Abstract
Background
Microsatellite instability (MSI) is one of the leading mechanisms for the carcinogenesis of gastric cancer. Its prognostic value is controversial.
Methods
Between May 1988 and Oct 2003, a total of 214 gastric cancer patients undergoing curative surgery were enrolled, and their MSI statuses were classified as MSI-H (high) or MSI-L/S (low/stable). The clinicopathologic characteristics of MSI-H and MSI-L/S gastric cancers were compared.
Results
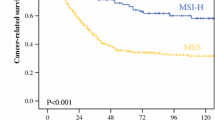
The MSI-H tumors accounted for 11.7 % (n = 25) of the 214 total gastric cancers. Although not statistically significant, the MSI-H gastric cancers were more frequently located in the lower third of the stomach (64 % vs. 49.2 %) and were more often the intestinal type (72 % vs. 61.4 %) compared to the MSI-L/S gastric cancers. The MSI-H gastric cancers had a significantly better 5-year overall survival (OS) rate (68 % vs. 47.6 %, p = 0.030) and a trend of a better 3-year disease-free survival rate (71.8 % vs. 55.2 %, p = 0.076) compared to the MSI-L/S gastric cancers. A multivariate analysis revealed that pathologic TNM stage and MSI status were the independent prognostic factors for OS after curative surgery.
Conclusions
Compared to MSI-L/S tumors, MSI-H tumors are associated with a better OS rate for gastric cancer patients after R0 resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the worldwide incidence of gastric cancer has been declining, it remains the fourth most common cancer and the second most common cause of cancer deaths [1]. Surgical resection and lymphadenectomy are the mainstays for curing gastric cancer, and the tumor stage provides an important prognostic prediction. Similar to colorectal cancer, there are two major pathways involved in the pathogenesis of gastric cancer: the chromosomal instability pathway and the microsatellite instability (MSI) pathway [2–5]. Somatic alterations demonstrate a DNA mismatch-repair (MMR) deficiency. Microsatellites are DNA regions containing short tandem repeats of one to six nucleotide motifs that frequently occur in the human genome. MSI is a common feature of gastric cancers and reflects an underlying MMR deficiency in the tumor, which is most commonly caused by the MMR genes (hMLH1, hMSH2). Alternatively, MSI is caused by the inactivation of hMLH1 (but not hMSH2) via epigenetic promoter methylation. The incidence of MSI in gastric cancer has been reported to be 8 % to 25 % [6–12].
The phenotype of MSI stomach cancer is associated with the following characteristics: (1) it is almost always of the intestinal, rather than the diffuse Lauren’s type of stomach cancer, which is much more frequently located in the distal (antrum) regions of the stomach instead of the proximal (body and cardia) regions; (2) it tends to be large and expansive; (3) it seldom gives rise to lymph node metastases [3, 13–18].
With respect to gastric cancer, some studies have reported that MSI is associated with a better survival [7, 8, 19–22], and other studies have found no significant differences between MSI and MSS [12, 14, 20, 23, 24]. Whether MSI can serve as a useful biomarker for survival prediction in gastric cancer is still unknown.
The aim of this study was to investigate the incidence of MSI-H tumors in Taiwanese gastric cancer patients and to clarify their clinicopathologic features and prognostic value.
Materials and methods
Between May 1988 and Oct 2003, gastric cancer patients who underwent curative resection were enrolled in this study. The institutional review board at the Taipei Veterans General Hospital approved this study, and written informed consent for tissue collection was obtained from all of the patients. Any patients with a history of gastric surgery or a pathologic diagnosis other than adenocarcinoma were excluded from the study. A total of 214 gastric cancer patients were enrolled in the study.
The pathologic staging of the gastric cancer was assessed after the surgery according to the seventh American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) TNM classification [25]. Specialized gastric cancer surgeons performed all of the operations. The data were prospectively collected and recorded in a computer, and the patients’ follow-up conditions were regularly updated.
