Abstract
Background
This study evaluated the impact of tumor regression grading (TRG) and other pathologic variates in a cohort of rectal carcinoma patients treated with neoadjuvant chemoradiotherapy (CRT). The value of a grading less than pCR for predicting survival is unknown. Tumor budding has not been systematically studied in rectal cancer after neoadjuvant therapy.
Methods
Pathologic risk factors for survival were evaluated on surgical specimens of 237 patients with stages I, II, and III rectal cancer treated between 1996 and 2006. All patients underwent preoperative CRT followed by surgical resection 6–8 weeks later. TRG, tumor grade, budding, venous invasion, radial margin, and nodal status were evaluated. The prognostic value of TRG categories was calculated with Cox regression models and validated with resampling methods.
Results
TRG of <25% occurred in 61 (25.7%) and a complete response in 39 (16.4%) of the resected specimens. TRG of <25% was shown to be a statistically significant predictor for cancer-specific survival (CSS) and recurrence-free survival (RFS) compared to TRG ≥25% (P = 0.013). Tumor budding was present in 24 (10.1%) of the patients and was negatively associated with CSS (P = 0.013). Lymph node involvement was observed in 83 (35.0%) patients. TRG and nodal status (P < 0.001) were the most significant predictors associated with outcome.
Conclusion
Partial pathologic response ≥25% was a superior predictor compared to pCR for improved survival after preoperative CRT. CSS and RFS were adversely affected by the presence of lymph node metastases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pathologic response to radiotherapy and chemotherapy given prior to surgical resection can be assessed by grading histologic changes in the resected specimens. The recently published 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual [1] recommends recording the tumor regression grade (TRG). Pathologic complete response (pCR), which means a specimen without viable tumor cells, varies from 8% to 30% [2–4]. pCR has been associated with improved recurrence-free survival (RFS), and factors predicting pCR have been evaluated [5–7]. Most patients do not achieve pCR. TRG has been proposed as an alternate assessment and has been shown to be associated with RFS [2, 8, 9]. Pathologic features such as the radial margin, venous invasion, lymph node involvement, and tumor stage have been shown to be associated with disease recurrence and/or survival [2, 7, 8] Other pathologic features, including the presence of acellular mucin in patients with pCR [7], tumor budding [9, 10], and the impact of TRG [8, 11–14] have either limited or no data in this setting. These features were specifically identified and studied in this endeavor with unique analysis as it relates to CSS and RFS in a continuous cohort of rectal carcinoma patients treated with CRT followed by curative resection and adjuvant chemotherapy.
The aims of this study were to assess the predictive value of pathologic features such as budding and TRG for rectal cancer resected after neoadjuvant therapy and to define the methods for assessing those features. The predictive findings discussed here could be used in a postsurgical treatment algorithm for patients with rectal cancer.
Methods
Patients
The medical records of all patients undergoing curative resection—abdominal perineal resection (APR), anterior resection (AR), low anterior resection (LAR)—for stages I, II, and III rectal cancer at the Mayo Clinic, Rochester during the years 1996–2006 were reviewed. All stage I patients were treated with radiation therapy at outside institutions but had their surgery performed at the Mayo Clinic. Because of the lack of sensitivity of preoperative endoscopic ultrasonography (EUS) staging, an “analytical stage” was determined from either the clinical or pathologic stage, whichever was worse. The operative techniques have been described in previous work from our institution [15]. Institutional review board approval was obtained to review records, confirm records, and report results.
Patients with neoadjuvant chemoradiotherapy (CRT) were selected for this study. Preoperative therapy consisted of a combination of irradiation and 5-fluorouracil (5-FU) chemotherapy. At 6–8 weeks after completion of the preoperative CRT, all patients underwent curative resection. Postoperative 5-FU chemotherapy was started 4 weeks after surgery. Of the 276 patients, 39 were removed before analysis. Among them, 26 had stage IV disease, 2 had missing ypT stage, and 11 had inconsistent tumor regression grading compared to ypT stages. The primary outcomes were cancer-specific survival (CSS) and RFS. CSS was defined as the time from operation to death due to rectal cancer. RFS was defined as the time from operation to the first event of either recurrence or death due to rectal cancer.
