Abstract
Background
Intraoperative detection of new nodules is common in patients undergoing hepatectomy for colorectal liver metastases, although the value of intraoperative diagnosis is not well assessed.
Methods
A prospectively collected and recorded database was retrospectively analyzed. Helical computed tomography (CT) results were correlated with those of the intraoperative diagnosis in 183 consecutive patients undergoing 254 consecutive hepatectomies, including repeated resection for colorectal liver metastases.
Results
In total, 270 nodules were newly detected during 65 hepatectomies. The sensitivity of CT to detect metastatic nodules was 72.8% (722/992), but it decreased to 34.6% (125/361) for small (≤1 cm diameter) tumors. Intraoperative visual inspection and/or palpation detected 207 of 270 nodules. Intraoperative ultrasonography (IOUS) played an important role in identifying deep (≥1 cm from the surface) and comparatively small (≤1 cm diameter) nodules (4/9 vs. 16/18, respectively, for those >1 cm vs. ≤1 cm diameter). The likelihood of intraoperative detection of new nodules increased from 10 in 112 to 6 in 9 when the preoperative tumor number increased from solitary to ≥10, resulting in an overall likelihood of 65 in 254 (25.6%). Of 65 patients with new nodules, 21 had at least one nodule that was detected only by IOUS. Preoperatively scheduled hepatectomy was altered in 47 (72%) patients, although additional limited resection(s) were sufficient to remove these nodules in 43 (91%) of them.
Conclusions
Visual inspection, palpation, and IOUS had equally indispensable roles in detecting new nodules during hepatectomy. Detection was common and usually necessitated alteration, albeit moderately, of the surgical plan.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than half of the patients with colorectal carcinoma develop liver metastatic disease [1, 2]. The liver is the most common and usually the first site of distant metastases [3]. Given that the primary disease is controlled and in the absence or with limited numbers of extrahepatic metastases, liver resection offers the only chance of cure for these patients. The reported 5-year survival after this surgery ranges from 25 to 50% [4–8].
The surgical strategy for hepatic colorectal metastases has changed drastically during the past two decades toward a more aggressive posture [9]. Specifically, the number of metastases is no longer considered a contraindication to surgery [10, 11]. Recent studies reported that the width of the surgical margin has no effect on survival so long as a microscopically negative margin is secured [12, 13]. Also, repeated liver resection for hepatic recurrence has become an accepted procedure that may result in long-term survival [10, 14, 15].
These innovations have inevitably led to more complex hepatic resections being carried out. Although a microscopically positive surgical margin (R1 resection) was reportedly still associated with survival benefit in the present era with more adjuvant therapy options, hepatectomy that leaves some of the tumor nodules unresected (i.e., resection that results in merely reduction surgery) is strongly contraindicated [16, 17]. Hence, accurate localization of all intrahepatic lesions is essential to plan the appropriate type of resection. Preoperative imagining techniques have improved largely during the corresponding period, including the development of helical and then multidetector row computed tomography (MDCT) and contrast-enhanced magnetic resonance imaging (MRI) [18–21]. Nevertheless, additional lesions are detected intraoperatively even after careful preoperative staging by these imaging methods.
The accuracy of preoperative imaging studies for colorectal liver metastases has not been well scrutinized in the current era of surgical strategy. Likewise, although intraoperative ultrasonography (IOUS) is thought to be the most accurate modality for detecting metastatic colorectal liver tumors [21–28], the contribution of classic maneuvers (i.e., visual inspection and bimanual palpation) to the intraoperative diagnosis has not been correctly assessed. Such methods should never be underestimated because tumors on the surface of the liver, which are easily detectable by inspection and/or palpation, are hardly identifiable by IOUS.
We undertook a retrospective cohort study investigating the details for 254 consecutive liver resections during 10 years to address three clinical questions: (1) We assessed the accuracy of preoperative imaging studies in patients undergoing liver resection for metastatic colorectal carcinoma, paying particular attention to those with multiple metastatic lesions. (2) We examined the relative contribution of visual inspection, bimanual palpation, and IOUS to the intraoperative diagnosis. (3) We evaluated the impact of intraoperative detection of new tumor nodules on the scheduled hepatectomy procedures.