Before surgery, all of the patients underwent chest radiography, abdominal sonography, or computer tomography (CT) for tumor staging. The patients were evaluated on the basis of their sex, age, tumor size, tumor location, operative methods, combined organ resection, pathologic tumor and lymph node stage, lymphovascular invasion, stromal reaction type, gross appearance, and recurrence pattern. For the stromal reactions, a cancer with a small number of stromal cells was classified as medullary, and a cancer with a large number of stromal cells was classified as scirrhous. An intermediate cancer was histologically intermediate (between the medullary and scirrhous types). The gross appearance of a tumor was classified as well defined (superficial or Bormann types I and II) or ill-defined (Bormann types III and IV).
A total or distal subtotal gastrectomy was performed depending on the distance between the cardia and the tumor. A margin of 3 cm was needed for superficial and well-defined tumors; a margin of 5 cm was needed for advanced or poorly defined tumors. A subtotal gastrectomy is the standard procedure for distal gastric cancer, whereas a total gastrectomy is the more common procedure for proximal gastric cancer. For early gastric cancer, at minimum a D1 + α dissection was performed. For advanced gastric cancer patients, the minimum of a D2 lymph node dissection was performed, except in those for whom curative resection was not possible. For D2 resection, combined-organ (i.e., en bloc) resection—including hemipancreaticosplenectomy, splenectomy alone, partial liver resection, and transverse colectomy—was sometimes performed to facilitate curative resection.
Follow-up
Overall survival was calculated from the time of surgery until death or the last follow-up contact. None of the patients received preoperative chemotherapy. Before 2008, adjuvant chemotherapy or radiotherapy after curative surgery was not routinely performed. Rather, it was performed only when tumor recurrence was diagnosed or highly suspected. Since 2008, adjuvant therapy (e.g., TS-1) was prescribed for stage II or stage III patients in our hospital after curative surgery because of its proven survival benefit [26].
Follow-up assessments were performed every 3 months for the first 5 years after surgery and every 6 months thereafter until the patient’s death. The follow-up procedures included medical histories, physical examinations, routine blood tests, liver function tests, measurements of the patients’ tumor marker levels—carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9—chest radiographs, and other imaging studies. All routine procedures were performed by a surgeon; the upper endoscopies were performed by a gastroenterologist; and the upper gastrointestinal series, abdominal sonography, and CT scans were performed by a radiologist.
Biopsy sampling confirmed recurrent disease or distant metastases. We did not perform biopsies of new, multiple pulmonary lesions or lesions characteristic of osseous metastases that were noted during CT or whole-body bone scans. A tumor recurrence in the hepatoduodenal ligament, celiac axis, or peripancreatic region was considered a locoregional recurrence. We defined remote lymphatic metastasis (paraaortic, Virchow’s, and inguinal nodes) and pulmonary lymphangitic spread as distant lymphatic recurrences. Using metallic staples at both the proximal and distal cut ends, we were able to readily identify the anastomotic sites and therefore could diagnose a recurrence at the anastomotic sites or duodenal stumps. Recurrence was classified as locoregional, hematogenous, distant lymphatic, or peritoneal. Patients who had recurrence of gastric cancer after surgery underwent chemotherapy with cisplatin 20 mg/m2, fluorouracil 450 mg/m2, and leucovorin 90 mg/m2, given simultaneously in 0.5 liter of normal saline. It is infused intravenously over 96 h every 21 days. This treatment was delayed or modified according to the protocol if the patient displayed toxic effects.
Microsatellite instability analysis
Normal and tumor DNA was extracted from the formalin-fixed, paraffin-embedded (FFPE) tissues or fresh frozen tissues stored at −80 °C or in liquid nitrogen. After DNA was purified using the QIAamp Tissue Kit (Qiagen GmbH, Hilden, Germany), the quantitative DNA analysis was performed by measuring the optical density (OD) at a wavelength of 260 nm and 280 nm. The DNA quality was confirmed by the OD ratio of 260/280.
