Abstract
Objective
This study was designed to evaluate the clinical efficacy of pyloric digital fracture for the prevention of early delayed gastric emptying (DGE) after high-level esophagogastrostomy.
Methods
From January 2004 to March 2009, we sequentially enrolled 78 patients after esophagogastrostomy: 48 patients with pyloric digital fracture (DF group) and 30 patients without any drainage procedure (non-DF group). Intraoperative manometric study was performed in 48 patients of the DF group. Postoperative evaluation was performed, including symptomatic questionnaire, radiographic study, and gastric scintigraphy.
Results
Intraoperative manometric study revealed that basal pyloric pressure and peak pressure of pylorus in phase III of the migrating motor complex increased significantly after gastric conduit was made and anastomosed, but decreased appreciably following digital fracture. Compared with the peak pressure of IPPW before digital fracture (88.52 ± 19.88 mmHg), it appreciably decreased following digital fracture (40.45 ± 13.52 mmHg). Occurrences of IPPW (in 10 min) and duration time of each occurrence (s) had similar trends for before and after digital fracture (11.5 ± 4.5 vs. 5.0 ± 3.5 and 7.0 ± 2.0 vs. 3.0 ± 1.0, respectively). Postoperative evaluation demonstrated that early DGE occurred in four patients in the non-DF group (13.3%), and there was no DGE patient in the DF group. There was significant difference regarding gastric scores between the DF group and the non-DF group (10.5 ± 3.4 vs. 16.7 ± 3.8, t = 2.8271, P < 0.05). Gastric scintigraphy revealed that either semi-emptying-time or percent of retention at 4 h of the DF group was significantly lower than that of the non-DF group.
Conclusion
Pyloric digital fracture can prevent early DGE after high-level esophagogastrostomy efficaciously and conveniently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For patients with esophageal cancer, high-level esophagogastrostomy can offer larger resection margins but also can be prone to leading to severe delayed gastric emptying (DGE) [1], which was defined as the presence of persistent nausea or vomiting combined with endoscopic or radiological gastric distension. Pathophysiologically, weak pressure difference between stomach and pylorus, due to a decrease of gastric motility or an increase of pyloric resistance, cannot propel food bolus from stomach to duodenum. Therefore, early gastric outlet obstruction (within 1 week after surgery) occurs, which might lead to kinking or tension at the anastomosis, predisposing to ischemia, impaired healing, and even anastomotic leak [2, 3].
Two major factors were previously thought to be relevant to early DGE [2, 4]: (1) patients who undergo esophagectomy will have truncal vagus ablated inevitably, and (2) DGE is closely related to the type of gastric conduit: emptying can be better with a long gastric conduit than that with the whole stomach. With respect to the first point, Collard et al. [5] indicated that intrinsic mesenteric plexus-origin-motor activity can be “recovered” gradually, and even complete migrating motor complexes can be generated. With regard to the second point, the proper width and length of gastric conduit can be easily controlled by an experienced operator using linear stapling devices. However, early DGE presently is still an untoward complication, especially for patients after high-level esophagogastrostomy [6].
This study was designed to clarify why there is a higher risk of early DGE without any pyloric drainage in patients after high-level esophagogastrostomy, and to verify whether “pyloric digital fracture,” a little reproducible technique, can be conducive to the prevention of early DGE.
Patient experiments
Patients and operations
The study protocol was reviewed and approved by the Research Ethics Board in Daping Hospital (TMMU-DPH/IRB-2004-010), and informed consents were obtained from all patients who agreed to participate in the study. Inclusion criteria were patients with high-level esophagogastrostomy (cervical anastomosis). Exclusion criteria were patients with definite gastric-pyloric-duodenal diseases, including malignant dissemination, benign ulcer, and hypertrophic pyloric stenosis.
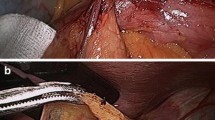
Between January 2004 and March 2009, we sequentially selected 48 patients with digital fracture (DF group) and 30 patients without drainage procedure (non-DF group). All 78 patients underwent cervical anastomosis according to the published protocol [7]. The gastric conduit was constructed by using linear stapling devices (TLC, Ethicon, Sweden) inserted into the lesser curvature 5 cm proximal to the pylorus and the width of gastric conduit was designed to be approximately 5 cm [2]. After esophagogastrostomy, pyloric digital fracture was taken as follows (Fig. 1): (1) clarification of pyloric canal; (2) pinching pyloric anterior wall with the first finger and index finger following perpendicularity of pyloric sphincter; and (3) 10–20 s exertion of pressure (It is extremely difficult to precisely depict the force, which is variable from one surgeon to another. It is only based on the “feeling” of pyloric relation and chalasis). The nasal-duodenal feeding tube and nasal gastric tube were put into all patients intraoperatively (duodenal feeding tube was placed in descendant duodenum and gastric tube was located in the body of stomach). To reduce experimental deviation, all of these operations were performed by a single surgical team.
