Abstract
Background
Laparoscopic resection is increasingly being performed for rectal cancer. However, few data are available to compare long-term outcomes after open versus laparoscopic surgery for early-stage rectal cancer.
Methods
Included in this retrospective study were 160 patients who underwent surgery for stage I rectal cancer between 2001 and 2008. Perioperative outcomes, overall survival (OS), and disease-free survival (DFS) were compared for open versus laparoscopic surgery.
Results
Altogether, 85 patients were treated using open surgery and 80 with laparoscopic surgery. Postoperative mortality (0 vs. 1.3 %; p = 1.00), morbidity (31.3 vs. 25.0 %; p = 0.38), and harvested lymph nodes (22.5 vs. 20.0; p = 0.84) were similar for the two groups. However, operating time was longer (183.8 vs. 221.0 min; p = 0.008), volume of intraoperative bleeding was less (200.0 vs. 150.0 ml; p = 0.03), time to first bowel movement was shorter (3.54 vs. 2.44 days; p < 0.001), rate of superficial surgical-site infection was lower (7.5 vs. 0 %; p = 0.03), and postoperative hospital stay was shorter (11.0 vs. 8.0 days; p < 0.001) in the laparoscopy group than in the open surgery group. At 5 years, there was no difference in OS (98.6 vs. 97.1 %; p = 0.41) or DFS (98.2 vs. 96.4 %; p = 0.30) between the open and laparoscopy groups.
Conclusions
Long-term outcomes of laparoscopic surgery for stage I rectal cancer were comparable to those of open surgery. Laparoscopic surgery, however, produced more favourable short-term outcomes than open surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic surgery has been approved as a standard treatment for colon cancer [1]. Its safety and oncologic outcomes have been proven for colon cancer in several randomized clinical trials [2–5]. However, there is no clear evidence of the benefits of laparoscopic resection for rectal cancer in surgical practice. In particular, long-term data regarding laparoscopic rectal resection are insufficient. Compared with colon resection, rectal cancer surgery involves difficult technical points with regard to total mesorectal excision and autonomic nerve preservation. Therefore, laparoscopic procedures for rectal cancer are regarded as technically demanding [6].
Several randomized studies have compared laparoscopic versus open surgery for rectal cancer [7–11]. Although these studies suggested the technical feasibility and short-term benefits of laparoscopic rectal resection, there remains a lack of level I evidence regarding long-term oncologic outcome. Several retrospective studies that enrolled patients with rectal cancer of various stages have reported long-term outcomes for laparoscopic rectal surgery compared with those for open surgery [12, 13]. They showed that laparoscopic rectal surgery is feasible, having few complications and similar long-term oncologic outcomes. However, the oncologic safety of laparoscopic rectal surgery must be verified in patients with stage I rectal cancer prior to applying it in patients with advanced rectal cancer. We need to make more data available on long-term outcomes of laparoscopic versus open rectal surgery even for early rectal cancer.
Methods
Patients
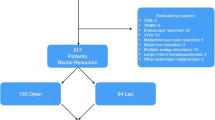
This study included 160 patients who underwent surgery for stage I rectal cancer within 15 cm of the anal verge at the National Cancer Center in Korea from June 2001 to December 2008. Stage I was defined as pathologic T1 or T2 N0 M0 cancer based on the TNM classification system [14]. No patient received chemoradiotherapy preoperatively or postoperatively. All patients underwent lower anterior resection. Abdominoperineal resections were excluded in this study. The medical records of all patients were retrospectively reviewed, including patient demographics, surgical procedures, pathologic findings, postoperative complications, and long-term follow-up data. The institutional review board of the Korean National Cancer Center approved the study protocol.
Surgical procedures
Six colorectal surgeons experienced in laparoscopic colorectal surgery performed all of the surgery. Because laparoscopic colorectal surgery was adopted only in 2006 at our institution, all of the patients who underwent surgery from 2001 to 2005 were included in the open group. After 2006, the surgeons could decide whether to perform laparoscopic surgery. Preoperative staging was performed using routinely computed tomography (CT) with additional magnetic resonance imaging (MRI) or rectal sonography. Furthermore, preoperative biopsy using colonoscopy was performed routinely to verify the cancer.
All patients underwent bowel preparation with polyethylene glycol electrolyte solution before surgery aimed at mechanical cleansing of bowels and reducing the risk of postoperative infection. Second-generation cephalosporin along with additional anti-anaerobe coverage such as cefotetan were given for prophylactic antibiotic coverage. The surgical techniques for open and laparoscopic total mesorectal excision (TME) have been previously described [8]. All patients underwent TME, and the extent of resection was same for the open and laparoscopic methods. We used five trocars: one for pneumoperitoneum and a 30º scope and four for manipulation and dissection of tissue during laparoscopic surgery.
