Abstract
Background
Patients with peritonitis undergoing emergency laparotomy are at increased risk for postoperative open abdomen and incisional hernia. This study aimed to evaluate the outcome of prophylactic intraperitoneal mesh implantation compared with conventional abdominal wall closure in patients with peritonitis undergoing emergency laparotomy.
Method
A matched case-control study was performed. To analyze a high-risk population for incisional hernia formation, only patients with at least two of the following risk factors were included: male sex, body mass index (BMI) >25 kg/m2, malignant tumor, or previous abdominal incision. In 63 patients with peritonitis, a prophylactic nonabsorbable mesh was implanted intraperitoneally between 2005 and 2010. These patients were compared with 70 patients with the same risk factors and peritonitis undergoing emergency laparotomy over a 1-year period (2008) who underwent conventional abdominal closure without mesh implantation.
Results
Demographic parameters, including sex, age, BMI, grade of intraabdominal infection, and operating time were comparable in the two groups. Incidence of surgical site infections (SSIs) was not different between groups (61.9 vs. 60.3 %; p = 0.603). Enterocutaneous fistula occurred in three patients in the mesh group (4.8 %) and in two patients in the control group (2.9 %; p = 0.667). The incidence of incisional hernia was significantly lower in the mesh group (2/63 patients) than in the control group (20/70 patients) (3.2 vs. 28.6 %; p < 0.001).
Conclusions
Prophylactic intraperitoneal mesh can be safely implanted in patients with peritonitis. It significantly reduces the incidence of incisional hernia. The incidences of SSI and enterocutaneous fistula formation were similar to those seen with conventional abdominal closure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients undergoing emergency surgery for peritonitis are at increased risk of abdominal wall-related complications. The risk of incisional hernia in patients with peritonitis is elevated, with an incidence of up to 54 %, compared with an incidence of 11–26 % in the general surgical population [1–3]. Furthermore, up to 24.1 % of patients with peritonitis undergoing emergency laparotomy may develop fascial dehiscence [4].
Prophylactic mesh implantation has been shown to reduce the incidence of incisional hernia in patients undergoing vascular or bariatric procedures [5–7]. However, it remains unclear if nonabsorbable intraperitoneal mesh implantation in an infected abdominal cavity is safe because of the theoretical increased risk of chronic mesh infection and enterocutaneous fistula [8–10].
In a previous study, we demonstrated the feasibility and safety of nonresorbable intraperitoneal mesh placement in patients with postoperative fascial dehiscence or an open abdomen [11]. The present study aimed to evaluate the safety and feasibility of prophylactic intraperitoneal mesh implantation compared with conventional abdominal wall closure in patients with peritonitis undergoing emergency laparotomy.
Methods
A matched case–control study was performed. To analyze a high-risk population for incisional hernia formation, only patients with at least two of the following risk factors were included in the study: male sex, body mass index (BMI) >25 kg/m2, malignant tumor, or previous abdominal incision [12, 13]. Exclusion criteria were no clinical signs of peritonitis, no midline incision, previous laparoscopic surgery, presence of incisional hernia, open abdomen, elective surgery, and previously implanted mesh. Between 2005 and 2010, prophylactic mesh implantation was performed in 63 patients with peritonitis. Patient data were prospectively collected in a database and analyzed retrospectively. In 2008, a total of 401 patients underwent emergency operation at our institution. Among them, 70 patients (17.5 %) underwent conventional abdominal closure without mesh implantation, met the study inclusion and exclusion criteria, and were used as a control group. Clinical long-term follow-up investigations were performed between September and December 2011 at our institution by a single investigator who was not involved in the medical care of the patients. In all, 27 patients (20.3 %) died before the long-term follow-up investigation was performed: 15 patients (23.8 %) in the mesh group and 12 patients (17.1 %) in the control group (p = 0.391). Four patients (6.3 %) in the mesh group and four (2.9 %) in the control group were lost to long-term follow-up. If the patients were unwilling or unable to undergo ambulatory consultations at the referral center (16/133; 12 %), their general practitioners completed the clinical examinations and filled out a questionnaire.