Materials and methods
Patients
From January 1994 to December 2003, a total of 183 patients underwent 254 consecutive liver resections for the treatment of colorectal liver metastasis identified by preoperative imaging modalities at the University of Tokyo Hospital, Tokyo, Japan. They comprised the base population of this study. Their data were prospectively collected and recorded in a specific database and analyzed retrospectively. Table 1 shows the characteristic of these patients and primary tumor. Patients undergoing hepatectomy after 2004 for metastatic colorectal carcinoma participated in the prospective study evaluating the value of contrast-enhanced IOUS and were thus excluded from the present study. The ethics committee did not require its approval or informed consent for this retrospective study, which was compliant with Declaration of Helsinki principles.
Preoperative imaging studies
The preoperative diagnostic imaging for metastatic lesions included ultrasonography and contrast-enhanced helical CT to stage the liver and abdominal involvement, helical CT, and chest plain radiography to detect the presence of pulmonary metastases. In addition to contrast-enhanced helical CT, colonoscopy and/or barium enema radiographic examination were performed to rule out the local recurrence. During intravenous contrast-enhanced helical CT scan with 5 mm reconstruction interval, iopamidol 370 (2 ml/kg, maximum 100 ml) was injected at a rate of 2.5–3.0 ml/s with a power injector. Scan delay was set at 80–90 s, resulting in portovenous enhancement. MDCT was introduced in 2001, and all CT examinations were carried out using this equipment thereafter. All of these CT imaging studies were conducted in our institution within 1 month of hepatectomy. MRI, CT during arteriography/CT during portography, and positron emission tomography with 18F-fluorodeoxyglucose were not routinely carried out because of the logistic circumstances and limited access, and it was ordered only when decided necessary. Two independent radiologists (M.A. and another doctor) performed the examinations of all CT images in a blinded manner. Disagreement between readers was resolved by consensus reevaluation. For the present analyses, equivocal lesions on CT images were classified as positive (i.e., metastatic lesions). We did not give the patients neoadjuvant chemotherapy in principle because we were concerned about the management of vanishing liver metastases resulting from it and chemotherapy-induced liver injury. We applied preoperative portal vein embolization to patients with small future remnant volumes [8, 10].
Intraoperative diagnosis and hepatectomy
At laparotomy, visual inspection, bimanual palpation, and then IOUS were carefully carried out to scan the whole liver by the attending surgeons (M.M., N.K., Y.B., H.I., Y.S.) in all patients after mobilizing the liver off the diaphragm. For IOUS, T-shaped linear or microconvex type US probes were used at frequencies of both 5.0 and 7.5 MHz. If the nodules identified preoperatively and/or intraoperatively were judged as malignant by the attending surgeons, they were resected. Based on this information, the final hepatectomy procedures were planned so as to resect all the metastatic tumors with negative histologic margins while preserving sufficient functional parenchyma [10]. Although the performance of an anatomic resection was not adhered to [29], this procedure was adopted on a case-by-case basis [30].
Removed specimens were sliced at intervals of 1 cm, and each slice was inspected and palpated to detect any occult lesions that were not detected intraoperatively. The malignant nature of the resected tumors were confirmed histologically. Histologic examination results and the intraoperative diagnosis, including IOUS of the nonresected part of the liver, constituted the standard references in the present study.
Analyses
We first conducted lesion-by-lesion analyses of the accuracy of preoperative imaging studies. Hepatic lesions that were recorded as metastases in the reports of preoperative CT scans and that had the same location and a similar size at CT and at surgery or histologic study were considered to be true-positive (TP). Metastatic lesions detected at surgical or histologic examination but missed at CT were considered to be false-negative (FN). Hepatic lesions that were benign at surgical or histologic examination and had been misclassified as metastatic lesions at helical CT were considered to be false-positive (FP). Sensitivity was defined as the number of TP lesions/the number of (TP + FN) lesions. We then evaluated the contribution of respective intraoperative modalities to the detection of new tumors at surgery (i.e., visual inspection and/or palpation vs. IOUS).
Secondly, we conducted the patient-by-patient analyses in a similar manner. Sensitivity was defined as the number of patients without FN lesions/the total number of patients. Specificity was defined as the number of patients without FP lesions/the total number of patients. Sensitivity and specificity were calculated stratifying the patients according to the number of preoperatively diagnosed tumor nodules. The relative contribution of visual inspection and/or palpation against IOUS to the intraoperative detection of new tumors was evaluated on a patient-by-patient basis.