Pathologic findings
At surgery, the resected specimen was dissected and sampled using a standard pathology technique. The subsequent pathology report made note of the presence of residual tumor and documented its size, grade, and depth of invasion. Angiolymphatic invasion, if present, was recorded. The radial margin was assessed. All regional lymph nodes were sampled and reviewed. For the current study, all glass slides prepared at the time of surgery were recalled and assessed by a single gastrointestinal pathologist (T.C.S.). Pathology parameters reported here were scored in real time by one or more pathologists and were re-reviewed by T.C.S. Disagreements were rare, but when there were disagreements we considered T.C.S. the expert reviewer and used his result. In addition to reviewing the previously documented parameters, the pathologist assessed tumor budding, tumor regression, and the presence of acellular mucin. A tumor bud is a group of five or fewer tumor cells at the tumor edge. Tumor buds were counted at × 200 magnification using a routine hematoxylin and eosin (H&E) stain. Budding was scored as negative if no field had more than 9 buds and positive if any field had 10 or more buds [16]. Tumor regression of the primary tumor was determined by the amount of viable tumor versus the amount of fibrosis, ranging from grade 0 (no regression) to grade 4 (complete regression), as described by Dworak et al. [17].
Follow-up
Patients with stage II and III disease were followed up every 3 months for 2 years and then every 6 months for the next 3 years. Physical examination, history taking, and computed tomography (CT) of the chest, abdomen, and pelvis were performed every 6 months; the carcinoembryonic antigen (CEA) level was recorded every 3 months. Colonoscopy was performed 1 year after surgery and every 3 years thereafter.
Statistical methods
The χ2 trend test for ordered categories was used for factors with more than two ordered categories. Kaplan–Meier and Cox proportional hazard models were used to estimate the association of pathologic variables—TRG, tumor budding, venous invasion, presence of acellular mucin, residual tumor, pathologic T stage, number of positive nodes, grade, and clinical stage—with CSS and RFS. The magnitudes of the association of each preoperative and pathologic feature with CSS and RFS were evaluated using univariate Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% confidence intervals (CIs). The features that were significantly associated with each outcome in a multivariable setting were determined using stepwise Cox proportional hazards best subsets regression algorithm. Clinical and pathologic features were used in the selection procedure if they attained P < 0.1 in univariate associations. Patients were grouped into TRG classes 0 + 1 (<25% response), TRG 2 + 3 (25% to >50% response), and pCR (complete regression) [8].
To test the pathologic response as a prognostic measure for CSS, the concordance index 18 was used to select optimal change point values to classify patients into low-risk or high-risk groups. The concordance index (or C-statistic) is a measure of model discrimination in survival analyses. A completely random prediction would have a concordance of 0.5, and a perfect rule has concordance 1. Potential change point values for TRG were defined as the value of 0 or 1; 2 or 3; 4 (=complete) leading to a maximum concordance index in univariate Cox models. To estimate the robustness of the results, we used 1000 bootstrap samples [18], which applies the Cox models to random samples with replacements of the patients. All statistical tests were two-sided, and a level of 0.05 was considered statistically significant. All data analyses were performed using R version 2.10.1 software [19].
Results
All 237 patients underwent preoperative CRT, and 233 (98.3%) were treated with postoperative chemotherapy. The mean age was 60.0 (SD 12.5) years. There were 160 (67.5%) men and 77 (32.5%) women. The number of patients in each stage were 8 (3.4%) with stage I, 64 (27.0%) with stage II, 165 (69.6%) with stage III. The distribution of pathologic stages (yp) was 35 (16.5%) with stage 0, 21 (8.9%) with stage I, 79 (33.3%) with stage II, and 102 (43%) with stage III. Median follow-up time was 3.5 years, with 25th and 75th percentiles of 2.1 and 4.8 years.
Pathologic findings
The preoperative T stage (uT) was stage 1 in 4 patients (1.8%), stage 2 in 29 (12.9%), stage 3 in 177 (79.0%), stage 4 (bad) in 14 (6.3%). There were 86 patients (38.4%) preoperatively identified as node-negative, and 138 (61.6%) as node-positive. The pathologic T stage (ypT) was 0 in 39 patients (16.5%), stage 1 in 14 (5.9%), stage 2 in 25 (10.5%), stage 3 in 144 (60.8%), and stage 4 in 15 (6.3%). The differences between preoperative and postoperative staging were as follows. A lower pathological stage compared to the US stage occurred in 17 patients (7.5%), whereas 79 patients (34.8%) had a lower pathologic stage.