First, the inferior mesenteric vessels were ligated high near the origin with clips, using the medial approach. For TME, the surgeon performed fine dissection using monopolar cautery from the Toldt fascia into the presacral space while maintaining the proper plane of dissection between the fascia propria of the rectum and the presacral fascia. The mesorectum was mobilized cautiously to avoid damaging the underlying hypogastric nerve plexus. For low anterior resection, the distal lumen of the tumour was clamped after identifying the distal resection margin, and rectal washout was performed with a 5 % povidone-iodine solution. One or more linear stapling devices were then introduced through the right lower quadrant port, and the rectum was transected. Surgical specimens were extracted via a 4- to 6-cm extended incision in the left lower quadrant port using a wound protector sleeve. Bowel anastomoses were performed by the double-staple technique or by transanal suture.
Conversion to an open procedure was defined as creation of an abdominal incision larger than that necessary for specimen retrieval. For the open technique, the traditional procedure was performed with the same extent as that in the laparoscopic procedure. A protective loop ileostomy was not performed routinely but was left to the surgeon to decide according to the height of the anastomosis and/or the patient’s co-morbidities, such as use of steroid therapy, diabetes, or immune deficiency.
Diet was started after the first flatus had been passed. Patients were discharged if they considered themselves sufficiently recovered, had been tolerating food for at least 24 hours, and when they met the following criteria: were analgesia-free, could ambulate safely, had afebrile status, and had no major complications. Postoperative morbidity was graded using the Dindo classification [15]: grades I or II for minor surgical morbidity; grades III, IV, or V for major morbidity. Postoperative mortality was defined as death occurring within 30 days after the operation.
Patient follow-up
We performed patient follow-up regularly at 3 or 6 month intervals for 5 years and then yearly thereafter. Carcinoembryonic antigen (CEA) level, digital rectal examination, chest radiography, and abdominal pelvic CT were checked at each follow-up visit. Surveillance colonoscopy was performed at 1–3 year intervals. In the present analysis, we included data up to the last follow-up in October 2010. The median follow-up was 34.0 months [interquartile ratio (IQR) 28.0–45.5 months] for laparoscopic surgery and 70.0 months (IQR, 55.5–89.5 months) for open surgery. Recurrence was diagnosed pathologically by surgical resection, biopsy, or cytology and/or radiologically. Local recurrence was defined as any recurrence diagnosed in the pelvic cavity. Distant metastasis was defined as any recurrence occurring outside the pelvis. Disease-free survival time was defined as the time interval between surgery and any type of recurrence.
Statistical analysis
All analyses were performed using SAS version 9.1.3 for Windows (SAS Institute, Cary, NC, USA). Clinical and pathologic variables were analyzed with the χ 2 test (or Fisher’s exact test) and Student’s t-test (or Mann-Whitney U-test), depending on the distribution of the variables. Overall survival (OS) and disease-free survival (DFS) curves were analyzed by the Kaplan-Meier method and compared using the log-rank test. Multivariate analysis was performed with a stepwise Cox proportional hazards regression model. A value of p < 0.05 was considered statistically significant.
Results
Patient characteristics
Of the 160 patients, 80 underwent open surgery and 80 underwent laparoscopic surgery. The two groups were balanced in terms of their baseline characteristics [sex, age, body mass index, American Society of Anesthesiologists (ASA) score, tumor height, surgical procedures, ileostomy, preoperative CEA level, histologic and pathologic findings] except for the T classification (Tables 1, 2). The laparoscopic group had more T1 lesions than did the open group (53.8 vs. 33.8 %, p = 0.01).
Perioperative outcome
Operating time was significantly longer in the laparoscopy group than in the open surgery group (mean 220.98 vs. 183.81 min; p = 0.008). However, intraoperative blood loss, time of first flatus, and hospital stay were significantly less [estimated blood loss (EBL) 200.0 vs. 150.0 ml, p = 0.03; first bowel movement 3.54 vs. 2.44 days, p = 0.03; hospital stay 11.0 vs. 8.0 days, p < 0.001] in the laparoscopy group than in the open surgery group (Table 3). One case (1.25 %) in the laparoscopy group required conversion to open surgery because of mechanical failure of the circular stapler.