Finally, we assessed how the intraoperative findings of the hepatic tumors altered the procedures of planned hepatic resections. This assessment was also done from the viewpoint of how the recent innovation in the preoperative imaging technique has changed the significance of intraoperative examinations. In addition, we evaluated the relevance of the resection of new tumors found by intraoperative examination in the context of later clinical profiles of the patients.
Statistics
The Cochran-Armitage test for trend was used to examine the difference in detection rates of metastatic lesions between preoperative and intraoperative modalities. Fisher’s exact test was used to examine the difference in detection rates between the primary and repeated hepatectomies. The log-rank test was used to examine the survival difference of patients. A difference of P < 0.05 was accepted as statistically significant. The Bonferroni correction was adopted for multiple comparisons.
Results
Preoperatively, 748 lesions were diagnosed with a mean number of 2.8 lesions per patient (median 2, range 1–21) in a total of 254 hepatectomies. Intraoperatively, 270 nodules were newly detected in 65 (24.1 %) patients, and 26 nodules were diagnosed as false-positive, resulting in a total of 992 metastatic lesions with a mean number of 3.8 lesions per patient (median 3, range 1–30), all of which were resected (Table 2).
Lesion-by-lesion analyses
Table 3 shows the number of TP, FN, and FP lesions and the sensitivities of preoperative CT studies stratified according to the tumor diameter. Sensitivity decreased with a decrease in tumor diameter. The contribution of each intraoperative modality to the detection of new tumors is shown in Fig. 1a. Figure 1a also depicts the comparison between initial and repeated hepatectomies. Figure 2 reveals the distribution of new lesions as functions of the tumor diameter and location (i.e., depth from the liver surface).
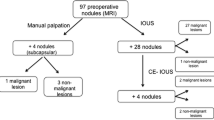
Modalities to detect new tumors intraoperatively. a Lesion-by-lesion analyses. New tumors that were both palpable and visible were classified as visible tumors. New tumors that were palpable but not visible were classified as being identified by palpation. New tumors detected by intraoperative ultrasonography (IOUS) that were not palpable or visible were classified as being identified by IOUS alone. *P = 0.089 between the first and repeated hepatectomies for the proportion of the number of tumors identified only with IOUS. b Patient-by-patient analysis. The numbers of newly detected tumor nodules in 65 patients were as follows: a single additional nodule in 24 patients, two or three nodules in 22 patients, four or five nodules in 8 patients, and six or more nodules in 11 patients. Patients in whom all the newly identified tumors could be detected by visual inspection and/or bimanual palpation were placed in the visual inspection and/or bimanual palpation group, whereas those who had at least one new tumor nodule that could be identified only through IOUS or by examining the resected specimen were classified into the IOUS alone group or as being identified in the specimen, respectively
Distribution of new lesions in regard to the tumor diameter and location. The analysis was done in 240 tumors excluding 30 nodules that were detected by examination of the resected specimen. *Numbers indicate the “number of new lesions detected by only IOUS/number of new lesions” (percentage of new lesions detected only by IOUS)
Patient-by-patient analyses
Table 4 showed the sensitivity and specificity of preoperative CT diagnoses stratified according to the number of tumor nodules that were diagnosed preoperatively. The likelihood with which the new tumors were detected intraoperatively increased with the increased number of tumor nodules that were identified preoperatively. Likewise, the specificity decreased with an increased number of tumor nodules. Figure 1b depicts the modalities to identify these tumor nodules.
Significance of intraoperative diagnosis of hepatic tumors in patient management and long-term outcome
Figure 3 shows the profiles of how the scheduled hepatectomy procedures were altered based on the intraoperative findings in 254 hepatectomies.
