Abstract
Background
Restless legs syndrome (RLS) is a common and poorly understood movement disorder that leads to unpleasant leg sensations. Although RLS can be idiopathic, secondary etiologies such as iron deficiency and renal failure are common. The aim of this prospective cohort study was to evaluate whether RLS is a common feature in patients undergoing parathyroidectomy for renal hyperparathyroidism (rHPT) and if RLS-related symptoms can be influenced by surgery.
Methods
After providing written consent, patients who underwent a parathyroidectomy for rHPT between January and November 2011 answered a validated RLS-screening-questionnaire (RLSSQ). If this was suggestive for RLS a confirming questionnaire (IRLS) was also completed on the day before surgery, on the fifth postoperative day, and again during follow-up (minimum 12 months). Perioperative parathyroid hormone and calcium levels, as well as the scores of the questionnaires were analyzed.
Results
Twenty-one patients (14 men, 7 women) with a mean age of 47.8 ± 3.2 years underwent total parathyroidectomy with bilateral cervical thymectomy and parathyroid autotransplantation for rHPT. The mean score of the RLSSQ of all 21 patients prior to operation was 6.1 ± 0.5. In 10 of 21 patients (47.6 %) the results of the RLSSQ were suggestive for RLS with a mean score of 8.0 ± 0.3. The consecutive scores of the IRLS in these latter patients significantly dropped from 26.6 ± 1.4 to 19.0 ± 2.2 between the preoperative and postoperative settings (p < 0.05). After a mean follow-up of 17.3 ± 3.7 months the mean scores of the RLSSQ and the IRLS were 6.1 ± 0.6 and 16.3 ± 1.8.
Conclusions
rHPT may play a major role in the severity of RLS-associated symptoms in patients with renal failure. Consequently, parathyroidectomy may prove to be a valuable tool to reduce RLS-associated morbidity in affected patients. However, larger prospective trials are required to confirm the possible relation between RLS and rHPT seen in the present study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restless legs syndrome (RLS) is a common and poorly understood movement disorder that can cause significant sleep disruptions due to unpleasant distressing leg sensations, especially at rest and at bedtime [1–3]. Symptoms may obviate sleep and may significantly impair overall quality of life [4–6]. The overall prevalence of RLS is reported to be 5–10 % [4], but it may be even higher as symptoms are frequently misinterpreted because the attending physicians are unfamiliar with the diagnosis. As could be demonstrated within the “RLS Epidemiology, Symptoms and Treatment” (REST) study, the diagnosis of RLS is rarely established, and symptoms are frequently attributed to other medical conditions, such as back pain, arthritis, or peripheral neuropathy [7, 8]. To establish the diagnosis of RLS, standardized criteria have been formulated by the International Restless Legs Syndrome Study Group (IRLSSG) [1].
Essential diagnostic criteria for RLS include (1) urge to move the legs, (2) worse during rest or inactivity; (3) relieved by activity such as walking, and (4) worse in the evening or night. Additional diagnostic procedures such as validated questionnaires [4, 9], polysomnographic studies, or an L-dopa-test can be helpful in confirming the diagnosis [10].
The underlying pathophysiology responsible for the development of RLS is unknown. It may be inherited, as genetic changes have been detected at chromosome 12q [1, 11, 12]. However, RLS may also be idiopathic or occur in association with other disorders, such as abnormalities in iron storage and consecutive dopaminergic dysfunction [13, 14], pregnancy [15], Parkinson’s disease [16], and end stage renal disease [17–19].
The prevalence of RLS in uremic patients is reported to be between 6.6 and 62 % [20–22]. However, RLS in uremic patients is usually associated with markedly pronounced symptoms within a short period of time after starting dialysis, and the response to dopaminergic agonists is not as good as in patients with idiopathic RLS [3].
Within the last two decades, some of our patients with RLS who underwent parathyroidectomy for renal hyperparathyroidism (rHPT) reported a remarkable improvement in RLS symptoms after surgery. A literature search (applying “restless legs” and “hyperparathyroidism”) revealed only two case reports linking primary hyperparathyroidism with the development of RLS [2, 23].
The present prospective cohort study aimed to objectify the aforementioned observations. Patients undergoing parathyroidectomy for rHPT were screened with validated questionnaires for RLS and, after establishing the diagnosis, were re-evaluated with the same questionnaires to delineate possible changes in symptoms after surgery.