Retrospective review by the expert gastrointestinal pathologist found some disagreement with the original report. Venous invasion had been missed in 5 cases; the expert reviewer changed the grade of 8 tumors from high to low, and 12 tumors were changed from low to high grade. Where tumor response was commented on in the original report, the reviewer did not have any disagreements with the original. Acellular mucin was seen in 15.4% of those without residual tumor. Tumor budding was seen in 24 patients (10.1%). The radial margin was reported as negative in all 237 patients. Eight patients had a radial margin of 1–2 mm, 96 patients had a radial margin >2 mm, and the remaining 133 patients were recorded as negative. The median number of lymph nodes examined was 11 with 25th and 75th percentiles of 7 and 15 (range 0–50). In all, 83 patients (45%) had metastatic disease in lymph nodes at the time of operation; 56 were ypN1, and 27 were ypN2. TRG 0 (no response) was observed in 7.2% of the patients, and TRG 4 (pCR) was found in 16.4% (Table 1).
The association of TRG with clinical and postoperative pathologic factors is outlined in Table 2. No association with TRG groups was found for age, sex, or stage. There was no difference in the surgical approach (APR or AR/LAR) for patients in the various TRG categories. However, there were more lymph nodes involved by metastatic disease for TRG 0 + 1 groups than for subjects with a better pathologic response (P < 0.001). The proportion of subjects with positive lymph nodes was 44.3% for TRG 0 + 1, 38.7% for TRG 2 + 3, and 7.7% for cPR. Tumor budding was significantly more common in tumors with less regression, being seen in 23% of tumors with TRG 0 + 1 compared to 7.3% of those with TRG 2 + 3 (7.3%) (P < 0.001).
Recurrence and cancer-specific survival
Of the 237 patients, 6 (2.5%) had local recurrence and 43 (18.1%) had distant recurrence. The median time from surgery to first recurrence was 38 months (25th–75th percentiles 20.7–54.8 months). Association of clinicopathologic features with CSS and RFS are summarized in Tables 3 and 4, respectively. Age, sex, pathologic T stage (ypT), and venous invasion were not significantly associated with CSS and RFS. A higher number of positive nodes was detrimental to CSS and RFS with an HR of 1.26 (95% CI 1.13–1.41), P < 0.001 for CSS (Fig. 1) and 1.18 (95% CI 1.09–1.28), P < 0.001 for RFS.
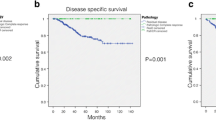
The optimal change point for pathologic response to identify groups of patients with respect to risk for survival was <25% (TRG 0 + 1: high risk) and ≥25% (TRG 2, 3, 4: low risk). Figure 2 shows the distribution of the TRG cutoff points that resulted in the largest concordance index in Cox models based on 1000 bootstrap iterations. The histogram indicates the number of times any given cutoff point was selected. In most iterations a pathologic response of <25% (TRG 0 + 1) versus ≥25% (TRG 2, 3, 4) was the most discriminating value for both CSS and RFS. The corresponding survival curves are shown in Fig. 3. Comparing survival curves of TRG 0 + 1, TRG 2 + 3 with TRG 4 (Fig. 4), the 95% CI of the hazard ratio are overlapping for TRG 2 + 3 and TRG 4. Thus, these classes could not be well distinguished. A lower response rate (TRG ≤ 25%) negatively affected survival with a hazard ratio of 2.73 (95% CI 1.10–6.77), P = 0.013 for CSS and 2.06 (95% CI 1.08–3.92), P = 0.014 for RFS. In the multivariate model selection algorithm, only positive lymph node and TRG categories were selected for the final model, and both were statistically significantly associated with CSS and RFS. The C-indices for the Cox models with nodal status, two TRG classes, and for the multivariable model combining TRG classes and nodal status were 0.70, 0.63, and 0.73, respectively. Tumor budding was negatively associated with CSS in univariate models with HR 2.56 (95% CI 1.30–9.75), P = 0.014 (Fig. 5).