Intraoperative manometric study
In the 48 patients of DF group, manometry was studied by standard, water perfusion, stationary manometry (Medtronic DPT-6000, Smith Medical, Sweden) with computer-assisted analysis (Polygram 2.0, Smith Medical, Sweden) of the tracings according to our published protocol [8]. A 0.5-cm incision was made on the anterior gastric wall (5 cm above pyloric canal). The sensor was passed through the hole to detect the manometric data, including gastric antrum, pylorus, and duodenum, in the three periods (10-min detection in each period) as follows: (A) the manometric data were performed promptly following laparotomy, but before gastric vagotomy; (B) data were obtained after the gastric tube had been made and anastomosed, but the fracture had not been performed yet; and (C) manometric data were obtained 5 min after the pyloric fracture.
Basal pyloric pressure was defined as the difference between the basal pressure recorded by the sleeve sensor and the distal antral pressure [9]. The peak pressure of pylorus and gastric antrum in phase III of the migrating motor complex were recorded intraoperatively. Isolated pyloric pressure wave (IPPW) was defined as phasic pressure wave detected by the sensor that was seen simultaneously in not more than one side hole over the length of the sleeve and that occurred in the absence (±5 s) of the onset of an associated pressure wave of any magnitude that was ascribable to gastric or duodenal contraction [9]. The manometric parameters regarding IPPW included peak pressure (mean value of every wave peak), frequency (occurrences in 10 min), and duration time.
Postoperative DGE evaluation
Symptomatic questionnaire and radiological study
Enteral nutrition was given through nasal-duodenal feeding tube from the postoperative third day to sixth day. Water swallow test was performed on the seventh day. Thereafter, clinical sequels, including pneumonia, reflux, and fevers, were evaluated. Normally, nasal-duodenal feeding and nasal gastric conduits were withdrawn on the eighth day. Semi-fluid feeding was given orally on the tenth day.
On postoperative day 10, we used a specific questionnaire to evaluate DGE, which was adopted from Horowitz et al. [10]. Anorexia, nausea, early satiety, vomiting, upper abdominal discomfort or distention, abdominal pain (gastric symptoms), dysphagia, heart burn, and acid regurgitation (esophageal symptoms) were scored as the following scheme: 0 = none; 1 = mild (symptom could be ignored if the patient did not think about it); 2 = moderate (symptom could not be ignored but did not influence daily activities); 3 = severe (symptom influenced daily activities).The global symptom score was calculated as the sum of the number and severity of symptoms. The maximum possible total was 18 for “gastric” symptoms and 9 for “esophageal” symptoms.
All 78 patients underwent barium swallow on postoperative day 10 to evaluate macroscopic aspects of anastomosis.
Gastric scintigraphy
A standardized gastric emptying procedure as the universally acceptable 99m technetium sulfur-colloid labeled low-fat, egg-white meals (solid food) [11] had been given to all patients without any anastomotic complication (leak, stricture, and etc.) on the postoperative day 14. Simultaneously, 20 healthy volunteers were subjected to gastric scintigraphy.
Medications that alter gastric emptying were discontinued 48–72 h in advance. Scinti-scanning with SPECT (GCA2 7100A/D1) at 4 h after test meal ingestion was performed in the upright position. When more than 10% of the scintigraphic meal is still in the stomach at 4 h, the diagnosis of delayed gastric emptying is confirmed [12]. Semi-emptying time of all patients was calculated and analyzed by computer.
Data analysis
All data entry and analysis was performed with SPSS 13.0 software (Apache Software Foundation, Chicago, IL). Analysis of variance and Dunnett test was implemented to analyze intraoperative manometric data in 48 patients of the DF group, before gastric vagotomy, and before and after pyloric digital fracture. Analysis of variance and Student–Newman–Keuls test was implemented to analyze gastric emptying time in healthy volunteers, cases in the DF and non-DF groups.