The purified DNA was amplified using the fluorescent polymerase chain reaction (PCR). According to the international criteria for the determination of MSI [27], five reference microsatellite markers were used: D5S345, D2S123, BAT25, BAT26, D17S250. The primer sequences were obtained from GenBank (www.gdb.org). The PCR products were denatured and analyzed via electrophoresis on 5 % denaturing polyacrylamide gels, and the results were analyzed using an ABI 3730 automated sequencer (Applied Biosystems, Foster City, CA, USA). The presence of novel alleles that were observed in the PCR products from the tumor DNA but not observed in PCR products from the corresponding normal DNA were scored as MSI at that particular locus, as reported in a previous study [28]. Any samples with two or more loci of instability of five markers were classified as MSI-H, and any samples with one MSI or no evidence for MSI were classified as MSI-L/S. The analysis was repeated if the results were equivocal.
Statistical analysis
The statistical analyses were performed using SPSS software (version 16.0 for Windows; SPSS, Chicago, IL, USA). All of the results in the text and tables are presented as means ± standard deviations (SD). The categoric data were compared using a χ2 test with a Yates correction or Fisher’s exact test. Multivariate logistic regression analysis was conducted to determine the differences between the two groups. The overall survival (OS) was measured from the operation date to the date of death or the final follow-up. The disease-free survival (DFS) was defined as the length of time after surgery for gastric cancer during which a patient survives without tumor recurrence. The distributions of OS and DFS were estimated using the Kaplan–Meier method. Cox proportional hazards models were used to explore the association of clinical parameters with OS and DFS. A p value of <0.05 was considered statistically significant.
Results
The mean age of all of the gastric cancer patients was 66.8 ± 12.4 years. The mean follow-up duration was 25.5 months (range 1–243 months). Of the 214 patients, 152 (71 %) were men, and 62 (29 %) were women. Of all of the gastric cancers, 28 were located in the upper third of the stomach (13.1 %), 68 in the middle third of the stomach (31.8 %), 109 in the lower third of the stomach (50.9 %), and 9 in the entire stomach (4.2 %). The cancer stage distribution was as follows: 68 (24.4 %) stage I, 71 (25.4 %) stage II, and 140 (50.2 %) stage III. MSI-H and MSI-L/S gastric cancers accounted for 11.7 % (n = 25) and 88.3 % (n = 189) of the patients, respectively. The clinicopathologic characteristics of these patients are summarized in Table 1. The MSI-H gastric cancers were more frequently associated with lower-third gastric tumors (64 % vs. 49.2 %, p = 0.419); and they were more strongly associated with intestinal-type cancers than were the MSI-L/S gastric cancers (72 % vs. 61.4 %, p = 0.381). However, the clinicopathologic characteristics were not significantly different between MSI-H and MSI-L/S tumors.
Disease-free survival
The MSI phenotype was not associated with DFS at each stage in our gastric cancer patients. However, MSI-H tumors were associated with a trend of a better 3-year DFS compared to the MSI-L/S tumors in all patients who underwent curative resection (71.8 % vs. 55.2 %, p = 0.076).
We used Kaplan–Meier survival analysis to assess six prognostic factors: age, sex, tumor size, Lauren’s classification, pathologic TNM stage, and MSI status. Univariate analysis showed that the tumor size and pathologic TNM stage were significantly associated with DFS (Table 2). The two variables were included in a multivariate Cox proportional hazards model with forward logistics regression stepwise procedure to adjust for the effects of covariates (Table 3). In this model, we demonstrated that only pathologic TNM stage was an independent prognostic factor for DFS.
Overall survival
There is a trend of better 5-year OS rates for MSI-H tumors than for MSI-L/S tumors at each TNM stage. We found that although all patients were enrolled the 5-year OS rate was significantly higher for patients with MSI-H tumors than for those with MSI-L/S tumors (68 % vs. 47.6 %, p = 0.030).
