Abstract
Background
Perioperative hemorrhage and postoperative bile leakage are severe complications of liver surgery. They may be related to the techniques used to divide the tissue. We designed a randomized clinical trial to compare the cavitron ultrasonic surgical aspirator (CUSA) and an endoscopic stapler device applied in routine clinical hepatic surgical practice.
Methods
All consecutive patients admitted for elective hepatic resective surgery—at least bisegmentectomy of the liver—were assessed for enrollment in the study. A total of 100 patients were subsequently randomized. There was a good balance between the study groups concerning issues that may be of relevance for the perioperative and postoperative courses. The primary objective of the study was to achieve an approximately 25 % reduction in perioperative blood loss and postoperative bile leakage. Secondary outcome variables were operating time, general postoperative morbidity, length of hospital stay, and direct medical costs.
Results
The amount of perioperative or postoperative blood loss did not differ significantly between the two groups. We observed a trend toward shorter transection and operating time for patients in whom staplers were used, but the difference did not reach statistical signifcance. The postoperative courses were close to identical in the respective study arms with no difference in bile leakage rates or in the total morbidity profiles. The direct medical costs were nonsignificantly lower in the group where staplers were used for liver transection.
Conclusions
The results show that the use of endoscopic vascular staplers in liver surgery is feasible and safe. It offers an attractive alternative for division of the liver parenchyma during routine hepatic surgery, being comparable to the use of CUSA without adding extra costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The attitudes toward, and experiences from, hepatic surgery have changed during the last decades. Nowadays, these surgical procedures can be performed with few serious perioperative events and with low postoperative morbidity [1, 2]. Specialized high-volume centers, with their expertise and implementation of novel technologies and therapies, however, drive hepatic surgery toward more complex procedures with their associated perioperative and postoperative challenges [3, 4]. One of these challenges is the increased preoperative blood loss observed in patients submitted to neoadjuvant chemotherapy due to colorectal cancer metastases [5].
Transection of hepatic tissue represents a critical part of hepatic surgery. Control of operative blood loss is of immediate concern when performing liver resection. Excessive hemorrhage and blood transfusion in patients undergoing liver resection are associated with increased postoperative morbidity and, in patients with colorectal metastasis, with a shorter disease-free survival [1, 3–5]. Low central venous pressure seems to be important to control bleeding and facilitate transection of the parenchyma [6, 7]. Moreover, there has been substantial experimental and clinical research on procedure-oriented issues—e.g. vascular inflow and outflow control and technologies used to transect the liver parenchyma—aiming at better bleeding control [6–12]. However, few if any data have been presented to suggest that, for example, one transection technique has advantages over another [11, 13, 14]. In fact, even today the standard of care for hepatic surgery is to divide the tissue using simple devices such as Kelly’s clamp or by finger fracture technique. Despite these ambiguities, ultrasonic dissectors such as the cavitron ultrasonic surgical aspirator (CUSA) have for many years been accepted as the standard technology for dividing the parenchyma. Ultrasonic dissectors are generally thought to allow the hepatic surgeon to complete and master difficult, meticulous dissections, particularly along the hepatic pedicles and major vessels.
As primarily metastatic liver surgery rapidly expands, however, the number of procedures that mandate refined dissection techniques and devices represents only a small number of cases [2–4]. Negative aspects of the CUSA is that it is time-consuming and sometimes difficult to master. Also, it contains several technical components that may malfunction. The technique has not solved the problems of postoperative bile leakage and enhanced blood loss when addressing a liver adversely affected by preoperative chemotherapy.
Uncontrolled studies have suggested that the use of vascular stapler devices, developed for laparoscopic use, may be feasible and have advantages for this clinical application [11, 13, 14]. The stapler technique’s advantages seem to be that it is simple and is thus easy to learn and master. Also, the transection can be accomplished quickly, and dividing the parenchyma and hepatic vein with the same device might save time. The aim of this study was to compare the CUSA to an endovascular stapler during routine clinical hepatic surgical practice.
Patients and Methods
Patients
All consecutive patients admitted for elective hepatic resective surgery—at least bisegmentectomy of the liver—from April 2008 and onward were assessed for enrollment in the study. A precondition for both devices (CUSA and stapler) was that the parenchyma from a surgical technique perspective was applicable and feasible for transection. All patients were randomized during surgery following intraoperative contrast-enhanced ultrasonography (CEUS). The randomization was completed by the use of opaque, sealed envelopes with computer-generated random numbers in blocks of 10 (5:5). All surgeons participating in the trial were familiar with both transection devices.