Disposition of 254 patients. The total number and summary of intraoperative findings are outlined. Four patients in whom the new tumor nodules were first identified by examining the resected specimens were classified as patients with no new findings. The numbers of patients who underwent planned operative procedures or modified operative procedures are shown at the bottom, classified according to the summary of intraoperative findings. *Two patients had both new lesions and false-positive (FP) lesions, and they are counted in both groups. As a result, the sum of each group was 256. Of 14 patients with FP lesions, 11 underwent reduced surgery because the lesion was revealed to be FP intraoperatively. Another three patients underwent planned liver resection because the location of intraoperatively diagnosed FP did not necessitate a change in the planned hepatectomy (n = 2), or the lesion was proven histologically to be FP postoperatively (n = 1)
The major revolution in CT scanning during the present study period was the introduction of MDCT, which occurred during 2000–2001 at our institution. Therefore, we divided the present study period into two phases: an early phase (1993–2000) and a late phase (2001–2003). The rate of new tumor detection during hepatectomy and subsequent alteration of the surgical plan did not change significantly during the two phases of the study: 30 of 117 (25.6 %) versus 35 of 137 (25.5 %) for the detection of new tumor nodules and 30 of 117 (25.6 %) versus 28 of 137 (20.4 %) for alteration of surgical plan during the early phase versus the late phase, respectively.
Table 5 demonstrates the posthepatectomy clinical courses of 65 patients in whom the new nodules were detected during liver resection and that were accordingly resected.
Discussion
The optimal imaging techniques that should be routinely used for preoperative evaluation of colorectal hepatic metastases must be discussed from every conceivable angle, including the economic consideration. In the present study, the sensitivity of portal-dominant helical CT in the depiction of hepatic tumors decreased with decreasing tumor diameter particularly in tumors ≤1 cm (Table 3). In parallel, most FP or intermediate lesions were ≤1 cm. Although former studies appear to favor the superiority of MRI to CT in detecting metastatic nodules, especially with the use of a liver-specific contrast medium such as superparamagnetic iron oxide [31, 32], the sensitivity of detection for tumors ≤1 cm has been equally unsatisfactory [20, 23, 24, 33–35]. It should be emphasized, however, that most of the newly found tumor nodules were small and were located on or underneath the liver surface; these nodules did not have a major impact on surgical management from the viewpoint of conducting multiple metastasectomies with a minimally acceptable surgical margin irrespective of the number of tumor nodules (Figs. 2, 3). This result is in line with the report by Wiering et al. [23].
The CT scan is widely available, is less expensive, and is the gold standard for identifying lung metastases [36, 37]; it is indispensable for the preoperative workup. Taking these issues into account, we insist that a portal-dominant single-phase helical CT scan is the minimum, optimal preoperative imaging modality for daily practice. The contention that a certain degree of limitation in preoperative liver imaging is acceptable for colorectal liver metastases in the availability of intraoperative diagnostic modalities should further be emphasized because the presence of multiple liver metastases by itself is not a contraindication to hepatectomy and the newly found nodules during hepatectomy rarely lead to a major change in the scheduled operative procedure. In any event, the diagnostic ability of preoperative imaging studies decreased in patients with increasing tumor nodules (Table 4). Scaife et al. [38] and Regge et al. [39] reported similar observations. MRI using liver-specific contrast medium is recommended when it is thought that an equivocal lesion detected by CT will have a major impact on the surgical management if it is malignant considering the time and economic efficacy. The role of further body imaging, including MRI as a problem-solving tool, may become more important in the era of neoadjuvant chemotherapy when borderline resectable cases and unclear lesions resulting from chemotherapy increase.
It is noteworthy that most of the newly detected nodules in our study were located within 1 cm from the liver surface (Fig. 2), which is partly attributable to the drawback of preoperative imaging modalities in delineating nodules located at the liver surface [28, 40]. Of interest, most of them could be identified by visual inspection and/or palpation; visible and/or palpable nodules comprised more than 75% of the nodules misidentified preoperatively (Figs. 1, 2). This observation differs from that of hepatocellular carcinoma (HCC), for which most new lesions are located deep from the liver surface; 88% of these new lesions were detected by IOUS [41]. The significant contribution of inspection and palpation to the detection of new nodules of colorectal liver metastases as compared with HCC is thought largely due to the soft background liver parenchyma in contrast to hard tumor tissue, even being small in diameter. Jarnagin et al. also reported the significant contribution of inspection and/or palpation to the alteration of the planned hepatic resection (34/42, 81%) compared with that by IOUS (8/42, 19%) in a cohort in whom the most common lesion was metastatic colorectal carcinoma [42]. The higher “apparent” sensitivity of preoperative imaging for the repeated hepatectomies than that for the initial resections can be explained by the difficulty of visual inspection and/or palpation due to the adhesion and/or scars on the liver surface (Table 3; Fig. 1). Considering that the outer layer with 1 cm of thickness over the whole liver represents nearly 50% of total hepatic mass [43, 44] the role of intraoperative inspection and palpation can never be underestimated. Although they are low-tech, these modalities are ready available, the cost is low, and they do not require a skilled hand. They thus play important roles in liver surgery even in the era of MDCT or IOUS. The role of bimanual investigation can never be underestimated when more and more liver resections are being done laparoscopically. Bimanual inspection should be done whenever hepatectomy is laparoscopically assisted where manual palpation can still be done with one hand.