Results
Intraoperative manometric study
Intraoperative manometric data are presented in Table 1, which indicates that basal pyloric pressure and peak pressure of pylorus in phase III of the migrating motor complex increased significantly after vagotomy. However, the two aforesaid pressures decreased significantly following digital fracture.
Additionally, there were significant differences regarding the parameters of IPPW before and after pyloric digital fracture. Compared with the peak pressure of IPPW before digital fracture (88.52 ± 19.88 mmHg), it significantly decreased after digital fracture (40.45 ± 13.52 mmHg). Occurrences of IPPW (in 10 min) and duration time of each occurrence (s) had the same trend before and after digital fracture (11.5 ± 4.5 vs. 5.0 ± 3.5 and 7.0 ± 2.0 vs. 3.0 ± 1.0, respectively).
The typical manometric tracings in the three aforementioned periods in one patient are shown in Fig. 2a–c. Figure 2a unveils phase III of the migrating motor complex in gastric antrum, pylorus, and duodenum in period A. Figure 2b unveils a distinct IPPW in period B. Figure 2c unveils that the occurrence of IPPW decreased in period C.
a In period A, phase III of the migrating motor complex was recorded in gastric antrum, pylorus, and duodenum. b In period B, a distinct IPPW was recorded. c In period C, the occurrence of IPPW decreased. Period A: manometric data were performed promptly after laparotomy, but before gastric vagotomy; Period B: data were obtained after gastric tube had been made and anastomosed, but fracture was not been performed yet; Period C: manometric data were obtained 10 min after the pyloric fracture
Postoperative clinical/radiological assessments
Among the 48 patients (33 men, 15 women; median age, 63 years) with pyloric digital fracture and the 30 patients (25 men, 5 women; median age, 60 years) without drainage procedure, 8 cases and 7 cases had radiologically cervical anastomotic leaks, respectively; 2 cases and 3 cases had cardiac-pulmonary complications, respectively. However, mortality of either group was zero.
Four patients of the non-DF group (13.3%) had an early DGE confirmed by clinical evaluation and scintigraphy. Intriguingly, no patient suffered from early DGE in the DF group. In the aforementioned four patients with DGE, their gastric and esophageal symptom score ranged from 16 to 18 (median: 17.5) and 7 to 9 (median: 8.5), respectively. Nutritional supporting and prokinetic medications were given to the aforementioned four patients with DGE via the nasal-duodenal feeding tube. And symptoms improved gradually on postoperative day 30.
There was significant difference regarding gastric scores between the DF group and non-DF group (10.5 ± 3.4 vs. 16.7 ± 3.8, t = 2.8271, P < 0.05). However, there was no appreciable difference regarding esophageal scores between these two groups (5.8 ± 2.1 vs. 6.2 ± 2.7, t = 1.1843, P > 0.05).
Follow-up varied from 5 years to 8 months (median: 1 year). Among the DF group and non-DF group, 18 and 11 patients had local or systemic recurrence, respectively; 34 patients and 24 patients are still alive.
Postoperative gastric scintigraphy
Postoperative gastric scintigraphy was given to 58 patients (38 subjects of DF group and 20 subjects of non-DF group), who had no surgical complications and agreed to undergo the study. Additionally, 20 healthy volunteers were enrolled. Semi-emptying time and percentage of retention at 4 h is shown in Table 2, which reveals that semi-emptying time or percentage of retention at 4 h in the DF group was significantly lower than that of the non-DF group.
Discussion
It is well accepted that cervical esophagogastrostomy can offer larger resection margins and decrease tumor residual significantly. However, early DGE contributes to significant morbidity and delayed recovery in patients with high-level anastomosis [6, 13]. Nevertheless, some other surgeons do not think it is necessary to implement pyloric drainage routinely because probably there is not any difference with or without the procedure [14]. We designed the study to verify why there is a higher risk of early DGE without any pyloric drainage in patients after high-level esophagogastrostomy.
From intraoperative manometric study, we can see that basal pyloric pressure and peak pressure of pylorus in phase III increased significantly after vagotomy. Therefore, DGE was theoretically more prone to occur due to the weak pressure difference between stomach and pylorus (P difference = P stomach − P pylorus). Compared with the parameters regarding IPPW before gastric vagotomy, either increasing incidence or duration of IPPW after gastric tube had been made and anastomosed, without pyloric fracture, predicted the higher risk of DGE associated with pyloric dysfunction [11, 15]. Actually, four patients of the non-DF group (13.3%) had an early DGE confirmed by clinical evaluation and scintigraphy. As a result, we think it is necessary to establish a convenient and safe technique to prevent early DGE.