We used Kaplan–Meier survival analysis to assess six prognostic factors: age, sex, tumor size, Lauren’s classification, pathologic TNM stage, and MSI status. Univariate analysis showed that the following five factors were associated with overall survival: age, sex, tumor size, pathologic TNM stage, and MSI status (Table 2). All five variables were included in a multivariate Cox proportional hazards model using the forward logistics regression stepwise procedure to adjust for the effects of covariates (Table 3). In that model, we demonstrated that pathologic TNM stage and MSI status were independent prognostic factors for OS.
Discussion
To our knowledge, this hospital-based study investigating the MSI status of gastric cancer patients after curative surgery is one of the largest series from a single institution. Our results showed that MSI-H tumors account for 11.7 % of gastric cancers. The reported incidence of MSI in gastric cancer varies by 8 % to 25 % [6–12]. The range of the incidence is primarily due to selection bias and varying definitions of MSI-H (i.e., the choice of microsatellite markers and the threshold for MSI-H tumors). As shown in Table 4, the markers for MSI testing ranged from 2 [7] to 11 [21] mononucleotide microsatellite markers. In an attempt to overcome this confusion, the National Cancer Institute proposed a panel of five microsatellite markers for the uniform analysis of MSI [28]. This panel included three dinucleotide (D5S346, D2S123, D17S250) and two mononucleotide (BAT26, BAT25) repeats.
Similar to previous reports [3, 13–18] this study demonstrated that MSI-H tumors are frequently associated with a distal stomach tumor location and an intestinal Lauren’s type of stomach cancer.
To clarify the role of MSI in gastric cancer, studies that were published through August 31, 2011 were searched and identified using the electronic PubMed database (http://www.pubmed.com). The key words included “gastric cancer,” “prognosis,” and “MSI.” The characteristics of the eligible studies are summarized in Table 4. In the literature review, for which 10 studies were included, the present study and two other studies [7, 19] performed multivariate analyses of OS; they all revealed a significant role for MSI, resulting in better survival rates. Only An et al. [12] and Perez et al. [24] analyzed DFS in gastric cancer with R0 resection. Their results indicated no significant differences between the MSI-H and MSI-L/S tumors in terms of DFS. Interestingly, although not statistically significant, our data showed a trend of better OS rates for patients with MSI-H tumors than for those with MSI-L/S tumors at each stage. However, the difference in OS between MSI-H and MSI-L/S gastric cancer was significant only when all patients were enrolled. Furthermore, our study showed a trend of better DFS for patients with MSI-H tumors than for those with MSI-L/S tumors after curative resection. This may be explained by the small number of MSI-H tumors in our series, which did not provide sufficient statistical power to achieve a statistically significant difference. The chief limitation of this study is that few MSI-H gastric cancer cases were included, which rendered the subgroup analyses difficult. A larger sample size may be needed to clarify the actual role of MSI-H in DFS in gastric cancer patients.
MSI-H colon cancer is frequently located in the right-sided colon and is associated with a mucinous histology [29, 30]. The inactivating mutations in MSI-H colon cancers cluster to similar hypermutable repetitive DNA sequences present in the coding regions of genes important to cell growth and cell survival, such as transforming growth factor-β receptor type II (TGFβ-RII), insulin-like growth factor receptor type II (IGF-RII), transcription factor E2F-4, Bcl-2 protein (BAX), caspase-5, and methyl-CpG binding domain protein 4 (MBD4) [31–36]. MSI-H gastric cancer is associated with antral tumor location and intestinal-type differentiation. Similar to colon cancer, some genes with simple, tandem repeat sequences within their coding regions have been found to be specifically altered in gastric cancers displaying MSI, including BAX, hMSH3, hMSH6, E2F-4, TGFβ-RII, and IGF-RII, which are known to be involved in the regulation of cell-cycle progression and apoptotic signaling [37, 38]. Moreover, similar to colorectal cancer, the presence of methylation of the hMLH1 gene is strongly associated with loss of hMLH1 protein expression and the MSI-H phenotype in gastric cancer [39–41]. Hypermethylation of CpG islands in the promoter region of the hMLH1 gene is associated with decreased hMLH1 protein expression and often occurs in MSI-H gastric cancer, indicating that epigenetic inactivation of this gene in association with promoter methylation may underlie MSI [40, 41]. Consequently, we will make an effort to investigate the molecular profile of MSI-H gastric cancer in our future work. The limitation of this study is that the biological markers are not validated internally with a validation set. External validation is also needed.