Methods
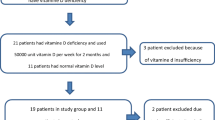
All patients with ongoing dialysis who underwent total parathyroidectomy with bilateral cervical thymectomy and parathyroid autotransplantation (PTX) between January and November 2011 were asked to participate and, after written consent, to complete a validated RLS-screening-questionnaire (RLSSQ). A pathologic result within the RLSSQ was defined as a score of seven or more points. Patients with a pathologic result were asked to proceed with an additional international RLS Severity Scale (IRLS) questionnaire on the day before surgery, five days after parathyroidectomy, and at follow-up (minimum 12 months post surgery). The results of the RLSSQ and IRLS questionnaires as well as the perioperative parathyroid hormone (PTH) and serum calcium levels were analyzed. Follow-up calcium and PTH levels were acquired via the attending nephrologists. To achieve the follow-up data of the questionnaires, all patients with a pathologic preoperative RLSSQ were contacted by mail and asked to repeat the RLSSQ and IRLS questionnaires.
Indications for surgery were intact PTH levels higher than ten times the upper normal limit (>650 pg/l). In all patients, age, gender, and perioperative total serum calcium and PTH values were analyzed. Parathyroid hormone was measured with a commercially available immunoluminescence assay that has a normal range of 11–65 pg/l. Total serum calcium was measured with a normal range of 2.2–2.7 mmol/l. Both PTH and calcium were measured one day before PTX and again on the third postoperative day.
The RLSSQ is a diagnostic 10-item patient self-rating questionnaire developed on the basis of the clinical features of RLS (Table 1) and validated in a study comparing patients with a definitive diagnosis of RLS according to the International RLS Study Group criteria with control subjects from the general population in whom RLS was excluded by an RLS expert [4, 24]. In the present study the diagnostic value of the RLSSQ was demonstrated using a cut-off of seven points (out of a maximum of ten points) as a pathological result referring to RLS with a sensitivity of 97.9 % and a specificity of 96.2 % [4]. The items of the RLSSQ are displayed in Table 2.
The IRLS has been developed by the International Restless Legs Syndrome Study Group and comprises a 10-item scale to enable a well-validated and easily-administered measurement of RLS severity. The IRLS has been shown to have a high degree of reliability, validity, and internal consistency [9]. The items of the IRLS are displayed in Table 3. The maximum score is 40 points.
Data were analyzed with SPSS software (Statistical Product and Services Solutions, version 16.0, SPSS Inc, Chicago, IL). All results were expressed as mean values ± standard error of the mean and percentages. Fisher’s exact test was used to determine statistically significant differences; p values less than 0.05 were considered to be statistically significant.
Results
A total of 21 patients (14 men, 7 women) with a mean age of 47.8 ± 3.2 years who underwent initial PTX between January and November 2011 at a single tertiary referral center were analyzed. The postoperative management comprised a routine supplementation of 3 × 0.5 μg oral calcitriol and 3 × 1,000 mg oral calcium. In case of calcium levels below 2.0 mmol/l, a supplemental 2–12 g calcium was administered intravenously. Patients were discharged only if calcium levels were >2.0 mmol/l. Mean duration of hemodialysis in these 21 patients was 76.7 ± 3.7 months.
The mean score of the RLSSQ of all 21 patients was 6.1 ± 0.5. In 10 of 21 patients (47.6 %) the RLSSQ revealed a pathological score with seven or more points. These ten patients (six men, four women) were further analyzed with an additional IRLS and a repetition of both RLSSQ and IRLS questionnaires on the fifth day post surgery and within the follow-up.
The mean patient age was 46.7 ± 5.3 years, and the preoperative mean score of the RLSSQ was 8.0 ± 0.3 in this subgroup of patients. The proportion of “yes” answers of the preoperative RLSSQ is displayed in Fig. 1. The total preoperative scores of the IRLS ranged from 20 to 33 (of a possible 40), with a mean of 26.6 ± 1.4, representing moderate to severe RLS in these patients.
Postoperatively, the mean scores of the RLSSQ and the IRLS were 6.2 ± 0.7 and 19.0 ± 2.2 (range 10–30), representing easy to moderate RLS. The results of each item of the IRLS are displayed in Fig. 2. The perioperative change of the scores of the RLSSQ (p = 0.021) as well as of IRLS questions 1 (p = 0.0403), 2 (p = 0.0145), 5 (p = 0.0408), 6 (p = 0.0134), and 10 (p = 0.0150) showed a statistically significant drop.
