Abstract
Background
Prophylactic neck dissection (PND) for papillary thyroid cancer is controversial. The objective of this study was to analyze the influence of PND on the rate of retreatment.
Methods
In this retrospective case-control study, papillary thyroid carcinomas >10 mm without ultrasonographic evidence of nodal disease (cN0) were treated with total thyroidectomy (TT) or TT with bilateral central compartment PND. All received postoperative radioactive iodine (131I) and were followed for at least 1 year. We compared the rate of retreatment (surgery or 131I).
Results
Altogether, 246 patients (mean age 46 years, 78 % women) underwent TT (n = 91) or TT + PND (n = 155). The groups were similar in age, sex, tumor size, and follow-up (median 6.3 years) (p > 0.05). Overall, 11 (12 %) of the patients in the TT group underwent reoperation in the central compartment for recurrence versus 3 (2 %) in the TT + PND group (p < 0.001). There were 1.18 administrations of 131I for the TT group versus 1.08 for the TT + PND group (p = 0.08). The average cumulative dose of 131I was 3.9 ± 1.8 GBq for the TT group and 3.8 ± 1.3 GBq for the TT + PND group (p = 0.52). Actuarial (Kaplan-Meier) 5-year retreatment rates were 14.7 % in the TT group and 6.5 % in the TT + PND group (p = 0.01, log-rank). The rate of permanent recurrent nerve paralysis was 2 % for the TT group and 1 % for the TT + PND group (p = 0.98). The rates of permanent hypoparathyroidism were 7 versus 3 %, respectively (p = 0.12).
Conclusions
Five-year retreatment rates were lower in patients treated with PND, with no added permanent morbidity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma carries a high risk of occult lymph node metastases—up to 80 %—whereas follicular carcinoma is associated with a low rate of lymph node metastases—<15 % [1, 2]. Therapeutic neck dissection, performed for patients with known lymph node metastases discovered by preoperative ultrasonography (US) scanning or physical examination [3, 4], is advocated to resect all gross disease and improve regional control [5, 6]. In two large database studies, the presence of lymph node metastases was related to a decrease in long-term survival, providing evidence in favor of more extensive surgical resection for these patients [7–9].
Prophylactic central neck dissection (PND) performed for patients with papillary carcinoma and no known lymph node involvement remains a subject of debate [10]. There is no convincing evidence showing improved survival whether a lymph node dissection was performed in patients who had total thyroidectomy followed by radioactive iodine (131I) administration [11]. The 20-year survival rates are in the range of 90 % for patients with stage I and II disease [12]. PND for microscopic metastatic nodes did not have an effect on the regional recurrence rate [6], but patients who did not undergo PND had a higher rate of reoperation in the central compartment (6.1 vs. 1.5 %, p = 0.004) [13].
Opponents of PND also cite an increased risk of permanent hypoparathyroidism and permanent recurrent nerve paralysis in contrast to what is seen with total thyroidectomy alone, although a recent meta-analysis has shown that experienced surgeons can perform PND safely [14]. Proponents of PND cite a higher risk of complications after reoperative surgery in the central compartment compared to that with primary surgery. Recent retrospective studies, however, have shown that experienced surgeons can perform reoperation in the central compartment safely [15, 16]. More recently it has been shown in three retrospective studies that PND can reduce the doses of 131I administered—or obviate it entirely—for low-risk patients shown to have no micrometastases (pN0) [17–20]. Thus, PND may be beneficial in some patients (pN0) by avoiding or reducing doses of 131I and in others (pN1) by decreasing the risk of reoperative surgery in the central compartment. The objective of this study was to analyze the influence of PND on the rate of reoperation in the central compartment and the rate of retreatment with 131I for papillary thyroid cancer.
Patients and methods
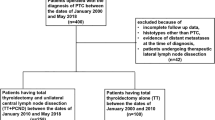
We conducted an institutional review board-validated retrospective study of files from 1995 to 2010 of patients treated at our institution for differentiated thyroid carcinoma >10 mm with no US evidence of nodal disease (cN0). We excluded patients treated for follicular, poorly differentiated, anaplastic, or medullary carcinoma; patients <18 years old; and patients with a prior history of radiation therapy to the neck. Patients treated with total thyroidectomy (TT) or TT with PND were included. The PND addressed the central compartment bilaterally and ipsilateral lateral neck levels III and IV [4, 21]. Patients with suspicious nodes on preoperative US, proven metastatic lymph nodes preoperatively, or suspicious nodes intraoperatively were excluded, as were patients with locally invasive disease (T4) and those with distant metastases diagnosed preoperatively.