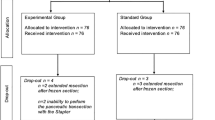
As seen in the patient flow chart (Fig. 1), 100 of 149 patients were found eligible for randomization. Eight patients were not allocated to randomization because of completion of a concomitant pilot project with a novel device for parenchyma transection. In all, 49 patients could not be enrolled in the study because neither transection technique was applicable for various reasons (for more detail see Fig. 1). In one case, the CUSA did not work accurately, which necessitated transection with the alternative technique (included in the intention-to-treat analysis). The most prominent reason for exclusion was the anatomic location of the lesions, which excluded the use of staplers (n = 16). It became clear that the surgical strategy often had to be changed after completion of the intraoperative CEUS, making the final operative approach more suitable for the use of the CUSA device. In 12 patients, for example, simple resection of only one liver segment was considered sufficient. In eight patients, disseminated disease was the reason for exclusion. In four cases, the preoperative assessment suggested gallbladder carcinoma, and the patient was scheduled for bisegmentectomy. At the final intraoperative evaluation by the operating team, however, simple cholecystectomy was considered sufficient.
The demographic characteristics of the patients are presented in Table 1, which shows a balance between the two study groups concerning issues that may be of relevance for perioperative and postoperative courses. About half of the patients in the respective study group had received preoperative chemotherapy.
Procedures
The procedures were all performed through a conventional laparotomy using an extended subcostal right incision. The surgeon made an initial estimate of the degree of steatosis and fibrosis in addition to the presence of possible macroscopic cirrhosis (Table 2). Conventional right and left hemihepatectomies were performed by division of the respective arterial and venous inflow and outflow before transection of the hepatic tissue. In most cases, the portal venous branch was divided and closed by vascular staplers, as were the right or left hepatic vein. According to our operative procedure protocol, a Pringle maneuver was not applied routinely, but it was registered whenever it was required to control bleeding during the transection. Before starting division of the liver, the central venous pressure was stabilized to ≤5 cm H 2 O. After incising the liver capsule, the dissection progressed through the tissue. The respective vascular and other intra-parenchymal structures were secured by use of clips or suture-ligation. In patients allocated to transection with staplers, we used the technique introduced by Fong and Blumgart [12], wherein the hepatic tissue was divided using an endoscopic vascular stapler (Covidien Sweden AB, Stockholm). To allow subsequent dissection of the hepatic parenchyma, the liver tissue was fractured in steps with a clamp and subsequently divided with the vascular endoscopic stapler [12, 14]. The procedure we followed when dividing the hepatic parenchyma with the ultrasonic dissector CUSA (CUSA, Electa) was according to the generally applied and practiced principles [1].
Before closure of the abdominal incision in all patients, a drain was inserted that drained the transection surface of the liver. During at least the first 3 postoperative days, bilirubin concentrations and the total volume output of the drains were recorded. We used the bile leakage definition of the international consensus group [15]: Bile leakage is present when bilirubin concentrations in the drain fluid exceeded the serum bilirubin level by at least three times, or after postoperative day 3 or if radiologic and/or operative interventions were required to drain an intraabdominal bile collection with or without peritonitis.
A new validated liver function assessment system, called composite scores, was used outside the predefined study endpoints [16]. It incorporates liver failure, ascites formation, bile leakage and intra-abdominal abscess formation. Because of the relevance of blood loss for this type of surgery and the design of this study, the need for more than two units of transfused blood during the first 48 h after the operation was also added to the composite scores. This amounted to a theoretical maximal score of 5 for each individual patient.
The direct medical costs of the operation were calculated from the price per minute in the operating theater (16 euros/minute). To that were added all retail prices for the instruments used, including the investment costs for the CUSA, as capital service costs spread out over a period of 3 years. All extra equipment and gear used for the respective operations as well as specialized pharmacologic interventions were recorded and added to the total costs of the procedure. Concerning the postoperative course, the interventions and hotel costs were virtually identical between the study groups, so we did not incorporate them into the final analysis.
Statistics and Ethics
The ethics committee of Karolinska Institutet approved the study protocol. Informed consent was obtained preoperatively from each participating patient. The primary objective of the study was to achieve an approximately 25 % reduction in preoperative blood loss and postoperative bile leakage. A total of 100 patients had to be enrolled in the trial to document such a difference with 95 % probability with a power of 80 %.
Secondary outcome variables were the operation time, general postoperative morbidity as assessed by use of Clavien’s scoring system [17], and the length of hospital stay (LOS).
Intergroup differences were assessed by use of the analysis of variance (ANOVA) variance test adjusted for multiple testing, the χ 2 test, and the t test. Wilcoxon nonparametric tests were added when applicable. If not otherwise stated, the data are presented as the median with a 95 % confidence interval (CI).