After a mean follow-up of 17.3 ± 3.7 months the mean scores of the RLSSQ and the IRLS were 6.1 ± 0.6 and 16.3 ± 1.8, confirming a stable improvement of parameters post surgery. The results of each item of the IRLS are also displayed in Fig. 2. The change from the preoperative results to the follow-up results were statistically significant for the scores of the RLSSQ (p = 0.0131) and for questions 1 (p = 0.0013), 2 (p = 0.0036), and 6 (p = 0.0010) of the IRLS. These significant changes were already evident in the postoperative evaluation. Question 5 was the only question that showed statistical significance from the preoperative response to the postoperative response, but not when we compared the preoperative status with the follow-up analysis.
Parathyroid hormone levels (normal range 11–65 pg/ml) perioperatively dropped from 1,317 ± 201 to 24 ± 16 pg/ml, and calcium levels (normal range 2.2–2.7 mmol/l) dropped from 2.34 ± 0.08 to 1.89 ± 0.07 mmol/l (Figs. 3, 4). Follow-up PTH and calcium levels were 38 ± 20 pg/ml and 2.11 ± 0.12 mmol/l, respectively. Follow-up examinations revealed no persistent or recurrent diseases in any of the patients examined in this study.
Discussion
Restless legs syndrome is well known to be associated with chronic kidney disease (CKD) [1, 3, 14]. In uremic patients it seems to progress more rapidly, and subjective severity of the RLS symptoms is worse than in patients with idiopathic RLS [3]. Although the underlying pathophysiology of RLS in uremic patients is unknown, the role of chronic kidney insufficiency is confirmed by the resolution or improvement of RLS symptoms after kidney transplantation and by their recurrence after graft failure [17, 18, 25].
To the best of our knowledge this is the first study to examine the association between RLS and rHPT and the influence of total PTX on the signs and symptoms of RLS. The RLSSQ questionnaire used is considered a valuable tool to accurately diagnose or exclude RLS [4].
In our study 21 patients were screened by the RLSSQ with a mean score of 6.1 ± 0.5 points. The mean score of the ten patients with a pathological score (seven or more points) was 8.0 ± 0.3 points. All patients with a result suggestive for RLS complained about unpleasant sensations (RLSSQ, question 1), symptoms in relaxed situations (RLSSQ question 3), and symptoms during the daytime (question 7). None of our patients reported family members with similar symptoms (question 10), pointing to a higher likelihood toward a secondary etiology like uremia or hyperparathyroidism then toward idiopathic RLS. In other studies, about 40 % of patients with idiopathic RLS responded that they have family members with similar symptoms [4].
The IRLS, a validated questionnaire to objectify the severity of RLS symptoms, is a clinician-administered instrument that covers ten RLS features including five items relating to symptom frequency and intensity and five items relating to the impact of symptoms on aspects of daily life and sleep [9, 26]. Each item is rated on a five-point score (0–4), with higher scores representing enhanced RLS severity [9]. A large multicenter international study to validate the IRLS questionnaire found an excellent reliability and international consistency, as well as high levels of validity based on its ability to discriminate RLS patients from control subjects, and a high correlation with a clinical global rating of RLS severity [9, 26]. An additional factor analysis yielded two separate factors loading on symptom measures (items 1, 2, 4, 6, 7, 8) and disease impact measures (items 5, 9, 10) [9, 26]. Within the present study, total preoperative scores of the IRLS dropped from 26.6 ± 1.4 to 19.0 ± 2.2 postoperatively and to 16.3 ± 1.8 within the follow-up. In 3/6 items (50.0 %) loading on symptom measures (question 1, question 2, and question 6) and in 2/3 items (66.6 %) loading on disease impact measures (question 5 and question 10) a significant drop could be observed between the preoperative and the postoperative measures. Interestingly, this significant drop was also evident in the follow-up evaluation, so that possible postoperative influences like severe electrolyte disturbances due to the parathyroidectomy or pain killers can be excluded as influence factors.
Overall, the results of the present study link to a possible relationship between rHPT and RLS. A possible connection between rHPT and RLS would have influence in the management of affected patients, as RLS has been demonstrated to have a negative impact on outcomes in patients with end-stage CKD, with prominent effects on sleep quality, quality of life, precocious dialysis discontinuation, and a notable higher mortality at 2.5 years of follow-up [25–29].
However, the results of the present study have to be interpreted with caution in view of two major features. First, the validity of this study is limited by the small number of patients. Second, RLSSQ and IRLS questionnaires are validated in a “general population.” It could be questioned, if these instruments are reliable for the evaluation of symptoms in a uremic population, which is a multi-symptomatic, metabolic subgroup.
Therefore, further studies in a larger cohort of patients with ongoing dialysis obtained in a setting independent of a surgical intervention with an analysis of PTH and calcium levels in relation to scores obtained by validated RLSSQ and IRLS questionnaires are necessary to confirm or exclude the possible association between rHPT and RLS.