All of the study patients received postoperative radioactive iodine under thyroid hormone withdrawal or after administration of recombinant human thyroid-stimulating hormone (rhTSH). Patients with distant metastases diagnosed on a postoperative whole-body scan following the first radioactive iodine administration were subsequently excluded from the study. All remaining patients were followed for at least 1 year after ablation, with at least one evaluation with neck US and stimulated thyroglobulin (Tg) measurements. Patients with <1 year of follow-up or without stimulated Tg measurements were subsequently excluded from analysis.
During the course of follow-up, retreatment was defined as “reoperation in the central neck” or “repeat 131I treatment for persistent disease in the neck (uptake outside the thyroid bed seen on the whole-body iodine scan after the first treatment) or for recurrent disease with increasing Tg levels.” We compared the following parameters for the two patient groups: (1) Tg levels measured just before the first administration of 131I; (2) stimulated Tg levels at the first year of follow-up; (3) total cumulative dose of 131I received; (4) total number of administrations of 131I; (5) number of reoperations in the central compartment; (6) rates of permanent hypoparathyroidism and symptomatic recurrent nerve paralysis.
Quantitative data were expressed as the mean and standard deviation. Qualitative data were expressed in percents. Risk factors for retreatment were analyzed by univariate and multivariate logistic regression and included the following characteristics: age, pT [17], tumor size, PND, aggressive histologic variant, bilaterality of the tumor, extrathyroidal extension, and multifocality. All reported p values were two-sided. Analyses were performed using SAS statistical software (SAS Institute, Cary, NC, USA). Significance level was 0.05. Time to retreatment was defined as the time between initial surgery and the first retreatment (reoperation or 131I administration). Patients who did not undergo retreatment were censored at the date of their last follow-up visit. Time until first reintervention and time until first retreatment (surgery or 131I) were both estimated by the inverse Kaplan-Meier method.
Results
A total of 246 patients (mean age 46 years, range 18–77 years), with 193 (78 %) women, were included in the final analysis. A TT alone was performed in 91 cases (6 at our institution and 85 outside our institution) and TT + PND in 155 (all performed at our institution) (Table 1). The groups were similar in age, sex, tumor size, pT stage, and average follow-up (Table 1). In all, 80 patients were staged pN1 in the TT + PND group (52 %). The median follow-up was 6.3 years (range 1–14 years). Over the course of follow-up, one patient presented distant lung metastases in the TT group, whereas none in the TT + PND group developed distant metastases.
Rate of reoperation
In all, 11 (12 %) of the patients in the TT group underwent reoperation in the central compartment versus 3 (2 %) in the TT + PND group (p < 0.0001) (Table 2). Over the entire follow-up period, the total number of reoperations was 18 for the TT group versus 4 for the TT + PND group. Two patients in the TT group underwent reoperation in the central compartment four and five times, respectively. One patient in the TT + PND group underwent reoperation twice (Table 2). One patient (in the TT group) underwent reoperation in the lateral neck alone. Seven patients in the TT group underwent retreatment with both 131I and reoperation. Two patients in the TT + PND group underwent both types of retreatment.
The final rate of permanent hypoparathyroidism requiring continuous supplementation was not significantly different between the groups (7 vs. 3 %, p = 0.23). The final rate of permanent symptomatic recurrent nerve paralysis was similar in the two groups as well (2 % for the TT group and 1 % for the TT + PND group, p = 0.98).
Radioiodine treatment
A total of 27 patients were retreated with 131I (Table 2), each time at a dose of 3.5 GBq, with thyroid hormone withdrawal. Retreatment with 131I was performed because of (1) uptake outside the thyroid bed at the first treatment associated with detectable stimulated Tg, or (2) an increase in Tg during follow-up. In no cases were large thyroid remnants the reason for readministration of 131I. At last follow-up, 131I had been administered 1.18 times to TT patients versus 1.08 times to the TT + PND patients (p = 0.08). Excluding the patient in the TT group who developed distant metastases, the numbers of administrations were 1.17 and 1.08, respectively (p = 0.12).