Results
The operating time was somewhat (but not significantly) shorter in the staple group because of a shorter liver transection time (data not shown). The total blood loss during the operations was numerically larger in the CUSA group (although the difference was not significant), although the amount of blood transfused was identical in the two groups (Table 2). At the time of final histomorphologic assessment of the operative specimens, the radicality, reflected in the R1 resection rates, was the same in the two groups. The bile leakage rate was identical for the two study arms, as was the need for intervention in terms of additional percutaneous drainage or stent insertion to control ongoing leakage (Table 3). The postoperative courses were similar, as was the postoperative complications profile, scored according to Clavien. Also, after the composite outcome score had been calculated, virtually identical scores were seen in the two study groups. Accordingly, the postoperative loss did not differ (Table 3).
The direct medical costs for the respective operations are detailed in Table 4. They show that the baseline charges for the time spent in the theater constituted the major costs. Slightly lower costs were seen for the operation in the stapler group (although not significantly so). As expected, however, fewer vascular clips were consumed when this technique was practiced (p < 0.001). The total costs amounted to 7,188 € and 6,506 € in the CUSA and stapler groups, respectively, a difference that did not reach statistical significance.
Discussion
The results of this study suggest that the endoscopic stapler device can be used successfully for transecting the parenchyma during routine hepatic resection. The use of a stapler might even facilitate the procedure in terms of a shorter transection time. However, although there was a small numerical difference with a shorter operating time in the stapler group, it never reached statistical significance, indicating that this part of the operation has limited impact on the important cost-driving parameter—how much time the total procedure takes in the operating theater. The operating time is of particular importance because it has a direct impact on the direct medical costs.
The purchase cost of a stapling device is substantial, but it has been suggested in an uncontrolled study to be compensated for by the advantages of the technique [13]. On the other hand, it can be argued that the investment costs for the CUSA equipment is covered anyway as there is no substitute for this technology in centers where more advanced hepatic surgery is practiced. Importantly, we were unable to demonstrate a difference in direct medical costs between the CUSA and the stapler. Because we observed nearly identical postoperative courses and in-hospital stays for the two groups, an even more extensive description of the total in-hospital costs would not have created another picture.
Transection of the hepatic tissue represents a critical part of hepatic surgery. It has been emphasized here that keeping the central venous pressure stable and low is essential to minimize blood loss [1, 6, 7]. Otherwise, a number of other approaches, relevant to inflow and outflow control and transection technologies, have been critically evaluated to achieve this goal [6–12, 18]. Presently, it can be concluded that no specific tool and/or approach has been found superior to the other when it comes to division of the liver parenchyma. This was also the conclusion of a recent systematic review of the literature [9]. In fact, even today the standard of care in hepatic surgery is to divide the tissue by use of simple devices such as Kelly’s clamp or by finger fracture technique. Despite these conclusions, reached from an evidence-based platform, it is clear that many expert centers across the world, continue to use an ultrasonic dissector such as the CUSA to divide the parenchyma. A generally advocated opinion is that the CUSA allows the hepatic surgeon to complete and master more difficult and meticulous dissections, particularly along the hepatic pedicles and major vessels. On the other hand, at a time when primarily metastatic liver surgery is rapidly expanding, the number of procedures that truly mandate refined dissection techniques and devices represents only a small number of cases [19, 20].
In our hands, parenchymal transection with endoscopic vascular staplers is a feasible, safe technique for standard liver resection. The mortality and morbidity presently observed compare well with published large series of nonselected patients undergoing routine liver resection in high-volume surgical centers.
Control of operative blood loss is one of the most immediate concerns when performing liver resection. The impact and consequences of excessive hemorrhage and blood transfusion on patients undergoing liver resection is well documented [21–23]. Excessive blood loss is associated with increased postoperative morbidity and, in cases of colorectal metastasis, with a shorter disease-free survival. In contrast to most former series, only a small number of our patients were subjected to a Pringle maneuver, and no other inflow vascular control was applied during the formal resections. As expected, more blood loss was observed in patients in whom more extensive liver resection was required. Ideally, the transection procedure of choice would offer quick, smooth division of the parenchyma with minimal blood loss. The use of staples seems to offer a small step in that direction. In fact, in cases in which we had to complete the transection quickly to master a difficult intraoperative situation, we frequently switched to the stapler so the operative procedure could be completed more meticulously.
Bile leakage and biloma formation presents major obstacles for an uneventful recovery after liver resection [15, 24]. Although recently the overall complication rate has often been reported to be markedly decreased, bile leaks still occur at an unchanged rate, as seen in the present series. It is important to classify these bile leaks carefully. Grade B leaks dominated in our patients. None was classified as grade C. Our protocol used a liberal definition of bile leak: We included those with a drainage bile bilirubin concentration equal to or more than three times the bilirubin concentration in peripheral blood, which explains the relatively high number recorded in our trial. Moreover, in our institution, we promptly reoperate patients who present significant bile leakage during the first 24 h after the operation—i.e. before any other adverse reactions occur. This happened in only one of our cases.
It has been suggested that introduction of a composite outcome score for liver surgery might reduce the sample size required in many clinical trials [17]. When this was applied in the present series of patients, identical scores were calculated for the two study arms.
