional esophagectomy for cancer, a significantly better preserved leukocyte count and IL-8 level were observed in the MIE group than in the open group. Though the differences are small, both values might be related to fewer respiratory infections found postoperatively in the MIE group. Moreover, stress response was also better preserved in the MIE group, as expressed by the slightly different prolactin values at 1 week postoperatively. These findings indicate that less surgical trauma could lead to better preserved acute-phase response and fewer respiratory infections. Overall, further studies will be needed to subscribe to our findings.
References
Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, Proud G, Tayler RM (1985) The influence of surgical operations on components of the immune system. Br J Surg 72:771–776
Wakefield CH, Carey PD, Foulds S, Monson JR, Giullou PJ (1993) Changes in major-histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg 80:205–209
Bellon JM, Manzano L, Larrad A, Honduvilla GN, Bujan J, Alvarez-Mon M (1998) Endocrine and immune response to injury after open and laparoscopic cholecystectomy. Int Surg 83:24–27
Sietses C, Wiezer MJ, Eijsbouts QA, Beelen RH, van Leeuwen PA, von Blomberg BM, Meijer S, Cuesta MA (1999) A prospective randomized study of the systemic immune response after laparoscopic and conventional Nissen fundoplication. Surgery 1:347–350
Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ, vd Peet DL, vd Tol MP, Bonjer HJ, Cuesta MA (2011) The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis 26:53–59
Yamaguchi Y, Nihara J, Hironaka K, Ohshita A, Okita R, Okawaki M, Matsuura K, Nagamine I, Ikeda T, Ohara M, Hamai Y (2006) Postoperative immunosuppression cascade and immunotherapy using lymphokine-activated killer cells for patients with esophageal cancer: possible application for compensatory anti-inflammatory response syndrome. Oncol Rep 15:895–901
Yamada T, Hisanaga M, Nakajima Y, Kanehiro H, Watanabe A, Ohyama T, Nishio K, Sho M, Nagao M, Harada A, Matsushima K, Nakano H (1998) Serum interleukin-6, interleukin-8, hepatocyte growth factor, and nitric oxide changes during thoracic surgery. World J Surg 22:783–790. doi:10.1007/s002689900470
Scheepers JJ, Sietses C, Bos DG, Boelens PG, Teunissen CM, Ligthart-Melis GC, Cuesta MA, van Leeuwen PA (2008) Immunological consequences of laparoscopic versus open transhiatal resection for malignancies of the distal esophagus and gastroesophageal junction. Dig Surg 25:140–147
Kawahara Y, Ninomiya I, Fujimura T, Funaki H, Nakagawara H, Takamura H, Oyama K, Tajima H, Fushida S, Inaba H, Kayahara M (2010) Prospective randomized controlled study on the effects of perioperative administration of a neutrophil elastase inhibitor to patients undergoing video-assisted thoracoscopic surgery for thoracic esophageal cancer. Dis Esophagus 23:329–339
Bonten MJ, Froon AH, Gaillard CA, Greve JW, de Leeuw PW, Drent M, Stobberingh EE, Buurman W (1997) The systemic inflammatory response in the development of ventilator-assisted pneumonia. Am J Respir Crit Care Med 156:1105–1113
Biere SS, Cuesta MA, van der Peet DL (2009) Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 64:121–133
Biere SS, van Henegouwen MIB, Maas KW, Bonavina L, Rosman C, Garcia JR, Hollmann MW, Klinkenbijl JH, de Lange ESM, Bonjer HJ, van der Peet DL, Cuesta MA (2012) Minimally invasive esophagectomy: a randomized controlled trial. Lancet 379:1887–1892
Biere SS, Maas KW, Bonavina L, Garcia JR, van Henegouwen MIB, Rosman C, Sosef MN, de Lange ESM, Bonjer HJ, van der Peet DL, Cuesta MA (2011) Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial). BMC Surg 11:2
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Henegouwen MIB, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, Ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Bilgen EJS, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, CROSS Group (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084
Fujimori Y, Kataoka M, Tada S, Takehara H, Matsuo K, Miyake T, Okahara M, Yamadori I, Tanimoto M (2003) The role of interleukin-8 in interstitial pneumonia. Respirology 8:33–40
Rodriguez JL, Miller CG, DeForge LE, Kelty L, Shanley CJ, Bartlett RH, Remick DG (1992) Local production of interleukin-8 is associated with nosocomial pneumonia. J Trauma 33:74–81
Marana E, Scambia G, Maussier ML, Parpaglioni R, Ferrandina G, Meo F, Sciarra M, Marana R (2003) Neuroendocrine stress response in patients undergoing benign ovarian cyst surgery by laparoscopy, minilaparotomy and laparaotomy. J Am Assoc Gynecol Laparosc 10:159–165
Muzii L, Marana R, Marana E, Paielli FV, Meo F, Maussier ML, Sciarra M, Mancuso S (1996) Evaluation of stress-related hormones after surgery by laparoscopy or laparotomy. J Am Assoc Gynecol Laparosc 3:229–234
Yardeni IZ, Shavit Y, Bessler H, Mayburd E, Grinevich G, Beilin B (2007) Comparison of postoperative pain management techniques on endocrine response to surgery: A randomized controlled trial. Int J Surg 5:239–243
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maas, K.W., Biere, S.S.A.Y., van Hoogstraten, I.M.W. et al. Immunological Changes After Minimally Invasive or Conventional Esophageal Resection for Cancer: A Randomized Trial. World J Surg 38, 131–137 (2014). https://doi.org/10.1007/s00268-013-2233-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2233-0