The average total cumulative dose of 131I received was 3.9 ± 1.8 GBq for the TT group and 3.8 ± 1.3 GBq for the TT + PND group (p = 0.52). Excluding the patient who had developed distant metastases, the average total dose of 131I received in the TT group was 3.9 ± 1.7 versus 3.8 ± 1.3 for the TT + PND group (p = 0.76) (Table 2). Patients staged pN0 in the TT + PND group received 3.6 ± 1.4 GBq versus 4.0 ± 1.2 for the patients staged pN1 (p = 0.01). However, no significant difference was found between patients staged pNx (the TT group) versus patients staged pN0 or pN1 (p = 0.6 and p = 0.1, respectively, Wilcoxon test).
Rate of retreatment
Retreatment occurred at least once in 18 (20 %) patients in the TT group and 14 (9 %) patients in the TT + PND group (p = 0.02) (Table 2). Actuarial (Kaplan-Meier) 2- and 5-year retreatment rates were 12.2 % [95 % confidence interval (CI) 6.9–20.5] and 14.7 % (95 % CI 8.8–23.5) in the TT group and 5.9 % (CI 3.1–10.8) and 6.5 % (95 % CI 3.6–11.6) in the TT + PND group, respectively (p = 0.01, log-rank test) (Fig. 1). Figure 2 shows the time to reoperation and Fig. 1 the time to retreatment (reoperation or 131I). The 2-year retreatment-free survival was thus 87.8 % in the TT group versus 94.1 % in the TT + PND group. The 5-year retreatment-free survival was 85.3 % in the TT group versus 93.5 % in the TT + PND group. There were no deaths in either group during the follow-up period.
Of the factors analyzed on univariate analysis—PND, pT stage, extrathyroidal extension, tumor, multifocality, tumor bilaterality, aggressive histopathologic subtype (nine tall cell variants, one Hürthle cell variant, one diffuse sclerosing variant of papillary carcinoma)—we found that PND was the only factor significantly associated with a lower risk of retreatment (Table 3).
Thyroglobulin levels
Between 1995 and 2006, the sensitivity of our Tg measurement kit was 1 ng/ml. Beginning in 2007, the functional sensitivity dropped to 0.11 ng/ml. For patients without anti-Tg antibodies, preablation stimulated Tg levels were ≤1 ng/ml for 43 % (22/51) of patients in the TT group versus 63 % (78/123) of patients in the TT + PND group (p = 0.01). At the follow-up evaluation performed 8–12 months after radioiodine ablation, stimulated-Tg levels were ≤1 ng/ml for 88 % of (45/51) patients in the TT group versus 94 % (114/121) of patients without Tg antibodies in the TT + PND group (p = 0.17). Final stimulated Tg levels, after a median follow-up of 6.3 years, were ≤1 ng/ml for 79 % of patients in the TT group versus 96 % of patients in the TT + PND group (p = 0.04).
Discussion
In the absence of evidence demonstrating a survival advantage by performing PND, the low-level evidence for and against this practice has been widely used in an ongoing debate between its proponents and opponents [10, 22]. The present study is the second in our knowledge to show a benefit of PND in terms of reoperation in the central neck. The study by Popadich et al. also found that reoperation in the central compartment was performed more frequently in the group treated with TT alone, as compared to the group treated with TT + PND (6.1 vs. 1.5 %, for a difference of 4.6 %, p = 0.004) [13]. We also found a trend toward fewer administrations of 131I for patients treated with PND (p = 0.08), and a higher proportion of patients with low stimulated Tg (≤1 ng/ml) at last follow-up (p = 0.04)
Several studies have shown that a personalized approach to radioiodine administration can be based on the knowledge of the lymph node status following PND [18–20, 23]. In the study by Bonnet et al, the pN status changed the decision for adjuvant radioactive iodine treatment in 30 % of the cases, with more radioactive iodine in half of these, and less iodine for the other half [18]. In a larger study by our team, patients staged pN0 received a lower average cumulative dose of radioactive iodine than patients pN1 (30 vs. 100 mCi, p = 0.0001) [20]. In the study by Hughes et al. the pN status upstaged 28.6 % of the patients in the group treated with bilateral central PND who then received higher doses of radioactive iodine, but there was no difference in the final results between the groups in terms of stimulated Tg levels or in the rate of recurrent/persistent disease (5 % in each group) [19]. Similarly, Laird et al. retrospectively studied the effects of prophylactic bilateral central neck dissection on iodine doses, and found that a higher median dose of 131I was delivered to node-positive patients versus patients classified as pN0 [23]. Significantly higher doses of iodine were administered to patients pN1 in the study by Moo et al. as well [24]. In the present study, we found a trend toward fewer administrations of 131I in patients treated with TT + PND as compared to TT alone, but the total cumulative dose was not different.
