Abstract
Background
The present study was designed to investigate the efficacy and safety of continuous occlusion of the hepatic artery proper combined with intermittent use of the Pringle maneuver for reduction of blood loss during enucleation of giant liver hemangiomas.
Methods
A retrospective study was performed on 115 patients who underwent enucleation of giant liver hemangiomas with or without continuous occlusion of the hepatic artery proper at a tertiary care university hospital. The characteristics of patients and perioperative parameters including intraoperative blood loss, the degree of ischemia–reperfusion injury, the incidence and severity of postoperative complications, and the length of hospital stay were summarized and compared in the two groups.
Results
Seventy-three and 42 patients underwent enucleation of hepatic hemangiomas with and without continuous occlusion of the hepatic artery proper, respectively. The Pringle maneuver was routinely used in all patients in cycles of 15/5 min of clamp/unclamp times. Patient characteristics were comparable between the two groups. Intraoperative blood loss and blood transfusion in the continuous occlusion group were significantly lower than in the non-occlusion group (P < 0.001 and P = 0.012, respectively). In a comparison of the two groups, there were no significant differences in the changes of the perioperative serum aspartate transaminase and total bilirubin levels (P = 0.086, P = 0.829, respectively), and in the postoperative hospital stay and surgical complications according to Clavien’s classification (P = 0.378, P = 0.227, respectively).
Conclusions
Continuous occlusion of the hepatic artery proper when added to intermittent use of the Pringle maneuver significantly reduced intraoperative blood loss when compared with intermittent Pringle maneuver alone. Enucleation of giant hepatic hemangiomas using continuous occlusion of the hepatic artery proper in addition to intermittent application of the Pringle maneuver for up to 1 h was safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver hemangioma is the most common benign tumor of the liver. Most hemangiomas are asymptomatic and are diagnosed incidentally, and they may be managed with observation. Giant liver hemangiomas produce a variety of symptoms and signs, including pain (in the abdomen, back, or shoulder), fullness, early satiety, and abdominal mass. Surgical resection provides the only consistently effective treatment and is indicated for symptomatic lesions in patients with an acceptable surgical risk, lesions for which a diagnosis is equivocal despite appropriate preoperative evaluation, or lesions that increase in size rapidly and cause Kasabach–Merritt syndrome [1, 2]. Enucleation of liver hemangioma refers to resection of the lesion by creation of a plane between the normal liver parenchyma and the hemangioma without removal of any normal hepatic tissue. Enucleation is advocated as a curative treatment by most surgeons, because the maximum amount of normal liver parenchyma is preserved, blood loss is limited, and the risk of bile leak is decreased [3, 4]. However, massive blood loss remains a problem during operation for giant lesions owing to the lack of a well-defined capsule in most parts of the hemangioma, which is filled with blood [5–7]. The Pringle maneuver is usually used to reduce blood loss. To minimize ischemia–reperfusion injury to the liver remnant, intermittent Pringle maneuver in cycles of 15/5 min of clamp/unclamp times is commonly used. The hemangioma shrinks rapidly as the inflow is occluded and it balloons again as the clamp is released. Bleeding from the hemangioma can be excessive during the unclamp time despite manual compression of the liver. Previous studies have demonstrated that the blood supply of liver hemangioma comes mainly from the hepatic artery [8–10]. Therefore, since 2004 in difficult cases seen at our Institute, continuous occlusion of the hepatic artery in addition to intermittent Pringle maneuver has been used to control the blood supply to the hemangioma and reduce blood loss during enucleation. The efficacy and safety of this technique were evaluated in the present study. Outcomes included the degree of ischemia–reperfusion injury, intraoperative blood loss, and the incidence and severity of postoperative complications.
