Abstract
Background
Pancreatic cancer (PC) carries frequent chemoresistance and extremely dismal prognosis. The underlying mechanisms remain to be further elucidated. We here report the role of Notch1 in gemcitabine resistance and its prognostic significance in PC.
Methods
A small interfering RNA (siRNA) specifically targeting Notch1 was transiently transfected into three PC cell lines (AsPC-1, BxPC-3, and MIA PaCa-2), followed by examination of chemosensitivity to gemcitabine. On the other hand, Notch1 expression was evaluated immunohistochemically and correlated with clinicopathological and prognostic variables.
Results
Successful knockdown of Notch1 by specific siRNA induced increased chemosensitivity to gemcitabine in all three cell lines. Immunohistochemical staining revealed that Notch1 was highly expressed in PC tissues (54.8 %), in contrast to that in para-tumor tissues (16.4 %). In addition, Notch1 positivity was significantly correlated with early-term metastasis and shortened overall survival. Multivariate Cox regression identified Notch1 as an independent prognostic factor.
Conclusions
Notch1 contributes to chemoresistance to gemcitabine, and serves as a significant indicator of unfavorable prognosis in PC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer (PC) is one of the most fatal malignancies, because of its rapid invasiveness and progression. By 2010, PC had become the fourth leading cause of cancer-related mortality [1]. Radical surgical resection is the only hope for cure and prolonged survival; however, only 10–20 % of all patients have the chance to accept potentially curative surgery [2, 3]. Therefore, palliative chemotherapy remains an option for patients with unresectable PC [4]. Since 1997, when a phase III trial demonstrated a modest benefit in survival and alleviation of symptoms, gemcitabine (GEM) has been considered a first-line treatment for patients with advanced PC [5]. Unfortunately, the low response rate of GEM caused by inherent and acquired chemotherapy resistance has limited its applications and is a major reason for the gloomy outcome of PC [6]. So far, less is known about how chemoresistance to GEM exactly occurs, although numerous investigations have been conducted.
The Notch signaling pathway plays a pivotal role in a line of cell phenotypes, such as differentiation, proliferation, and apoptosis [7]. The mammalian Notch receptor family consists of four type I transmembrane receptors (Notch1–4), all of which have been implicated in human cancer [8]. After binding of the receptors to their ligands, the γ-secretase complex mediates cleavage of the transmembrane domain of the Notch receptor to release the intracellular domain of the Notch receptor (NICD). Then, NICD translocates into the nucleus and works as a transcriptional coactivator, thus regulating the expression of target genes, including the hairy enhancer of split (Hes) and the Hes-related (Hey) family. At present there is increasing evidence concerning aberrant Notch signaling in many solid malignancies [8, 9]. In PC, many investigations have suggested that Notch signaling contributes to tumor initiation, growth, invasion, and apoptosis inhibition in both cell lines and animal models [10–18]. However, the function of Notch1 protein in PC is still controversial, because different—even opposite—effects have also been indicated [19, 20]. In particular, the relationship between Notch1 and the sensitivity of GEM in PC remains to be clarified. It has been reported that Notch1 accumulated in PC specimens, but no association with clinicopathological features was found [11, 21]. Furthermore, its prognostic significance remains to be elucidated.
In the present study, we aimed to explore the role of Notch1 in GEM chemoresistance and its prognostic significance in PC.
Materials and methods
Cell culture
Human pancreatic cancer cell lines, AsPC-1, BxPC-3, and MIA PaCa-2 (kind gifts from Professor Helmut Freiss, Heidelberg University, Germany) were maintained in an atmosphere with 5 % CO2 at 37 °C in Dulbecco’s Modified Eagle Medium (DMEM) or Rosewell Park Memorial Institute 1640 (RPMI 1640) medium (Hyclone, Thermo Fisher Scientific Inc, Waltham, MA) supplemented with 10 % fetal bovine serum (FBS; Hyclone).