Surgical technique
For closure of the abdominal wall in the control group, a standard technique was applied using a running suture of PDS loop (Ethicon Sarl, Neuchatel, Switzerland). The distance of the sutures to the fascial border was 1 cm, and the distance between stitches was ≤1 cm. The total length of the suturing was at least four times the total length of the abdominal incision.
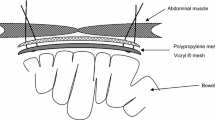
For abdominal wall closure in the mesh group, mesh was implanted intraperitoneally prior to closure. The types of nonabsorbable composite mesh used in 63 patients were as follows: Parietene (Covidien AG, Wollerau, Switzerland) in 45 (71.4 %) patients; Parietex (Covidien AG) in 10 (15.9 %) patients; and Dynamesh (Laubscher, Hölstein, Switzerland) in 8 (12.7 %) patients. Meshes were placed intraperitoneally and fixed with single knot fascial sutures (Prolene 2-0; Ethicon Sarl), endosurgical staples (Protack; Covidien AG), or a combination of the two. Meshes were tailored to overlap lateral and cranial borders of the incision by at least 5 cm. Afterward, the abdominal wall was closed as described for the control group.
The primary outcome measure was incisional hernia. The secondary outcome measures were an open abdomen, surgical site infections (SSIs), enterocutaneous fistula, mesh explantation, and hospital stay. SSIs were assessed up to 30 days after surgery according to the criteria developed by the Centers for Disease Control and Prevention [14]. Infections were categorized as incisional (superficial or deep) or organ–space infections. Superficial SSIs involved only skin and subcutaneous tissue and excluded stitch abscesses. Deep SSIs involved deeper soft tissues, such as fascia and muscle at the site of incision. Organ–space SSIs were defined as infections in any organ or space. Contaminated wounds were defined as acute nonpurulent infections and dirty wounds as having an active infection present. An incisional hernia was defined as any abdominal wall gap with or without a bulge in the area of a postoperative scar that was perceptible or palpable by clinical examination or imaging.
Statistical analysis
Analysis was by intention to treat. Student’s t test was performed to determine the significance between continuous variables and Fisher’s exact test to compare proportions. The p values were two-sided, and p < 0.05 was used as the threshold for statistical significance (NCSS 2007 for Windows; NCSS, Kaysville, UT, USA).
Results
A total of 133 patients with peritonitis who underwent emergency laparotomy fulfilled the inclusion and exclusion criteria. Demographic parameters—including sex, age, BMI, American Society of Anesthesiologists (ASA) score, and co-morbidities—were not significantly different between the two groups (Table 1). No difference was found in the immunosuppression status between the two groups. In the mesh group 11 of the 63 patients (17.5 %) received immunosuppression therapy, as did 9 of the 70 patients (12.9 %) in the control group (p = 0.51). There was a significant difference in the sum of risk factors for incisional hernia between the mesh and control groups: median 3 (range 2–4) versus 2 (2–4) (p = 0.013) (Table 2).
Table 3 reports the operative results. Grades of intraabdominal infection (dirty and contaminated) were comparable in the mesh and control groups (58.7/41.3 vs. 54.3/45.7 %; (p = 0.726). Operating time and duration of hospital stay were comparable in the two groups. In all, 22 patients (34.9 %) in the mesh group and 17 patients (24.3 %) in the control group were treated on the intensive care unit postoperatively (p = 0.188). In four patients (5.7 %) of the control group, mesh was implanted secondarily during reoperation for an open abdomen.