Postoperative morbidity and mortality were similar for the two groups. There was one death in the laparoscopy group. It was caused by pelvic sepsis due to anastomotic leakage. Superficial surgical-site infection was observed more frequently in the open group surgery than in the laparoscopy group (7.5 vs. 0 %, p = 0.03). Other major postoperative morbidities were similar in the two groups, including anastomosis leakage (open surgery 0 % vs. laparoscopy 2.5 %, p = 0.50).
The pathologic findings revealed that the distal resection margin (1.10 vs. 2.00 cm, p = 0.61), radial resection margin (1.00 vs. 1.00 cm, p = 0.34), and median number of harvested lymph nodes (22.50 vs. 20.00, p = 0.84) were similar for the open and laparoscopic surgery groups (Table 2). We found three patients with Dindo grade III or more in laparoscopy group. One patient underwent small bowel resection for bowel obstruction. The other two patients had anastomosis leaks. One of the latter two patients developed multiorgan dysfunction.
Long-term outcome
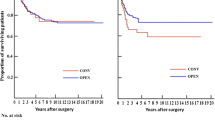
The median follow-up period was 51 months (range 1–109 months). At 5 years after surgery, there was no difference in OS (98.6 vs. 97.1 %, p = 0.410) or DFS (98.2 vs. 96.4 %, p = 0.296) between the two groups (Fig. 1). No local recurrence was detected in either group during the 5 year postsurgical period. One patient in the open surgery group and two patients in the laparoscopy group had recurrences with distant metastasis within a 5 year period after surgery. The distant recurrences were in the liver, lung, and paraaortic lymph nodes. Univariate analysis revealed tumor height as the only significant factor in terms of DSF (Table 4).
Discussion
Many studies, including multicenter randomized trials, have suggested that laparoscopic surgery is feasible for rectal cancer, with acceptable short-term outcomes. The long-term oncologic outcomes of laparoscopic surgery for rectal cancer remain unknown, however. Recent retrospective reports have stated that long-term outcomes for laparoscopic rectal cancer surgery are comparable to those achieved with open surgery. Laurent [12] reported a 5 year DFS rate of 70 % for laparoscopic intersphincteric resection in patients with rectal cancer of the lower third, which is comparable to the 71 % reported for open surgery. Their study included patients with advanced rectal cancer and patients with T3 or N+ tumors who underwent neoadjuvant chemoradiation therapy. The patient groups in most of these studies [12, 13] were heterogeneous, including patients with advanced rectal cancer. This varied patient population means that the results were strongly influenced by the chemotherapy regimen, pre- or post-operative radiation treatment, and differences in the difficulty of the surgical resection, resulting in a wide range of conversion rates and postoperative morbidity rates. Importantly, these factors can affect the feasibility and long-term oncologic outcomes of laparoscopic surgery for rectal cancer. Therefore, it is necessary to consider the long-term oncologic outcomes of laparoscopic rectal cancer surgery in early rectal cancer patients alone—before evaluating advanced rectal cancer patients.
Moreover, many colorectal surgeons first attempt laparoscopic rectal cancer surgery in patients who present with small tumors or early-stage disease. Until now, there have been reports by highly experienced laparoscopic surgeons that included patients with advanced rectal cancer. The present study indicates the feasibility of performing laparoscopic surgery for early rectal cancer. It yielded long-term outcomes that were similar to those achieved with open surgery, providing evidence-based information to novice laparoscopic surgeons for addressing rectal cancer.
Several randomized trials have reported the short-term benefits of laparoscopic surgery over open surgery for rectal cancer [7–11, 16]. The present study also showed that, compared with the open surgery group, the laparoscopic group had better perioperative outcomes, including fewer wound complications, earlier bowel movements, less intraoperative blood loss, and shorter length of hospital stay. The morbidity (25.0 %) and mortality (1.3 %) rates for the laparoscopy group in the present study were comparable to those reported in other studies, which ranged from 6.9 to 40.0 % and from 0 to 2.5 %, respectively [7, 9, 16, 17].
Concerning long-term outcome, the Medical Research Council (MRC) classic trial showed comparable long-term outcomes of laparoscopic surgery for rectal cancer, although these results were drawn from subgroup analysis [18]. Another rectal cancer trial showed that the laparoscopic procedure had oncologic outcomes similar to those attained with the open procedure [10], but the primary endpoint of that study was harvested lymph nodes. A comparative retrospective study [19] reported long-term outcomes with laparoscopic surgery for rectal cancer and found no difference in DFS between laparoscopic and open surgery (82 vs. 79 %; p = 0.52) at 5 years after surgery. Despite these findings, there are insufficient conclusive results regarding the long-term outcome of laparoscopic rectal surgery. We await the findings of long-term outcomes for advanced rectal cancer in several randomized clinical trials, including the COLOR II, ACOSOG-Z6051, and COREAN trials [8, 20, 21].