On the other hand, IOUS played an indispensable role in detecting tumors located deep from the liver surface (Fig. 2). Even though repeated hepatic resections for hepatic recurrence are justified currently [10, 14, 15], it must be borne in mind that misdiagnosed tumors located deep in the liver may necessitate a major anatomic resection at the subsequent repeated hepatectomy if the lesion has enlarged. In addition, nearly one-third of patients in whom new metastases were found at hepatectomy had at least one nodule that was identified only with the use of IOUS. This observation should be recalled when performing surgery on patients with multiple liver metastases (Fig. 3). In addition, the role of IOUS may become more important in the present era when neoadjuvant chemotherapy is increasingly being carried out. This is because chemotherapy causes changes in the liver parenchyma that make bimanual detection of tumors difficult.
In the present series, intraoperative scrutiny identified new nodules in 65 patients (Fig. 3). These findings led us to change the preoperatively planned operation in 47 (72%) of the patients. Nevertheless, these nodules were removed successfully by additional single or multiple limited resection(s) in 43 (91%) of the patients. Previous investigators showed that intraoperative findings led to new findings during surgery in 13–55% of patients, and these new findings altered the planned surgical management in 31–90% of those with new findings [21, 22, 25, 27, 28, 42] Overall, detection of new tumor nodules during hepatectomy were common events and led to modification of the planned surgical procedures in many of the patients. However, it rarely necessitated a major alteration in the scheduled hepatectomy.
It may be naively assumed that even though these new nodules had been undetected during the planned hepatic resection, it would not have resulted in a major clinical disadvantage considering the high frequency of postoperative hepatic recurrence as they could have been resected together with other occult tumors during the subsequent repeated resection. We tried to address this question (Table 5). Among 65 patients in whom new lesions were detected and resected at the time of planned hepatectomy, 6 (9%) remained free of recurrence to date (mean ± SD follow-up of 37.4 ± 29.8 months). Evidently, these patients benefited from the intraoperative detection of new tumor nodules. In addition, 35 (54%) patients have developed inoperable extrahepatic recurrences after the hepatectomies. It can be assumed that if the intraoperatively identified nodules had not been detected at the time of hepatectomies and they had grown up as postoperative hepatic recurrences before the emergence of the extrahepatic metastases these patients might have been candidates for repeated hepatectomy. They also might benefit from the detection of new nodules, although this remains a speculative consideration.
As a limitation of this study, the study period covered a relatively long span (10 years) and up to 2003. Although the overall tendency in the investigated parameters did not change from one era to the next—before and after use of multidetector helical CT—most of the current cohort did not receive neoadjuvant chemotherapy. The value of an intraoperative diagnosis during the era of neoadjuvant chemotherapy needs to be reevaluated in a future study. The CT conditions adopted in the present study (i.e., portal-dominant single-phase CT with 5 mm reconstruction thickness) may be another limitation. Although many institutions are currently using triple-phase CT scan registration with the aim of optimizing the sensitivity for detecting malignant nodules, the sensitivity and the overall diagnostic ability as evaluated areas under receiver operating characteristics curves were reportedly not different between portal-dominant single-phase and triple-phase CT scans [32]. Likewise, although thinner collimation (i.e., 2.5 mm) was reportedly associated with better detection of lesions <1 cm [45], other studies reported that the sensitivity for detection of small nodules was not different based on the collimations owing to the higher noise values at thinner collimations [46, 47]. These questions remain to be addressed in the future studies.