Factors speaking in favor of the use of staplers can be summarized as follows. The technique is simple and easy to learn and master. Transection of the hepatic tissue is quick and apparently can be accomplished with little blood loss or at least of the same magnitude as when the parenchyma is divided using the ultrasonic dissector. Another point that must be taken into account is that dividing the portal branches and the hepatic vein using the stapler is already a central part of many hepatic procedures. We also found that when the hepatic parenchyma must be divided rapidly to gain access to a bleeding source the operating surgeon chooses to complete the transection using a stapler or finger fracture.
It can be argued that the current preconditions entered into the sample size calculation—i.e. a difference of 25 % between the groups—is not clinically realistic. However, based on the current leakage and morbidity figures, when we calculate the number of patients needed to be studied to minimize the risk for type I and type IΙ errors to show significant results, 250 patients would be required in each arm. Similar calculations suggest that the present results are of clinical importance and relevance for the routine hepatic surgical practice.
Conclusions
The use of endoscopic vascular staplers is a feasible, safe, attractive approach for dividing liver parenchyma during routine hepatic surgery. The results are comparable to those obtained using the CUSA without additional cost.
References
Blumgart LH, Fong Y (2000) Surgery of the liver and the biliary tract, 3rd edn. Saunders, London
Jarnagin WR, Gonen M, Fong Y et al (2001) Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 236:397–407
Khatri VP, Petrelli NJ, Belghiti J (2005) Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol 23:8490–8499
Taketomi A, Kitakawa D, Itoh S et al (2007) Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute’s experience with 625 patients. J Am Coll Surg 4:580–587
Reissfelder C, Rahbari NN, Koch M et al (2009) Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol 16:3279–3288
Rahbari NN, Wente MN, Schemmer P et al (2008) Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br J Surg 95:424–432
Rahbari NN, Koch M, Mehrabi A et al (2009) Portal triad clamping versus vascular exclusion for vascular control during hepatic resection: a systematic review and meta-analysis. J Gastrointest Surg 13:558–568
Schmidt T, Koch M, Antolovic D et al (2008) Influence of two different resection techniques (conventional liver resection versus anterior approach) of liver metastases from colorectal cancer on hematogenous tumor cell dissemination: prospective randomized multicenter trial. BMC Surg 8:6
Rahbari NN, Koch M, Schmidt T et al (2009) Meta-analysis of the clamp-crushing technique for transection of the parenchyma in elective hepatic resection: back to where we started? Ann Surg Oncol 16:630–639
Rahbari NN, Zimmerman JB, Koch M et al (2009) IVC clamp: infrahepatic inferior vena cava clamping during hepatectomy—a randomized controlled trial in an interdisciplinary setting. Trials 10:94
Schemmer P, Friess H, Dervenis C et al (2007) The use of endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg 24:300–305
Fong Y, Blumgart LH (1997) Useful stapling techniques in liver surgery. J Am Coll Surg 185:93–100
Schemmer P, Friess H, Hinz U et al (2006) Stapler hepatectomy is a safe dissection technique: analysis of 300 patients. World J Surg 30:419–430
McEntee GP, Nagorney DM (1991) Use of vascular staplers in major hepatic resections. Br J Surg 78:40–41
Koch M, Garden JO, Padbury R et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading by the international study group of liver surgery. Surgery 149:680–688
Van den Brock MAJ, van Dam RM, van Breukkelen GJP et al (2011) Development of a composite endpoint for randomized controlled trials in liver surgery. Br J Surg 98:1138–1145
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg 240:205–213
Belghiti J, Noun R, Zante E et al (1996) Portal triad clamping or hepatic vascular exclusion for major liver resection: a controlled study. Ann Surg 224:155–161
Nordlinger B, Guiguet M, Vaillant JC et al (1996) Surgical resection of colorectal carcinoma metastasis to the liver: a prognostic scoring system to improve case selection, based on 1,568 patients. Cancer 77:1254–1262
Fong Y, Fortner J, Sun RL et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1,001 consecutive cases. Ann Surg 230:309–318
Yamamoto J, Kosuge T, Takayama T et al (1994) Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 115:303–309
Rosen CB, Nagorney DM, Taswell HF et al (1992) Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 216:493–505
Melendez JA, Arslan V, Fischer ME et al (1998) Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 187:620–625
Tanaka S, Hirohashi K, Tanaka H et al (2002) Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg 195:484–489
Acknowledgments
This study was supported by an unconditional research grant by Covidien Sweden AB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Savlid, M., Strand, A.H., Jansson, A. et al. Transection of the Liver Parenchyma With an Ultrasound Dissector or a Stapler Device: Results of a Randomized Clinical Study. World J Surg 37, 799–805 (2013). https://doi.org/10.1007/s00268-012-1884-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1884-6