Limitations of the present retrospective study are that the surgical policy of our institution changed in 2006, and there were no clear criteria for deciding whether a patient underwent laparoscopic or open surgery. Laparoscopic surgery was not performed for rectal cancer from 2001 to 2005, so all patients who had surgery before 2006 were included in the open surgery group. After we adopted the option of laparoscopic surgery for rectal cancer in 2006, the surgeons were responsible for patient selection. However, the relative homogeneity of patients (T1–2 and no classification) and relatively easy surgical resection of the tumors, regardless of the type of surgery (laparoscopic or open) may have minimized selection bias. For example, although the distance between the tumor and the anal verge was greater in the laparoscopy group and tumors were larger in the open surgery group, the differences were not statistically significant. There were significantly more T1 lesions in the laparoscopy group. This disparity may have been influenced by the surgeons’ preference for laparoscopic surgery in cases of early cancer, but no effect on oncologic outcomes was found in the prognostic analysis.
Although the present study was a retrospective, single-center study, our results suggest the possibility of using the laparoscopic approach for early rectal cancer. Randomized clinical trials are still required, however. A randomized trial for early rectal cancer is currently underway in Japan [22].
Conclusions
Our results show that the long-term outcomes of laparoscopic surgery are comparable to those of open surgery for stage I rectal cancer. Also, laparoscopic surgery for stage I rectal cancer is feasible, is safe, and has short-term benefits compared with open surgery. Further randomized trials are needed to evaluate the outcomes of laparoscopic surgery for rectal cancer, building on the results of the present study.
References
Luglio G, Nelson H (2010) Laparoscopy for colon cancer: state of the art. Surg Oncol Clin N Am 19:777–791
Lacy AM, Garcia-Valdecasas JC, Delgado S et al (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359:2224–2229
Nelson H, Group CS (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Buunen M, Veldkamp R, Hop WC et al (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10:44–52
Jayne DG, Guillou PJ, Thorpe H et al (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC trial group. J Clin Oncol 25:3061–3068
Nandakumar G, Fleshman JW (2010) Laparoscopy for rectal cancer. Surg Oncol Clin N Am 19:793–802
Guillou PJ, Quirke P, Thorpe H et al (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Kang SB, Park JW, Jeong SY et al (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645
Leung KL, Kwok SP, Lam SC et al (2004) Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 363:1187–1192
Lujan J, Valero G, Hernandez Q et al (2009) Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg 96:982–989
Ng SS, Leung KL, Lee JF et al (2008) Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol 15:2418–2425
Laurent C, Paumet T, Leblanc F, Denost Q, Rullier E (2012) Intersphincteric resection for low rectal cancer: laparoscopic vs open surgery approach. Colorectal Dis 14:35–41
Lam HD, Stefano M, Tran-Ba T et al (2011) Laparoscopic versus open techniques in rectal cancer surgery: a retrospective analysis of 121 sphincter-saving procedures in a single institution. Surg Endosc 25:454–462
Green FL, Balch CM, Fleming ID et al (2002) AJCC cancer staging manual: TNM classification of malignant tumors, 6th edn. Springer, New York
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Zhou ZG, Hu M, Li Y et al (2004) Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 18:1211–1215
Breukink S, Pierie J, Wiggers T (2006) Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev (4):CD005200
Ceelen WP (2007) Use of laparoscopy for rectal cancer: a word of caution. J Clin Oncol 25:5040
Laurent C, Leblanc F, Wutrich P et al (2009) Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg 250:54–61
Buunen M, Bonjer HJ, Hop WC et al (2009) COLOR II: a randomized clinical trial comparing laparoscopic and open surgery for rectal cancer. Dan Med Bull 56:89–91
Fleshman J (2010) American College of Surgeons Oncology Group (ACOSOG)- Z6051: a Phase III prospective randomized trial comparing laparoscopic-assisted resection versus open resection for rectal cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT00726622. Accessed 12 Nov 2010
Yamamoto S, Yoshimura K, Konishi F et al (2008) Phase II trial to evaluate laparoscopic surgery for stage 0/I rectal carcinoma. Jpn J Clin Oncol 38:497–500
Acknowledgment
This study was supported by a grant from the National Cancer Center of Korea (grant no. 0910200).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.D., Park, S.C., Park, J.W. et al. Laparoscopic Versus Open Surgery for Stage I Rectal Cancer: Long-term Oncologic Outcomes. World J Surg 37, 646–651 (2013). https://doi.org/10.1007/s00268-012-1846-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1846-z