Conclusions
The results of the present study link to a possible relationship between rHPT and RLS. Parathyroidectomy may have a significant influence on the severity of RLS-associated symptoms and may be able to improve the notable higher mortality of patients with CKD attributed to associated RLS. Larger prospective trials are required to analyze this observation in patients with rHPT.
References
Allen RP, Picchietti D, Hening WA et al (2003) Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 4:101–119
Agarwal P, Griffith A (2008) Restless legs syndrome: a unique case and essentials of diagnosis and treatment. Medscape J Med 10:296
Enomoto M, Inoue Y, Namba K et al (2008) Clinical characteristics of restless legs syndrome in end-stage renal failure and idiopathic RLS patients. Mov Disord 23:811–816
Stiasny-Kolster K, Möller JC, Heinzel-Gutenbrunner M et al (2009) Validierung des Fragebogens zum Screening auf Restless-Legs-Syndrom. Somnologie 13:37–42
Walters AS, Allen RP, Hening W (2003) The impact of restless legs syndrome on quality of life: a population-based survey in the USA. Sleep 26:A329
Thorpy MJ (2005) New paradigms in the treatment of restless legs syndrome. Neurology 64:S28–S33
Allen RP, Walters AS, Montplaisir J et al (2005) Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 165:1286–1292
Hening WA (2004) Restless legs syndrome: the most common and least diagnosed sleep disorder. Sleep Med 5:429–430
Wunderlich GR, Evans KR, Sills T et al (2005) An item response analysis of the international restless legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 6:131–139
Stiasny-Kolster K, Kohnen R, Moller JC et al (2006) Validation of the “L-DOPA test” for diagnosis of restless legs syndrome. Mov Disord 21:1333–1339
Desautels A, Turecki G, Xiong L et al (2004) Mutational analysis of neurotensin in familial restless legs syndrome. Mov Disord 19:90–94
Desautels A, Michaud M, Montplaisir J et al (2002) Restless leg syndrome: clinical aspects, etiology and genetic factors. Rev Neurol (Paris) 158:1225–1231
Hening WA (2004) Subjective and objective criteria in the diagnosis of the restless legs syndrome. Sleep Med 5:285–292
Allen RP, Earley CJ (2001) Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol 18:128–147
Manconi M, Govoni V, De Vito A et al (2004) Pregnancy as a risk factor for restless legs syndrome. Sleep Med 5:305–308
Nomura T, Inoue Y, Miyake M et al (2006) Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson’s disease. Mov Disord 21:380–384
Winkelmann J, Stautner A, Samtleben W et al (2002) Long-term course of restless legs syndrome in dialysis patients after kidney transplantation. Mov Disord 17:1072–1076
Winkelman JW, Chertow GM, Lazarus JM (1996) Restless legs syndrome in end-stage renal disease. Am J Kidney Dis 28:372–378
Huiqi Q, Shan L, Mingcai Q (2000) Restless legs syndrome (RLS) in uremic patients is related to the frequency of hemodialysis sessions. Nephron 86:540
Bhowmik D, Bhatia M, Gupta S et al (2003) Restless legs syndrome in hemodialysis patients in India: a case controlled study. Sleep Med 4:143–146
Hui DS, Wong TY, Ko FW et al (2000) Prevalence of sleep disturbances in Chinese patients with end-stage renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 36:783–788
Kawauchi A, Inoue Y, Hashimoto T et al (2006) Restless legs syndrome in hemodialysis patients: health-related quality of life and laboratory data analysis. Clin Nephrol 66:440–446
Lim LL, Dinner D, Tham KW et al (2005) Restless legs syndrome associated with primary hyperparathyroidism. Sleep Med 6:283–285
Auger C, Montplaisir J, Duquette P (2005) Increased frequency of restless legs syndrome in a French–Canadian population with multiple sclerosis. Neurology 65:1652–1653
Molnar MZ, Novak M, Ambrus C et al (2005) Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis 45:388–396
Walters AS, LeBrocq C, Dhar A et al (2003) Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 4:121–132
Merlino G, Piani A, Dolso P et al (2006) Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transpl 21:184–190
Mucsi I, Molnar MZ, Ambrus C et al (2005) Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transpl 20:571–577
Unruh ML, Levey AS, D’Ambrosio C et al (2004) Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis 43:900–909
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, R., Karakas, E., Bartsch, D.K. et al. The Influence of Parathyroidectomy on Restless Legs Syndrome in Patients with Renal Hyperparathyroidism. World J Surg 37, 2866–2871 (2013). https://doi.org/10.1007/s00268-013-2185-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2185-4