Our standard of care for re-treating patients was based not only on the post-iodine whole body scan, but also on the concomitant Tg level and ultrasound findings.
We did not re-treat patients unless there was sufficient evidence in favor of residual or recurrent disease. All patients who received additional radioactive iodine had detectable Tg and uptake in the neck outside of normal thyroid remnants. All of the patients who had surgery had detectable Tg and neck nodes that were visible on ultrasound and/or other imaging modalities.
There is a current trend toward the administration of lower doses of radioiodine or none at all for low-risk patients [25, 26]. In our study we chose, however, to include only patients having received ablation with 131I in order to avoid a selection bias and to compare stimulated Tg levels in the two patient groups. Low Tg levels following ablation have been shown to be predictive of a favorable outcome for differentiated thyroid cancer. Brassard et al. found that the negative predictive value for recurrence of a low post-ablation Tg titer (<.27 ng/ml while taking l-thyroxine) is 99 % [27]. In the study by Tuttle et al. patients with a stimulated post-ablation Tg < 1 ng/ml and a normal neck ultrasound had a risk of recurrence of only 2 %, and could thus be re-classified, whatever their initial classification, into the “low-risk” group [28]. Concerning PND, several retrospective studies have found that postoperative preablative Tg levels were lower in patients treated with TT + PND [13, 29–31].
In the present study, we similarly found a higher proportion of patients with low stimulated Tg measurements at the time of ablation for patients treated with PND, and a higher proportion of patients with low Tg levels at the last follow-up. We chose not to calculate the exact quantitative Tg measurements in each group in our study, for several reasons. First, our Tg assay kit changed between the periods 1995–2007 and 2007–2010, with a functional sensitivity of 1 ng/ml for the first period and a more sensitive assay with a functional sensitivity of 0.11 ng/ml for the second period. Thus the exact Tg levels of patients followed during these different periods were not entirely comparable. Second, the method of stimulation during follow-up changed over time, from thyroid hormone withdrawal to rhTSH, which also modified the sensitivity of the Tg measurements. Finally, Tg levels may slowly decrease over time, and the length of follow-up may also modifiy the relevance of the Tg measurements.
One bias in our study, as in the study by Popadich et al. [13] resided in the fact that a subset of patients in the group treated with TT alone were treated outside of our institution, possibly by lower-volume surgeons. The difference in preablation Tg levels may then be due to a larger thyroid remnant left by less experienced surgeons, rather than the effect of PND itself [32]. However, the difference between our two study groups persisted after ablation. In the study by Hughes et al., in which no difference in preablation Tg was found, all of the patients had been treated surgically at the same institution [19]. In the studies by Sywak et al. [29], Lang et al. [31], and So et al. [30], which found lower Tg levels for patients undergoing PND, the patients in the compared groups were treated by the same surgical teams.
It may be argued that despite the 10 % of patients who avoided reoperation in the central compartment in our study thanks to PND, the other 90 % of the patients treated with TT + PND were surgically overtreated. This is not a disadvantage if the complication rate is not higher than that for TT alone. Several studies have shown that for experienced surgeons the rate of permanent complications after reoperation is comparable to that for initial surgery [15, 16]. Indeed, in our study, even with more reoperations in the group treated with TT alone, there was no significant difference in the rate of permanent complications.