Discussion
This study shows that tumor regression grading and nodal status are independent predictors for CSS and RFS based on analysis of 237 patients with stages I, II, and III rectal cancer treated with preoperative CRT and adjuvant chemotherapy. Tumor budding was a significant predictor of CSS and RFS.
The use of TRG has been criticized because of poor reproducibility [20]. Studies have varied in terms of inclusion criteria, sample size, and TRG scale; and the effect of TRG has been either assessed for individual grades [2, 13] or subjectively combined in groups (no response or partial or complete response) [8, 11–14]. Beddy et al. [21] showed that a three-tiered system of TRG was a significant predictor of RFS in 126 patients with T3/T4, N1/N2 rectal cancer. A five-point scale of TRG and pN status were associated with RFS for 144 patients with cT3,4 rectal cancer in Vecchio et al. [14]. In a series of 102 rectal cancer patients, Bouzourene et al. [11] found that a five-point scale of TRG was significantly associated with RFS in univariate and multivariable models. Evaluating pathologic factors, including ypN and TRG, to predict long-term outcomes, ypN was the most important prognostic factor in Kim et al. [2]. In patients with persistent disease (no pCR), Bujko et al. [22] found limited prognostic value for TRG in a series of 131 patients with cT4/cT4 rectal cancer. Rodel et al. [8] reported that TRG alone is not significant and that ypN remains the most important prognostic marker. Our findings support the idea that nodal status is the strongest predictor for survival outcomes in patients with rectal cancer after preoperative CRT and adjuvant therapy. In a subset of 198 patients with incomplete tumor regression, TRG was significantly associated with CSS (HR 0.35, 95% CI 0.14–0.87, P = 0.024) and RFS (HR 0.48, 95% CI 0.26–0.91, P = 0.025). TRG remained an independent predictor in multivariate models with all 237 patients. Prognostic factors were chosen by best subset selection and resulted in the combination of TRG and nodal status as significant predictors for CSS and RFS. Our findings highlight the critical importance of including the TRG in the model with nodal status as a improvement for the prediction of survival outcomes.
Although some studies have compared pCR with other levels of regression [6], or “no response versus “some response” [2], or TRG groups 0 + 1, 2 + 3, and pCR [8], the extent of tumor response that would be associated with improved CSS or RFS has not been elucidated. We identified that a partial tumor response of ≥25% significantly improved survival outcomes. This result was robust, as indicated by applying the Cox models to 1000 bootstrap samples.
The term “tumor budding” has been used in the surgical pathology literature since 1993 [23], but only in the last decade or so has it been generally accepted as a robust predictor of adverse outcome. The current recommendations for reporting surgically resected specimens of colorectal carcinoma advise reporting the presence or absence of tumor budding [24]. The authors of that report, however, made no recommendation regarding how to define tumor budding, and the literature is variable in that regard, with some authors proposing defined cut points [16], others reporting counts as a continuous variate [25], and others devising scoring systems [26]. The literature also varies with regard to the site studied (colon versus rectum), tumor stage, study endpoints (lymph node metastases, recurrence, survival), and use of adjuvant therapy [27]. To our knowledge, this is the first study to assess tumor budding in patients who underwent neoadjuvant therapy.
The variability in methods for assessing budding translates into some variability in its reported prevalence. Most studies find that 25% to 40% of colorectal carcinomas show budding, although some reports are as low as 15% or as high as 75%. We observed tumor budding in 10% of rectal cancers resected after neoadjuvant therapy, suggesting that such therapy reduces the prevalence of tumor budding. When budding persists, it is has an adverse effect on outcome; and we found that it was a significant predictor of survival.
A limitation of this study was that some pathologic variables (e.g., acellular mucin, venous invasion, distance to radial margin) were either not present in most of the patients or could not be consistently assessed. The study was retrospective, and a follow-up of <5 years may result in underestimating the disease recurrence. Retrospective review by the expert gastrointestinal pathologist did turn up disagreements with the original report, but as the review was taking place years after the original surgery there were no discussions with the original pathologist. Another limitation was that all stage I patients had received their preoperative radiotherapy elsewhere. However, excluding 13 patients (9 patients with preoperative T stage 1 or 2 and uN0 as well as 4 patients with missing uT stage and ypT stage 1 or 2) did not change the conclusions.