Transfection of siRNA and GEM treatment
Cells were seeded into 6-well plates at 5 × 105 cells per well, then transiently transfected with Notch1 and control siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 80 pmol for 48 h, using lipofectamine 2000 (Invitrogen, Carlsbad, CA). Then, GEM was added at three concentrations (1 mmol/L, 1 μmol/L, 1 nmol/L) for 48 h.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA, extracted from the tested cells, was isolated by Trizol reagent (Invitrogen) according to the protocols of the manufacturer. Total RNA (2 mg) was subjected to cDNA synthesis with the Progema Reverse Transcription Systems (A3500, Promega, Madison, WI) in a total volume of 20 μl. The reverse transcription reaction was started at 42 °C for 15 min, followed by 95 °C for 5 min and 4 °C for 5 min. Real-time PCR performed with the TaqMan Gene Expression Assay (Notch1: Hs00413187_m1; GAPDH: Hs99999905_m1. Applied Biosystems) was undertaken with TaqMan Gene Expression Master Mix (Applied Biosystems) in triplicate with a 7500 Fast Real-Time PCR System (Applied Biosystems). The reaction conditions were as follows: 95 °C for 10 min, 40 cycles at 95 °C for 15 s, and 40 cycles at 60 °C for 1 min. A comparative CT method (2−ΔΔCT) was used to analyze the relative quantitative expression level of genes.
Protein immunoblot assay
Total protein lysates were separated by 8–10 % Tris–glycine gel electrophoresis and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). After blocking with 5 % non-fat dry milk, proteins were incubated with the primary antibodies (Hes-1 and β-actin; Santa Cruz) overnight at 4 °C. Subsequently, the membrane was incubated with appropriate horseradish peroxidase-conjugated secondary antibodies at room temperature. Protein bands were visualized by an ECL kit (Millipore), and β-actin was used as the internal control.
Cell growth inhibition assay
Cell growth was determined with a cell count kit (CCK-8). After incubation for 3 h with cell culture medium containing CCK-8 reagent, absorbance at 450 nm was detected with a microplate enzyme-linked immunosorbent assay reader (Wellscan MK3, Thermo/Labsystems, Helsinki, Finland). Inhibition rate, as the effect marker of treatments, was then calculated.
Patients and specimens
The matched tumor and adjacent normal tissues were collected from patients with pancreatic ductal adenocarcinoma (PDAC) who underwent surgical resection in our institution from September 2007 to July 2009. In total, 73 patients (41 men and 32 women) were enrolled in this study; the median age of the patients was 63 years (range: 38–80 years). TNM (Tumor-Node-Metastasis) staging produced the following results: stage I, 7 cases; stage II, 54 cases; stage III, 5 cases; and stage IV, 7 cases. Follow-up data were obtained in 67 patients (median: 12 months; range: 2–34 months). Of 55 patients who underwent curative resection 26 developed postoperative recurrence, and 11 received GEM-based chemotherapy after recurrence. Early-term metastasis (defined as metastasis within 3 months after surgery), overall survival (OS), and disease-free survival (DFS) served as endpoints. The institutional ethics committee gave approval for the study.
Immunohistochemical staining and results assessment
Immunohistochemical staining for Notch1 was performed in paraffin-embedded specimens from 73 patients with PDAC. Rabbit anti-human Notch1 polyclonal antibody (ab27526, Abcam, Cambridge, UK) and Power Vision two-step staining kit (PV-6001, Beijing Zhongshan Biotech Co, China) were used for staining. All sections were deparaffinized in xylene and dehydrated through a gradient concentration of alcohol before endogenous peroxidase activity was blocked with 3 % H2O2 in methanol for 10 min. Antigen retrieval was performed by both microwave and incubation with 0.1 % trypsin. The primary antibody at a dilution of 1:200 was incubated overnight at 4 °C. After washing in phosphate buffered saline (PBS) three times, horseradish peroxidase (HRP)-labeled secondary antibody was added for an incubation of 30 min at 37 °C. Diaminobenzidine was adopted as a chromogen. Finally, slides were counterstained with hematoxylin. Preimmune rabbit serum at the same dilution was used as the negative control. The immunoreactivity of Notch1 was evaluated independently by two pathologists (Q.C.C. and J.S.). Cytoplasmic immunoreactivity was considered positive when the specific staining was observed in more than 10 % of the cells, as previously reported [22].