Ideally, we need a more sensitive means of preoperatively staging the central neck without having to perform PND. Several surgical teams have investigated sentinel node biopsy in the treatment of thyroid cancer [33]. The detection rates are high (84–98 %) and the false-negative rates relatively low (0–16 %). However, none of these studies has shown an improvement in the rate of surgical complications compared to that achieved with PND.
Some studies have also shown a high rate of occult nodal metastases in the lateral neck, discovered on routine prophylactic lateral neck dissection [20, 34]. The relatively high rate of occult metastases to the lateral neck—51 % in the study by Ito et al. [34] and 23 % in our previous study [20]—suggests that complete staging of the neck may require PND of the lateral neck in addition to PND of the central compartment. Most patients with occult metastases in the lateral neck also have nodal disease in the central neck. However, “skip” metastases to the lateral neck occur in only 15–20 % of patients with metastatic nodes [35]. Thus, with the relatively low rate of “skip” metastases to the lateral neck, central compartment PND alone may suffice for staging the neck N0/N1 in most patients, keeping in mind that patients staged N1 in the central neck are at a relatively higher risk of harboring occult metastases in the lateral neck. Indeed, in our previous study, 55 % of the patients with nodal metastases had metastases in the lateral neck [20]. This number was 51 % in the study by Ito et al. [34]. In our current study, most patients underwent PND of the ipsilateral lateral neck levels III and IV as well as the central compartment, which may have influenced preablative Tg measurements but also doses of 131I administered. PD of the lateral neck probably did not influence the rate of reoperation, however, because only one patient in our study (in the TT-only group) underwent reoperation for an isolated recurrence in the lateral neck.
Ideally, to provide the advantage of personalized radioiodine treatment according to the pN stage while avoiding PND for patients without occult metastatic nodes (pN0), we need to improve our methods for staging the neck preoperatively. New techniques of sentinel node mapping and imaging based on contrast-enhanced US are being studied [36–38]. Studies of biologic prognostic markers may provide the means of noninvasively predicting occult lymph node metastases in the future.
Conclusions
Adding PND to TT decreased the number of reoperations in the central compartment and the rate of retreatment with surgery or 131I. It also increased the proportion of patients with low preablation Tg levels. Reoperation in the central compartment was avoided by PND in 10 % of patients. The rate of permanent complications was similar in the two groups. When it can be performed safely, PND may be advantageous to avoid reoperation and improve long-term prognosis by lowering preablation Tg levels. The benefits of PND could be maintained while avoiding surgery for patients without occult nodal metastases if nonsurgical staging of the central neck were to be improved by new imaging or biologic tools.
References
Alfalah H, Cranshaw I, Jany T et al (2008) Risk factors for lateral cervical lymph node involvement in follicular thyroid carcinoma. World J Surg 32:2623–2626. doi:10.1007/s00268-008-9742-2
Pisanu A, Deplano D, Pili M et al (2011) Larger tumor size predicts nodal involvement in patients with follicular thyroid carcinoma. Tumori 97:296–303
Robbins KT, Clayman G, Levine PA et al (2002) Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch Otolaryngol Head Neck Surg 128:751–758
Carty SE, Cooper DS, Doherty GM et al (2009) Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 19:1153–1158
Cooper DS, Doherty GM, Haugen BR et al (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
Bardet S, Malville E, Rame JP et al (2008) Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol 158:551–560
Podnos YD, Smith D, Wagman LD et al (2005) The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg 71:731–734
Zaydfudim V, Feurer ID, Griffin et al (2008) The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 144:1070–1077 discussion 1077–1078
Bilimoria KY, Bentrem DJ, Ko CY et al (2007) Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 246:375–381 discussion 381–374
Mazzaferri EL, Doherty GM, Steward DL (2009) The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid 19:683–689
Zetoune T, Keutgen X, Buitrago D et al (2010) Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a meta-analysis. Ann Surg Oncol 17:3287–3293