Table 4 reports outcome parameters. SSIs occurred in 30 patients (60.3 %) in the mesh group and 39 patients (61.9 %) in the control group (p = 0.603). The incidence of incisional hernia was significantly lower in the mesh group (2/63 patients) compared with the control group (20/70 patients) (3.2 vs. 28.6 %; p < 0.001). Enterocutaneous fistulas developed in three patients (4.8 %) in the mesh group and in two patients (2.9 %) in the control group (p = 0.667). One mesh was explanted in the mesh group because of a lack of mesh incorporation secondary to repeated reoperations for postoperative intraabdominal hemorrhage. The 30-day mortality rate for patients with an open abdomen was 20 % (1/5).
Discussion
Prophylactic intraperitoneal mesh implantation significantly reduces the incidence of incisional hernia in patients with peritonitis and is associated with a comparable rate of SSIs and enterocutaneous fistula formation compared to conventional abdominal closure. Abdominal wall-associated complications, such as fascial dehiscence and SSIs, are frequent in patients undergoing surgical therapy for peritonitis. Reinforcement of the abdominal wall with a prophylactic intraperitoneal mesh implantation is a reliable treatment strategy to reduce the incidence of incisional hernia. The present study demonstrates a significantly reduced incidence of incisional hernia in patients with peritonitis undergoing prophylactic intraperitoneal mesh implantation (3.2 vs. 28.6 %; p = 0.0001).
Few studies have explored the incidence of incisional hernia. In a retrospective trial, the incidence of incisional hernia was 54.3 % after a median follow-up of 6 years in patients undergoing emergency surgery for secondary peritonitis [1]. A lower incidence of 28.6 % was found in our control group, which may have been due to a shorter duration of follow-up. Prophylactic mesh implantation did not prevent incisional hernia completely in the present study. Incisional hernia was observed in two patients (3.2 %) despite prophylactic mesh implantation. Potential explanations include insufficient mesh fixation or implantation of an undersized mesh. Subgroup analysis showed no difference in the incidence of incisional hernia or SSIs with respect to the different meshes and types of fixation. However, we acknowledge a potential type two error with regard to the small size of the subgroups.
No patient with prophylactic mesh implantation had postoperative open abdomen compared to 5 of 70 patients (7.1 %) in the control group. This difference is not statistically significant.
Complications associated with mesh implantation in patients with peritonitis include SSIs and enterocutaneous fistulas with or without mesh explantation. In the present study, no statistically significant difference regarding the appearance of SSIs was found between patients with and without mesh implantation. No mesh explantations were performed because of chronic infection. SSIs were treated with local therapy, including wound dressing or vacuum-assisted therapy, in both groups.
A relevant difference between this study and previous case series is the mesh material used and the intraperitoneal position of the mesh. Polypropylene-based meshes are associated with significantly reduced ingrowths of bacteria compared to polyester and polytetrafluoroethylene (PTFE)-based meshes [15]. Biofilm produced by gram-positive bacteria provides protection against bacteria only in meshes with large surfaces, such as PTFE, and is thereby associated with chronic infection [15]. The second putative reason for the absence of chronic infection in our series is the mesh placement within the abdominal cavity and not in a preperitoneal space. Unlike preperitoneal tissue, when placed in the abdominal cavity the mesh is in direct contact with peritoneal macrophages and granulocytes, which immediately remove necrotic tissue and initiate a humoral and cellular immune response [16, 17]. In a previous study, mesh implantation in clean-contaminated and contaminated ventral hernia repairs was associated with increased postoperative complications [18]. This study, however, has a selection bias, as the database analyzed did not allow the authors to correct for the indication of mesh implantation [18]. Furthermore, specific mesh-associated complications—e.g., mesh explantation and enterocutaneous fistula—were not described in detail [18].
A limitation of the present study is lack of randomization. Intraperitoneal mesh implantation was performed in selected high-risk patients. Despite being at higher risk, however, the incidence of incisional hernia was reduced in the treatment group at long-term follow-up.