An et al. [12] reported that patients with MSS/MSI-L gastric cancers obtained potential DFS benefits from 5-fluorouracil (5-FU)-based adjuvant chemotherapy. By contrast, 5-FU-based adjuvant chemotherapy did not provide DFS benefits to stage II and III gastric cancers with MSI-H. Because adjuvant chemotherapy (e.g., TS-1) was initiated in our hospital only in 2008, our patients did not receive routine adjuvant chemotherapy before 2008. We could not compare the effect of chemotherapy on MSI-H versus MSI-L/S patients in this study.
Conclusions
Based on our hospital-based study, MSI-H gastric cancers were associated with significantly better OS and a trend of better DFS compared to MSI-L/S gastric cancers after curative surgery. A multivariate analysis revealed that sex, pathologic TNM stage, and MSI status are independent prognostic factors for OS.
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Ottini L, Falchetti M, Lupi R et al (2006) Patterns of genomic instability in gastric cancer: clinical implications and perspectives. Ann Oncol 17:vii97–vii102
Oliveira C, Seruca R, Seixas M et al (1998) The clinicopathological features of gastric carcinomas with microsatellite instability may be mediated by mutations of different “target genes”: a study of the TGFbeta RII, IGFII R, and BAX genes. Am J Pathol 153:1211–1219
Gu M, Kim D, Bae Y et al (2009) Analysis of microsatellite instability, protein expression and methylation status of hMLH1 and hMSH2 genes in gastric carcinomas. Hepatogastroenterology 56:899–904
Pedrazzani C, Corso G, Velho S et al (2009) Evidence of tumor microsatellite instability in gastric cancer with familial aggregation. Fam Cancer 8:215–220
Lee HS, Choi SI, Lee HK et al (2002) Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol 15:632–640
Beghelli S, de Manzoni G, Barbi S et al (2006) Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery 139:347–356
Corso G, Pedrazzani C, Marrelli D et al (2009) Correlation of microsatellite instability at multiple loci with long-term survival in advanced gastric carcinoma. Arch Surg 144:722–727
Oki E, Kakeji Y, Zhao Y et al (2009) Chemosensitivity and survival in gastric cancer patients with microsatellite instability. Ann Surg Oncol 16:2510–2515
Seo HM, Chang YS, Joo SH et al (2009) Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol 99:143–147
Vauhkonen M, Vauhkonen H, Sajantila A et al (2005) Differences in genomic instability between intestinal- and diffuse-type gastric cancer. Gastric Cancer 8:238–244
An JY, Kim H, Cheong JH, et al. (2011) Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. doi: 10.1002/ijc.26399. [Epub ahead of print]
Wu MS, Lee CW, Shun CT et al (2000) Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer 27:403–411
Ottini L, Palli D, Falchetti M et al (1997) Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res 57:4523–4529
Dos Santos NR, Seruca R, Constancia M et al (1996) Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology 110:38–44
Chung YJ, Song JM, Lee JY et al (1996) Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res 56:4662–4665
Yamamoto H, Perez-Piteira J, Yoshida T et al (1999) Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology 116:1348–1357
Buonsanti G, Calistri D, Padovan L et al (1997) Microsatellite instability in intestinal- and diffuse-type gastric carcinoma. J Pathol 182:167–173
De Manzoni G, Tomezzoli A, Di Leo A et al (2001) Clinical significance of mutator phenotype and chromosome 17p and 18q allelic loss in gastric cancer. Br J Surg 88:419–425
Gazvoda B, Juvan R, Zupanic-Pajnic I et al (2007) Genetic changes in Slovenian patients with gastric adenocarcinoma evaluated in terms of microsatellite DNA. Eur J Gastroenterol Hepatol 19:1082–1089