Conclusions
As the surgical indication for multiple liver metastases of colorectal origin has been expanded, the likelihood that unexpected new lesions are detected intraoperatively has become higher. Most of these new lesions were <1 cm and were located on or underneath the liver surface; and in most cases, they could be identified by visual inspection or bimanual palpation. On the other hand, IOUS played a crucial role in identifying small tumors located deep from the liver surface; and a significant proportion of patients had at least one lesion detected solely by use of IOUS. Detection of new nodules during surgery usually led to the modification, albeit to a mild extent, of planned surgical management.
References
Kemeny N, Kemeny M, Dawson L (2008) Liver metastases. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB et al (eds) Abeloff’s clinical oncology, 4th edn. Elsevier, Philadelphia, pp 885–923
Garden OJ, Rees M, Poston GJ et al (2006) Guidelines for resection of colorectal cancer liver metastases. Gut 55(Suppl III):iii1–iii8
Galandiuk S, Wieand HS, Moertel CG et al (1992) Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet 174:27–32
Elias D, Liberale G, Vernerey D et al (2005) Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol 12:900–909
Fong Y, Fortner J, Sun RL et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318
Abdalla EK, Vauthey JN, Ellis LM et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239:818–825
Pawlik TM, Choti MA (2007) Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 11:1057–1077
Minagawa M, Makuuchi M, Torzilli G et al (2000) Extension of frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg 231:487–499
Khatri VP, Petrelli NJ, Belghiti J (2005) Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol 23:8490–8499
Imamura H, Seyama Y, Kokudo N et al (2004) Single and multiple resections of multiple hepatic metastases of colorectal origin. Surgery 135:508–517
Weber SM, Jarnagin WR, DeMatteo RP et al (2000) Survival after resection of multiple hepatic colorectal metastases. Ann Surg Oncol 7:643–650
Pawlik TM, Scoggins CR, Zorzi D et al (2005) Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241:715–722; discussion 722–724
Kokudo N, Miki Y, Sugai S et al (2002) Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg 137:833–840
Adam R, Bismuth H, Castaing D et al (1997) Repeat hepatectomy for colorectal liver metastases. Ann Surg 225:51–62
Adam R, Pascal G, Azoulay D et al (2003) Liver resection for colorectal metastases: the third hepatectomy. Ann Surg 238:871–884
Malafosse R, Penna C, Sa Cunha A et al (2001) Surgical management of hepatic metastases from colorectal malignancies. Ann Oncol 12:887–894
Scheele J, Stangl R, Altendorf-Hofmann A et al (1991) Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 110:13–29
Kamel IR, Choti MA, Horton KM et al (2003) Surgically staged focal liver lesions: accuracy and reproducibility of dual-phase helical CT for detection and characterization. Radiology 227:752–757
Sahani D, Saini S, Pena C et al (2002) Using multidetector CT for preoperative vascular evaluation of liver neoplasms: technique and results. AJR Am J Roentgenol 179:53–59
Vogl TJ, Schwarz W, Blume S et al (2003) Preoperative evaluation of malignant liver tumors: comparison of unenhanced and SPIO (Resovist)-enhanced MR imaging with biphasic CTAP and intraoperative US. Eur Raidol 13:262–272
Sahani DV, Kalva SP, Tanabe KK et al (2004) Intraoperative US in patients undergoing surgery for liver neoplasms: comparison with MR imaging. Radiology 232:810–814
Zacherl J, Scheuba C, Imhof M et al (2002) Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg 26:550–554
Wiering B, Ruers TJ, Krabbe PF et al (2007) Comparison of multiple CT, FDG-PET and intra-operative ultrasound in patients with colorectal liver metastases selected for surgery. Ann Surg Oncol 14:818–826
Schmidt J, Strotzer M, Fraunhofer S et al (2000) Intraoperative ultrasonography versus helical computed tomography and computed tomography with arterioportography in diagnosing colorectal liver metastases: lesion-by-lesion analysis. World J Surg 24:43–47
Conlon R, Jacobs M, Dasgupta D et al (2003) The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound 16:211–216