Conclusions
Prophylactic intraperitoneal mesh implantation in patients with peritonitis should be considered as a therapeutic option to reduce significantly the incidence of incisional hernia. The low incidence of enterocutaneous fistula and mesh explantation seems to justify a prophylactic procedure to prevent a frequent complication such as incisional hernia even in patients with an infected abdomen. Randomized, controlled trials are warranted to confirm the safety of prophylactic intraperitoneal mesh placement in the infected abdominal cavity.
References
Moussavian MR, Schuld J, Dauer D et al (2010) Long term follow up for incisional hernia after severe secondary peritonitis: incidence and risk factors. Am J Surg 200:229–234
Sorensen LT, Hemmingsen UB, Kirkeby LT et al (2005) Smoking is a risk factor for incisional hernia. Arch Surg 140:119–123
Kurmann A, Beldi G, Vorburger SA et al (2010) Laparoscopic incisional hernia repair is feasible and safe after liver transplantation. Surg Endosc 24:1451–1455
van Ramshorst GH, Nieuwenhuizen J, Hop WC et al (2010) Abdominal wound dehiscence in adults: development and validation of a risk model. World J Surg 34:20–27. doi:10.1007/s00268-009-0277-y
Strzelczyk JM, Szymanski D, Nowicki ME et al (2006) Randomized clinical trial of postoperative hernia prophylaxis in open bariatric surgery. Br J Surg 93:1347–1350
Bevis PM, Windhaber RA, Lear PA et al (2010) Randomized clinical trial of mesh versus sutured wound closure after open abdominal aortic aneurysm surgery. Br J Surg 97:1497–1502
Curro G, Centorrino T, Low V et al (2012) Long-term outcome with the prophylactic use of polypropylene mesh in morbidly obese patients undergoing biliopancreatic diversion. Obes Surg 22:279–282
Campanelli G, Catena F, Ansaloni L (2008) Prosthetic abdominal wall hernia repair in emergency surgery: from polypropylene to biological meshes. World J Emerg Surg 3:33. doi:10.1186/1749-7922-3-33
Gray SH, Vick CC, Graham LA et al (2008) Risk of complications from enterotomy or unplanned bowel resection during elective hernia repair. Arch Surg 143:582–586
Hawn MT, Gray SH, Snyder CW et al (2010) Predictors of mesh explantation after incisional hernia repair. Am J Surg 202:28–33
Scholtes M, Kurmann A, Seiler CA et al (2012) Intraperitoneal mesh implantation for fascial dehiscence and open abdomen. World J Surg 36:1557–1561. doi:10.1007/s00268-012-1534-z
Hoer J, Lawong G, Klinge U et al (2002) Factors influencing the development of incisional hernia: a retrospective study of 2,983 laparotomy patients over a period of 10 years. Chirurg 73:474–480
Raffetto JD, Cheung Y, Fisher JB et al (2003) Incision and abdominal wall hernias in patients with aneurysm or occlusive aortic disease. J Vasc Surg 37:1150–1154
Anonymous (2004) National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 32:470–485
Engelsman AF, van der Mei HC, Busscher HJ et al (2008) Morphological aspects of surgical meshes as a risk factor for bacterial colonization. Br J Surg 95:1051–1059
Park SY, Jung MY, Lee SJ et al (2009) Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci 122:3365–3373
Wagner BJ, Lindau D, Ripper D et al (2011) Phagocytosis of dying tumor cells by human peritoneal mesothelial cells. J Cell Sci 124:1644–1654
Choi JJ, Palaniappa NC, Dallas KB et al (2012) Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann Surg 255:176–180
Conflict of interest
Drs. Anita Kurmann, Corina Barnetta, Daniel Candinas and Guido Beldi have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurmann, A., Barnetta, C., Candinas, D. et al. Implantation of Prophylactic Nonabsorbable Intraperitoneal Mesh in Patients With Peritonitis Is Safe and Feasible. World J Surg 37, 1656–1660 (2013). https://doi.org/10.1007/s00268-013-2019-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2019-4