Hayden JD, Cawkwell L, Quirke P et al (1997) Prognostic significance of microsatellite instability in patients with gastric carcinoma. Eur J Cancer 33:2342–2346
Schneider BG, Bravo JC, Roa JC et al (2000) Microsatellite instability, prognosis and metastasis in gastric cancers from a low-risk population. Int J Cancer 89:444–452
An C, Choi IS, Yao JC et al (2005) Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res 11:656–663
Perez RO, Jacob CE, D’Ottaviano FL et al (2004) Microsatellite instability in solitary and sporadic gastric cancer. Rev Hosp Clin Fac Med Sao Paulo 59:279–285
Sobin L, Gospodarowicz M, Wittekind C (eds) (2009) TNM Classification of Malignant Tumours, 7th edn. International Union Against Cancer (UICC), Wiley, New York
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) ACTS-GC Group: adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Boland CR, Thibodeau SN, Hamilton SR et al (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257
Chang SC, Lin JK, Yang SH et al (2006) Relationship between genetic alterations and prognosis in sporadic colorectal cancer. Int J Cancer 118:1721–1727
Raut CP, Pawlik TM, Rodriguez-Bigas MA (2004) Clinicopathologic features in colorectal cancer patients with microsatellite instability. Mutat Res 568:275–282
Söreide K, Janssen EA, Söiland H et al (2006) Microsatellite instability in colorectal cancer. Br J Surg 93:395–406
Markowitz SD, Wang J, Myeroff L et al (1995) Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268:1336–1338
Souza RE, Appel R, Yin J et al (1996) Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nature Genet 14:255–257
Souza RF, Yin J, Smolinski KN et al (1997) Frequent mutation of the E2F-4 cell cycle gene in primary human gastrointestinal tumors. Cancer Res 57:2350–2353
Rampino N, Yamamoto H, Ionov Y et al (1997) Somatic mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275:967–969
Schwartz S Jr, Yamamoto H, Navarro M et al (1999) Frameshift mutations at mononucleotide repeats in caspase-5 and other target genes in endometrial and gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res 59:2995–3002
Riccio A, Aaltonen LA, Godwin AK et al (1999) The DNA repair gene MBD4 (MED1) is mutated in human carcinomas with microsatellite instability. Nat Genet 23:266–268
Fiocca R, Luinetti O, Villani L et al (2001) Molecular mechanisms involved in the pathogenesis of gastric carcinoma: interactions between genetic alterations, cellular phenotype and cancer histotype. Hepatogastroenterology 48:1523–1530
El-Rifai W, Powell SM (2002) Molecular biology of gastric cancer. Semin Radiat Oncol 12:128–140
Toyota M, Ahuja N, Suzuki H et al (1999) Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res 59:5438–5442
Lee JH, Park SJ, Abraham SC et al (2004) Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene 23:4646–4654
Leung WK, Yu J, Ng EK et al (2001) Concurrent hypermethylation of multiple tumor-related genes in gastric carcinoma and adjacent normal tissues. Cancer 91:2294–2301
Acknowledgments
This research was supported by the Center of Excellence for Cancer Research at Taipei Veterans General Hospital (DOH101-TD-C-111-007) and funding from the National Science Council (99-2314-B-075-009).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, WL., Chang, SC., Lan, YT. et al. Microsatellite Instability Is Associated With a Better Prognosis for Gastric Cancer Patients After Curative Surgery. World J Surg 36, 2131–2138 (2012). https://doi.org/10.1007/s00268-012-1652-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1652-7