Ellsmere J, Kane R, Grinbaum R et al (2007) Intraoperative ultrasonography during planned liver resections: why are we still performing it? Surg Endosc 21:1280–1283
Wildi SM, Gubler C, Hany T et al (2008) Intraoperative sonography in patients with colorectal cancer and resectable liver metastases on preoperative FDG-PET-CT. J Clin Ultrasound 36:20–26
Mazzoni G, Napoli A, Mandetta S et al (2008) Intra-operative ultrasound for detection of liver metastases from colorectal cancer. Liver Int 28:88–94
Kokudo N, Tada K, Seki M et al (2001) Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg 181:153–159
Kawasaki S, Makuuchi M, Kakazu T et al (1994) Resection for multiple metastatic liver tumors after portal embolization. Surgery 115:674–677
Ward J, Robinson PJ, Guthrie JA et al (2005) Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology 237:170–180
Motosugi U, Ichikawa T, Nakajima H et al (2009) Imaging of small hepatic metastases of colorectal carcinoma: how to use superparamagnetic iron oxide-enhanced magnetic resonance imaging in the multidetector-row computed tomography age? J Comput Assist Tomogr 33:266–272
Matsuo M, Kanematsu M, Itoh K et al (2001) Detection of malignant hepatic tumors: comparison of gadolinium- and ferumoxide-enhanced MR imaging. AJR Am J Roentgenol 177:637–643
Soyer P, Poccard M, Boudiaf M et al (2004) Detection of hypovascular hepatic metastases at triple-phase helical CT: sensitivity of phases and comparison with surgical and histopathologic findings. Radiology 231:413–420
Bhattachariya S, Bhattachariya T, Baber S et al (2004) Prospective study of contrast-enhanced computed tomography, computed tomography during arterioportography, and magnetic resonance imaging for staging colorectal liver metastases for liver resection. Br J Surg 91:1361–1369
Miller DL (2002) Management of the subcentimeter pulmonary nodule. Semin Thorac Cardiovasc Surg 14:281–285
Povoski SP, Fong Y, Sgouros SC et al (1998) Role of chest CT in patients with negative chest x-rays referred for hepatic colorectal metastases. Ann Surg Oncol 5:9–15
Scaife CL, Ng CS, Ellis LM et al (2006) Accuracy of preoperative imaging of hepatic tumors with helical computed tomography. Ann Surg Oncol 13:542–546
Reggae D, Campanella D, Anselmetti GC et al (2006) Diagnostic accuracy of portal-phase CT and MRI with mangafodipir trisodium in detecting liver metastases from colorectal carcinoma. Clin Radiol 61:338–347
Hagspiel KD, Neidl KF, Eichenberger AC et al (1995) Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology 196:471–478
Zhang K, Kokudo N, Hasegawa K et al (2007) Detection of new tumors by intraoperative ultrasonography during repeated hepatic resections for hepatocellular carcinoma. Arch Surg 142:1170–1175
Jarnagin WR, Bach AM, Winston CB et al (2001) What is the yield of intraoperative ultrasonography during partial hepatectomy for malignant disease? J Am Coll Surg 192:577–583
Schultz W, Hort W (1981) The distribution of metastasis in the liver: a quantitative postmortem study. Virchows Arch 394:89–96
Berry CL (1987) Liver lesions in an autopsy population. Hum Toxicol 6:209–214
Weg N, Scheer MR, Gabor MP (1998) Liver lesions: improved detection with dual-detector-array CT and routine 2.5-mm thin collimation. Radiology 209:417–426
Haider MA, Amitai MM, Rappaport DC et al (2002) Multi-detector row helical CT in preoperative assessment of small (≤1.5 cm) liver metastases: is thinner collimation better? Radiology 225:137–142
Abdelmoumene A, Chevallier P, Chalaron M et al (2005) Detection of liver metastases under 2 cm: comparison of different acquisition protocols in four row multidetector-CT (MDCT). Eur Radiol 15:1881–1887
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research (B) (20390352).
Conflict of interest
None of the authors has a conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hata, S., Imamura, H., Aoki, T. et al. Value of Visual Inspection, Bimanual Palpation, and Intraoperative Ultrasonography During Hepatic Resection for Liver Metastases of Colorectal Carcinoma. World J Surg 35, 2779–2787 (2011). https://doi.org/10.1007/s00268-011-1264-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1264-7