With respect to postoperative balloon dilatation, we think it is less acceptable to the patients than intraoperative procedures, including routine pyloromyotomy or pyloroplasty, which was regarded as the safe and efficacious management by numerous surgeons [16, 17]. However, some other reports [18–20] recently presented “pessimistic” complications and results after pyloromyotomy or pyloroplasty. Zieren’s study [18] reported that two patients with pyloroplasty, but no patient of the control group, suffered 12 months postoperatively from severe vomiting due to fibrotic stricture of the pylorus. A case-controlled study that compared outcomes in 159 patients who underwent pyloromyotomy with 83 controls indicated no difference in the incidence of DGE [19].
Before the utilization of pyloric fracture, we had not taken any routine pyloric procedures intraoperatively due to the aforesaid inconclusive clinical outcomes, and incidence of early DGE ranged from 8 to 14% among various surgical teams in our institute. In China, the digital pyloric fracture was first utilized in 17 patients with carcinoma of gastric cardia who underwent proximal subtotal gastrectomy [21]. An intraoperative manometric and postoperative gastric emptying study indicated that proximal subtotal gastrectomy might lead to DGE, and pyloric digital fracture could be an efficacious management to improve remnant gastric emptying following proximal subtotal gastrectomy. We successfully evaluated the surgical security by animal experiment (data not shown), which microscopically revealed the fractured point in muscle layer and the intact subpyloric mucosa, and there was no appreciable complication confirmed by the observation of postoperative sequel lasting 2 months.
Additionally, clinical manometric study intraoperatively indicated that basal pyloric pressure and peak pressure of pylorus in phase III appreciably decreased after digital fracture, which predicts the increase of gastropyloric pressure difference and lower risk of DGE. Similarly, the decrease of peak pressure, frequency, and duration time of IPPW after fracture prognosticates the lower risk of DGE. Therefore, we presume that the clinical efficacy of digital fracture may result from the disappearance of “bottle neck” in pyloric sphincter. Actually, in the series, patients in the DF group had no early DGE. The postoperative scintigraphic study also indicated that the DF group had faster gastric emptying than the non-DF group.
Although the disrupted pyloric muscle may recover gradually and late fibrosis of pyloric channel may form finally, DGE does not reoccur in the long-term follow-up. Additionally, we do not think pyloric digital fracture can increase duodenogastric reflux, because gastric symptom scores in the DF group are much lower than those in the non-DF group and there is no appreciable difference regarding esophageal scores between the two groups.
We have presented our surgical experience with the technique: (1) Pyloric fracture should be performed after rather than before the anastomosis. Before the gastric conduit was made and anastomosed, the pinch pressure was very difficult to be handled and controlled properly due to “soft” and “pliable” pyloric canal without any tension. However, we felt tension exactly after anastomosis and applied proper pressure until pylorus relation and chalasis. (2) During pinching, the pyloric anterior wall should be squeezed between the first finger and index finger, and slipping and sliding of the two fingers should be avoided. For instance, other Chinese institutes have reported occurrences of mucosa impairment due to digital slipping and sliding. Longitudinal pressure should be avoided carefully to obviate anastomosis leakage.
Collectively, we conclude that pyloric digital fracture can prevent early DGE efficaciously and conveniently. This technique might be an alternative to routine pyloromyotomy or pyloroplasty. However, a prospective, multi-institutional, randomized trial regarding the aforementioned techniques is required urgently.