Statistical analysis
Data regarding continuous variables were presented as mean ± standard deviation (SD). An independent sample t test was used for continuous variables, and either the Chi square test or Fisher’s exact test (two-sided) was used for categorical variables. Patient survival was calculated by the Kaplan–Meier method and compared with the log rank test. Cox regression (proportional hazard model) was adopted for multivariate analysis. Statistical software SPSS 13.0 (SPSS Inc, Chicago, IL) was employed for all analyses. A statistically significant p value was defined as a value of <0.05.
Results
Knockdown of Notch1 enhanced chemosensitivity to gem in pc cell lines
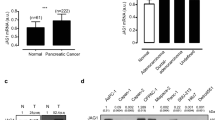
RNA interference that specifically targets to Notch1 was used. Transfection with Notch1 siRNA for 48 h in three cell lines successfully decreased the expressions of Notch1 and Hes-1, compared with that of control siRNA (Fig. 1a, b). After transfection, cells were treated with GEM at various concentrations (1 mmol/L, 1 μmol/L, 1 nmol/L) for an additional 48 h. In AsPC-1 and MIAPaCa-2 cells, inhibition rates were significantly increased in the Notch1 siRNA group at both 1 mmol/L and 1 μmol/L concentrations of GEM, compared with those in the control group (Fig. 1c). In contrast, no difference was observed at the level of 1 nmol/L. In BxPC-3 cells, significantly higher inhibition rates have been present in all three concentrations of GEM in the Notch1 siRNA group (Fig. 1c). On the other hand, Notch1 siRNA, compared with control siRNA, did not result in statistically changed inhibition rates (Fig. S1).
Expression and significances of Notch1 in PDAC
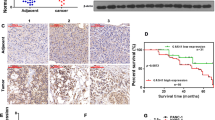
The Notch1 specific staining signal is located primarily in the cytoplasm of tumor cells. Positive staining of Notch1 was detected in tumor tissues from 40 patients (54.8 %; Fig. 2 b–d). It was also strongly expressed in tumor tissues with perineural invasion (Fig. 2e). In contrast, Notch1 staining was negative in 61 paratumoral tissue samples (83.6 %; Fig. 2a), with a positive rate significantly less than that in tumor tissues (p = 0.001). Among the 11 patients who received GEM-based chemotherapy after recurrence, 5 out of 7 with tumors carrying positive Notch1 expression showed progression of disease after the treatment, whereas 1 of 4 patients with tumors in which Notch1 expression was negative did not reach tumor control (p = 0.242; Fisher’s exact test).
Immunohistochemical expression of Notch1 in pancreatic ductal adenocarcinoma tissues and its prognostic implication. a Negative expression of Notch1 in para-tumor tissue (lower left quadrant) and positive in tumor tissue (upper right quadrant), original magnification ×100. b Low expression of Notch1 in tumor tissue, original magnification ×100. c Medium expression of Notch1 in tumor tissue, original magnification ×100. d High expression of Notch1 in tumor tissue, original magnification ×200. e Positive expression of Notch1 in tumor tissue with perineural invasion, original magnification ×100. f Negative control. Original magnification ×100
With either the Chi square test or Fisher’s exact test, no clinicopathological parameters including gender, age, tumor location, pathological T stage, N stage, and tumor differentiation grade were associated with Notch1 expression in tumor cells (Table 1).
For the endpoints, Notch1 expression significantly correlated with early-term metastasis (6/34 vs 0/33; p = 0.029). Besides, a statistically significant difference of overall survival was found in PDAC patients between positive and negative staining of Notch1 in tumor cells (Table 2). The median survival for negative Notch1 expression was 22 months, whereas that for those with positive expression was 13 months. Kaplan–Meier survival curves showed that patients with positive Notch1 staining had an obviously poorer OS (Fig. 3). In addition, OS was significantly associated with N stage and resection margin status (Table 2). Multivariate Cox regression analysis identified Notch1 expression and N stage as independent prognostic factors for OS (Table 2). However, Notch1 expression was not a significant indicator for DFS (data not shown).