Shaha AR (1998) Thyroid carcinoma: implications of prognostic factors. Cancer 83:401–402 discussion 403–404
Popadich A, Levin O, Lee JC et al (2011) A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery 150:1048–1057
Chisholm EJ, Kulinskaya E, Tolley NS (2009) Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope 119:1135–1139
Alvarado R, Sywak MS, Delbridge L et al (2009) Central lymph node dissection as a secondary procedure for papillary thyroid cancer: Is there added morbidity? Surgery 145:514–518
Shen WT, Ogawa L, Ruan D et al (2010) Central neck lymph node dissection for papillary thyroid cancer: comparison of complication and recurrence rates in 295 initial dissections and reoperations. Arch Surg 145:272–275
Sobin LH, Gospodarowicz MK, Wittekind CW (2010) TNM classification of malignant tumors. Wiley-Blackwell, West Sussex, pp 58–61
Bonnet S, Hartl D, Leboulleux S et al (2009) Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab 94:1162–1167
Hughes DT, White ML, Miller BS et al (2010) Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery 148:1100–1107
Hartl DM, Leboulleux S, Al Ghuzlan A et al (2012) Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg 255:777–783
Robbins KT, Shaha AR, Medina JE et al (2008) Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg 134:536–538
Hughes DT, Doherty GM (2011) Central neck dissection for papillary thyroid cancer. Cancer Control 18:83–88
Laird AM, Gauger PG, Miller BS et al (2012) Evaluation of postoperative radioactive iodine scans in patients who underwent prophylactic central lymph node dissection. World J Surg 36:1268–1273. doi:10.1007/s00268-012-1431-5
Moo TA, McGill J, Allendorf J et al (2010) Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg 34:1187–1191. doi:10.1007/s00268-010-0418-3
Schlumberger M, Catargi B, Borget I et al (2012) Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med 366:1663–1673
Mallick U, Harmer C, Yap B et al (2012) Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med 366:1674–1685
Brassard M, Borget I, Edet-Sanson A et al (2011) Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab 96:1352–1359
Tuttle RM, Rondeau G, Lee NY (2011) A risk-adapted approach to the use of radioactive iodine and external beam radiation in the treatment of well-differentiated thyroid cancer. Cancer Control 18:89–95
Sywak M, Cornford L, Roach P et al (2006) Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery 140:1000–1005 discussion 1005–1007
So YK, Seo MY, Son YI (2012) Prophylactic central lymph node dissection for clinically node-negative papillary thyroid microcarcinoma: influence on serum thyroglobulin level, recurrence rate, and postoperative complications. Surgery 151:192–198
Lang BH, Wong KP, Wan KY et al (2012) Impact of routine unilateral central neck dissection on preablative and postablative stimulated thyroglobulin levels after total thyroidectomy in papillary thyroid carcinoma. Ann Surg Oncol 19:60–67
McHenry CR (2011) Prophylactic central compartment neck dissection for papillary thyroid cancer: the search for justification continues. Surgery 150:1058–1060
Balasubramanian SP, Harrison BJ (2011) Systematic review and meta-analysis of sentinel node biopsy in thyroid cancer. Br J Surg 98:334–344
Ito Y, Tsushima Y, Masuoka H et al (2011) Significance of prophylactic modified radical neck dissection for patients with low-risk papillary thyroid carcinoma measuring 1.1–3.0 cm: first report of a trial at Kuma Hospital. Surg Today 41:1486–1491
Machens A, Holzhausen HJ, Dralle H (2004) Skip metastases in thyroid cancer leaping the central lymph node compartment. Arch Surg 139:43–45
Yang WT, Goldberg BB (2011) Microbubble contrast-enhanced ultrasound for sentinel lymph node detection: Ready for prime time? AJR Am J Roentgenol 196:249–250
De Giorgi V, Gori A, Grazzini M et al (2010) Contrast-enhanced ultrasound: a filter role in AJCC stage I/II melanoma patients. Oncology 79:370–375
Curry JM, Ezzat WH, Merton DA et al (2009) Thyroid lymphosonography: a novel method for evaluating lymphatic drainage. Ann Otol Rhinol Laryngol 118:645–650
Conflicts of interest
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was included in a poster presentation at the 82nd annual meeting of the American Thyroid Association, Quebec, Canada, September 19–22, 2012.
Rights and permissions
About this article
Cite this article
Hartl, D.M., Mamelle, E., Borget, I. et al. Influence of Prophylactic Neck Dissection on Rate of Retreatment for Papillary Thyroid Carcinoma. World J Surg 37, 1951–1958 (2013). https://doi.org/10.1007/s00268-013-2089-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2089-3