References
Sonett JR (2000) Esophagectomy. The role of the intrathoracic anastomosis. Chest Surg Clin N Am 10:519–530
Sutcliffe RP, Forshaw MJ, Tandon R, Rohatgi A, Strauss DC, Botha AJ, Mason RC (2008) Anastomotic strictures and delayed gastric emptying after esophagectomy: incidence, risk factors and management. Dis Esophagus 21:712–717
Burt M, Scott A, Williard WC, Pommier R, Yeh S, Bains MS, Turnbull AD, Fortner JG, McCormack PM, Ginsberg RJ (1996) Erythromycin stimulates gastric emptying after esophagectomy with gastric replacement: a randomized clinical trial. J Thorac Cardiovasc Surg 111:649–653
Wang Y-Q, Ye W-W, Lu T, Zhang W-M, Xu Y (2004) Application of mechanical dilatation of the pyloric sphincter in esophagectomy for esophageal carcinoma. Asian Cardiovasc Thorac Ann 12:19–22
Collard JM, Romagnoli R, Otte JB, Kestens PJ (1998) The denervated stomach as an esophageal substitute is a contractile organ. Ann Surg 227:33–39
Finley FJ, Lamy A, Clifton J, Evans KG, Fradet G, Nelems B (1995) Gastrointestinal function following esophagectomy for malignancy. Am J Surg 169:471–475
Blackmon SH, Correa AM, Wynn B, Hofstetter WL, Martin LW, Mehran RJ, Rice DC, Swisher SG, Walsh GL, Roth JA, Vaporciyan AA (2007) Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg 83:1805–1813
Deng B, Wang RW, Jiang YG, Tan QY, Liao XL, Zhou LH, Zhao YP, Gong TQ, Ma Z (2009) Diagnosis of chest pain with foregut symptoms in Chinese patients. World J Gastroenterol 15:742–747
Sun WM, Smout A, Malbert C, Edelbroek MAL, Jones K, Dent J, Horowitz M (1995) Relationship between surface electrogastrography and antropyloric pressures. Am J Physiol Gastrointest Liver Physiol 268:G424–G430
Horowitz M, Maddox AF, Wishart JM, Harding PE, Chatterton BE, Shearman DJC (1991) Relationships between esophageal transit and solid and liquid gastric-emptying in diabetes-mellitus. Eur J Nucl Med 18:229–234
Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, Shreve P, Szarka LA, Snape WJ, Ziessman HA (2008) Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 103:753–763
Waseem S, Moshiree B, Draganov PV (2009) Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol 15:25–37
Martin JT, Federico JA, McKelvey AA, Kent MS, Fabian T (2009) Prevention of delayed gastric emptying after esophagectomy: a single center’s experience with botulinum toxin. Ann Thorac Surg 87:1708–1713 discussion 1713-1704
Urschel JD, Blewett CJ, Young JEM, Miller JD, Bennett WF (2002) Pyloric drainage (pyloroplasty) or no drainage in gastric reconstruction after esophagectomy: a meta-analysis of randomized controlled trials. Digest Surg 19:160–164
Treacy PJ, Jamieson GG, Dent J, Devitt PG, Heddle R (1992) Duodenal intramural nerves in control of pyloric motility and gastric emptying. Am J Physiol 263:G1–G5
Fok M, Cheng SW, Wong J (1991) Pyloroplasty versus no drainage in gastric replacement of the esophagus. Am J Surg 162:447–452
Law S, Cheung MC, Fok M, Chu KM, Wong J (1997) Pyloroplasty and pyloromyotomy in gastric replacement of the esophagus after esophagectomy: a randomized controlled trial. J Am Coll Surg 184:630–636
Zieren HU, Muller JM, Jacobi CA, Pichlmaier H (1995) Should a pyloroplasty be carried out in stomach transposition after subtotal esophagectomy with esophago-gastric anastomosis at the neck? A prospective randomized study. Chirurg 66:319–325
Lanuti M, de Delva PE, Wright CD, Gaissert HA, Wain JC, Donahue DM, Allan JS, Mathisen DJ (2007) Post-esophagectomy gastric outlet obstruction: role of pyloromyotomy and management with endoscopic pyloric dilatation. Eur J Cardiothorac Surg 31:149–153
Cerfolio RJ, Bryant AS, Canon CL, Dhawan R, Eloubeidi MA (2009) Is botulinum toxin injection of the pylorus during Ivor Lewis [corrected] esophagogastrectomy the optimal drainage strategy? J Thorac Cardiovasc Surg 137:565–572
Liu J, Wang Q, Tian Z, Cao F, Zhao X, Zhang Y, Gu G (1999) Functional improvement of remnant stomach after proximal subtotal gastrectomy for cardiac cancer. Zhonghua Wai Ke Za Zhi 37:82–85
Author information
Authors and Affiliations
Corresponding author
Additional information
Bo Deng and Qun-You Tan are joint first authors.
Rights and permissions
About this article
Cite this article
Deng, B., Tan, QY., Jiang, YG. et al. Prevention of Early Delayed Gastric Emptying after High-Level Esophagogastrostomy by “Pyloric Digital Fracture”. World J Surg 34, 2837–2843 (2010). https://doi.org/10.1007/s00268-010-0766-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-010-0766-z