Discussion
Notch is a highly conserved family of genes that encode four transmembrane receptors, i.e., Notch1–4 [8]. When a Notch receptor binds to its ligand, the signaling pathway is initiated [8]. Through three cleavages, particularly a third cleavage (S3 cleavage).
mediated by the presenilin–γ-secretase complex, active NICD is released, thus initiating a transcriptional cascade that regulates expression of target genes, including Hes family [8]. So far, the role of each Notch receptor, as an oncogene or a tumor suppressor, is still controversial. In PC cells and/or animal models, most research reports that activation of Notch1 signaling accelerates growth, inhibits apoptosis, and promotes invasion [12–14, 17], thus contributing to formation and progression of the tumor. However, Mazur et al. [20] found that deficiency of Notch2, but not Notch1, stops pancreatic intraepithelial neoplasia (PanIN) progression, prolongs survival, and leads to a phenotypical switch toward anaplastic pancreatic cancer with epithelial-mesenchymal transition. Other authors even revealed that Notch1 functions as a tumor suppressor in a model of K-ras-induced PDAC [19]. Therefore, further evidence needs to be accumulated. In addition, the effect and mechanisms of Notch1 on sensitivity to GEM, a first-line agent in PC [3], have not been previously studied. In the present study, it was shown that successful knockdown of Notch1 by specific siRNA downregulated Hes-1 expression, thus decreasing the activity of the signaling pathway. Subsequently, the inhibition rates of cells to GEM were significantly enhanced, under the basis of nonsignificant differences caused by Notch1 siRNA alone, suggesting that the treatment made the cells more sensitive to GEM. Importantly, the same trend was present in all three cell lines, strongly indicating that the phenomenon is quite pronounced. Moreover, we found the trend concerning the association between positive expression of Notch1 and poor tumor control in the clinical application of GEM-based chemotherapy, although a statistically significant difference was not observed because of limited case numbers. The findings reported here provide the first evidence that Notch1 contributes chemoresistance to GEM in PC cells. It was found that Notch1 was involved in phenotypes of cancer stem cells (such as epithelial–mesenchymal transition) and development of pancreas [18, 23], indicating a possible explanation of our results. In addition, it was reported that inhibition of Notch3 enhances sensitivity to GEM in PC through an inactivation of PI3 K/Akt-dependent pathway [24]. Therefore, exact roles and mechanisms of Notch receptors in sensitivity of PC cells to GEM remain to be investigated in detail.
Thus far, PC, also known as PDAC, remains a malignant tumor with an extremely dismal prognosis [1–3]. Many clincopathologic variables, including lymph node metastases, high tumor grade, large tumor, high CA 19–9 level, and positive margin of resection, have been linked to poor prognosis of patients with PC [3]. On the other hand, a panel of molecular markers, for example, Ki-67, p27, p53, transforming growth factor β1, Bcl-2, survivin, vascular endothelial growth factor, cyclooxygenase 2, CD34, S100A4, and human equilibrative nucleoside transporter 1, have provided independent prognostic information in PC [25]. However, the correlation between Notch1, a prognostic factor in other malignant neoplasms, including colorectal cancer [26], breast cancer [27], lung cancer [28], and neuroblastoma [29], and outcome of patients with PDAC continues to be of interest, although preliminary clues for expression and clinicopathological relevance of Notch1 protein in PADC samples have been provided [11, 21]. In the present study, we examined the expression of Notch1 protein in tumor and paratumoral tissues of PDAC by immunohistochemistry, and we found that Notch1 expression was significantly increased in tumor tissues compared with adjacent normal pancreatic tissues. The different staining evaluation criteria might account, at least in part, for the result that was not consistent with prior findings [21], whereas no association with clinical and pathological parameters was observed in either of the investigations. As for prognostic significance of Notch1, our data revealed a positive correlation between Notch1 expression and two variables reflecting patient outcome, including early-term metastasis and OS. For OS, multivariate analysis identified positive expression of Notch1 as an independent prognostic factor of PDAC, accompanied by a conventional clinical variable, N stage. The results indicated that Notch1 might have potential relevance for clinical outcome in PC, although its implications in DFS were not shown in the present study. In the future, more prospective studies with larger sample size will be helpful for comprehensive evaluation of the prognostic value of the novel biomarker in patients with PDAC.
In summary, our data revealed that Notch1 contributed to GEM resistance in PC. Positive expression of Notch1 in tumor tissue was correlated with early-term metastasis and poor OS, and thus was of prognostic significance in PDAC. All the findings suggest that Notch1 serves as a promising therapeutic target and prognostic marker in PC.