There are several strengths of this study. All patients were treated with preoperative CRT and almost all with postoperative chemotherapy. A number of pathological features were collected and recorded, and grades of tumor regression were examined instead of limiting the study to patients with a complete pathological response. This enabled the distinction of possible risk groups based on TRG. However, the results need to be validated in larger scale studies at other institutions.
Conclusions
This study evaluated the impact of various pathologic features on the prognosis of rectal cancer. We showed that ypN, TRG, and tumor budding were significant markers for CSS and RFS. In particular, the extent of tumor response, TRG ≥25%, was associated with improved CSS and RFS, making it a better predictor than a pathologic complete response. Nodal status and TRG classification were both selected for predictive multivariate models. These findings may be used in the postsurgical treatment algorithm for patients with rectal cancer.
References
Anonymous (2010) AJCC cancer staging manual. In: Edge SB, Byrd DR, Compton CC et al (eds) Colon and Rectum. Springer, New York
Kim TH, Chang HJ, Kim DY et al (2010) Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys 77:1158–1165
Roh MS, Colangelo LH, O’Connell MJ et al (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27:5124–5130
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Capirci C, Valentini V, Cionini L et al (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72:99–107
Kalady MF, de Campos-Lobato LF, Stocchi L et al (2009) Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 250:582–589
Smith KD, Tan D, Das P et al (2010) Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg 251:261–264
Rodel C, Martus P, Papadoupolos T et al (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23:8688–8696
Okuyama T, Oya M, Ishikawa H (2003) Budding as a useful prognostic marker in pT3 well- or moderately-differentiated rectal adenocarcinoma. J Surg Oncol 83:42–47
Ueno H, Price AB, Wilkinson KH et al (2004) A new prognostic staging system for rectal cancer. Ann Surg 240:832–839
Bouzourene H, Bosman FT, Seelentag W et al (2002) Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer 94:1121–1130
Suarez J, Vera R, Balen E et al (2008) Pathologic response assessed by Mandard grade is a better prognostic factor than down staging for disease-free survival after preoperative radiochemotherapy for advanced rectal cancer. Colorectal Dis 10:563–568
Losi L, Luppi G, Gavioli M et al (2006) Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis 21:645–651
Vecchio FM, Valentini V, Minsky BD et al (2005) The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 62:752–760
Zaheer S, Pemberton JH, Farouk R et al (1998) Surgical treatment of adenocarcinoma of the rectum. Ann Surg 227:800–811
Ueno H, Murphy J, Jass JR et al (2002) Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40:127–132
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12:19–23
Harrell F, Lee KL, Califf RM et al (2006) Regression modelling strategies for improved prognostic prediction. Stat Med 3:143–152
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Nagtegaal I, Marijnen C (2008) The future of TNM staging in rectal cancer: the era of neoadjuvant therapy. Curr Colorectal Cancer Rep 4:147–154
Beddy D, Hyland JM, Winter DC et al (2008) A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol 15:3471–3477
Bujko K, Kolodziejczyk M, Nasierowska-Guttmejer A et al (2010) Tumour regression grading in patients with residual rectal cancer after preoperative chemoradiation. Radiother Oncol 95:298–302
Hase K, Shatney C, Johnson D et al (1993) Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 36:627–635
Jass JR, O’Brien J, Riddell RH et al (2008) Recommendations for the reporting of surgically resected specimens of colorectal carcinoma: Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol 129:13–23
Masaki T, Matsuoka H, Sugiyama M et al (2006) Actual number of tumor budding as a new tool for the individualization of treatment of T1 colorectal carcinomas. J Gastroenterol Hepatol 21:1115–1121
Wang LM, Kevans D, Mulcahy H et al (2009) Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 33:134–141
Prall F (2007) Tumour budding in colorectal carcinoma. Histopathology 50:151–162
Conflicts of interest
The authors do not have actual or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huebner, M., Wolff, B.G., Smyrk, T.C. et al. Partial Pathologic Response and Nodal Status as Most Significant Prognostic Factors for Advanced Rectal Cancer Treated With Preoperative Chemoradiotherapy. World J Surg 36, 675–683 (2012). https://doi.org/10.1007/s00268-011-1409-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1409-8