References
Jemal A, Siegel R, Xu J et al (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Gillen S, Schuster T, Meyer Zum Buschenfelde C et al (2010) Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 7:e1000267
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617
Shrikhande SV, Kleeff J, Reiser C et al (2007) Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 14:118–127
Burris HA 3rd, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Xie DR, Yang Q, Chen DL et al (2010) Gemcitabine-based cytotoxic doublets chemotherapy for advanced pancreatic cancer: updated subgroup meta-analyses of overall survival. Jpn J Clin Oncol 40:432–441
Tien AC, Rajan A, Bellen HJ (2009) A Notch updated. J Cell Biol 184:621–629
Ranganathan P, Weaver KL, Capobianco AJ (2011) Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 11:338–351
Takebe N, Harris PJ, Warren RQ et al (2011) Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 8:97–106
Miyamoto Y, Maitra A, Ghosh B et al (2003) Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 3:565–576
Buchler P, Gazdhar A, Schubert M et al (2005) The Notch signaling pathway is related to neurovascular progression of pancreatic cancer. Ann Surg 242:791–800
Wang Z, Banerjee S, Li Y et al (2006) Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res 66:2778–2784
Wang Z, Zhang Y, Li Y et al (2006) Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther 5:483–493
Kimura K, Satoh K, Kanno A et al (2007) Activation of Notch signaling in tumorigenesis of experimental pancreatic cancer induced by dimethylbenzanthracene in mice. Cancer Sci 98:155–162
De La OJ, Emerson LL, Goodman JL et al (2008) Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A 105:18907–18912
Mullendore ME, Koorstra JB, Li YM et al (2009) Ligand-dependent Notch signaling is involved in tumor initiation and tumor maintenance in pancreatic cancer. Clin Cancer Res 15:2291–2301
Ristorcelli E, Beraud E, Mathieu S et al (2009) Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer 125:1016–1026
Bao B, Wang Z, Ali S et al (2011) Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett 307:26–36
Hanlon L, Avila JL, Demarest RM et al (2010) Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res 70:4280–4286
Mazur PK, Einwachter H, Lee M et al (2010) Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A 107:13438–13443
Vizio B, Mauri FA, Prati A et al (2012) Comparative evaluation of cancer stem cell markers in normal pancreas and pancreatic ductal adenocarcinoma. Oncol Rep 27:69–76
Cheung HC, Corley LJ, Fuller GN et al (2006) Polypyrimidine tract binding protein and Notch1 are independently re-expressed in glioma. Mod Pathol 19:1034–1041
Murtaugh LC, Stanger BZ, Kwan KM et al (2003) Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100:14920–14925
Yao J, Qian C (2009) Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3 K/Akt-dependent pathway. Med Oncol 27:1017–1022
Ansari D, Rosendahl A, Elebro J et al (2011) Systematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with pancreatic cancer. Br J Surg 98:1041–1055
Chu D, Zhang Z, Zhou Y et al (2011) Notch1 and Notch2 have opposite prognostic effects on patients with colorectal cancer. Ann Oncol 22:2440–2447
Reedijk M, Odorcic S, Chang L et al (2005) High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 65:8530–8537
Donnem T, Andersen S, Al-Shibli K et al (2010) Prognostic impact of Notch ligands and receptors in nonsmall cell lung cancer: coexpression of Notch-1 and vascular endothelial growth factor-A predicts poor survival. Cancer 116:5676–5685
Chang HH, Lee H, Hu MK et al (2010) Notch1 expression predicts an unfavorable prognosis and serves as a therapeutic target of patients with neuroblastoma. Clin Cancer Res 16:4411–4420
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was supported by National Natural Science Foundation (81071693) and National Laboratory of Molecular Biology Special Foundation (2060204), China.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Impacts of Notch1 siRNA on growth inhibition in 3 cell lines. Supplementary material 1 (TIFF 208 kb)
Rights and permissions
About this article
Cite this article
Du, X., Zhao, YP., Zhang, TP. et al. Notch1 Contributes to Chemoresistance to Gemcitabine and Serves as an Unfavorable Prognostic Indicator in Pancreatic Cancer. World J Surg 37, 1688–1694 (2013). https://doi.org/10.1007/s00268-013-2010-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2010-0