Methods
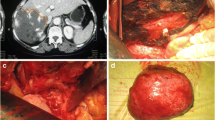
A retrospective study was performed on 115 patients who underwent enucleation of giant liver hemangiomas (Figs. 1, 2, 3) with or without continuous occlusion of the hepatic artery proper by a single team of surgeons at the Institute of Hepatobiliary Surgery, Southwest Hospital, Third Military Medical University, Chongqing, China, between January 2004 and September 2009. Intermittent Pringle maneuver in cycles of 15/5 min of clamp/unclamp times was used in all patients. Because of its obvious advantage, enucleation of liver hemangioma is used routinely in our institute instead of partial hepatectomy. The patients in this series were divided into two groups, depending on the operative approach (with or without continuous occlusion of the hepatic artery). The decision for arterial occlusion was entirely determined by the operating surgeon at the time of laparotomy. When enucleation of the hepatic hemangioma was considered technically difficult and likely to incur excessive bleeding, continuous occlusion of the hepatic artery was used by all surgeons. The medical records were obtained from a prospectively maintained database and retrospectively reviewed for demographics, blood biochemistry, characteristics of the hemangioma (location, size, and number), surgical variables (surgery time, inflow occlusion time, amount of blood loss, and transfusion requirements), length of hospital stay, and postoperative complications. The study was approved by the Institutional Ethical Review Board.
Surgery started with a right subcostal incision or midline incision with a right horizontal extension. The reverse Trendelenberg position was used to reduce the pressure on the inferior vena cava. After the abdominal cavity was explored, the liver was fully mobilized. Intraoperative ultrasonography was performed routinely to delineate the extent of the hemangioma and to plan the enucleation. The hepatic artery, or any collateral arteries from the left gastric artery or the superior mesenteric artery, was mobilized and controlled by a bull-dog clamp, if required. Intermittent Pringle maneuver, consisting of cycles of 15/5 min of clamp/unclamp times, was routinely used by tightening and untightening a 4 mm tape tourniquet around the portal triad. Liver transection was carried out using a Kelly clamp. Small bridging vessels were coagulated with diathermy and divided, and the few relatively larger vessels were controlled with ligation and divided. A silicone drain was placed near the raw area after enucleation. All patients received the same postoperative care by the same team of surgeons and were nursed in the intensive care unit during the early postoperative period. Parenteral nutritional support was provided for patients with liver cirrhosis. Early enteral nutrition was given once bowel activity returned. All intraoperative and postoperative complications were recorded prospectively. Surgical complications were summarized according to Clavien’s classification [11]. Biliary leakage was diagnosed when the total bilirubin level in the drainage fluid exceeded 21 μmol/l (the upper limit of normal for serum bilirubin in our laboratory) after surgery.
Statistical analysis
Statistical analysis was performed using the χ2 test or Fisher’s exact test to compare discrete variables, and the Mann–Whitney test was used to compare continuous variables. A value of P < 0.05 was considered to be significant. Statistical analyses were performed with SPSS 10.0 for Windows computer software (SPSS Inc., Chicago, IL).
Results
A total of 115 patients with liver hemangiomas (85 women and 30 men) were enrolled and analyzed. Seventy-three and 42 patients underwent enucleation of the hepatic hemangiomas using continuous or no occlusion of the hepatic artery proper, respectively. The demographics, liver function tests, hemoglobin levels, platelet counts, cirrhosis, location, size, and number of hemangiomas were compared between the two groups (Table 1). There were no significant differences in these parameters between the two groups. Although there were more hemangiomas located centrally in the continuous occlusion group than in the non-occlusion group, the difference was insignificant.
The surgical variables, outcomes, and postoperative liver function tests are shown in Table 2. Intraoperative blood loss and blood transfusion were significantly lower in the continuous occlusion group than in the non-occlusion group (P < 0.001 and P = 0.012, respectively). The number of patients without blood transfusion in the continuous occlusion group was markedly higher than in the non-occlusion group (P = 0.006). The median Pringle time was around 1 h in the two groups (P = 0.126). The maximum continuous occlusion time of the hepatic artery proper was 96 min. Although the postoperative serum aspartate transaminase (AST) on day 3 in the continuous occlusion group was higher than in the non-occlusion group, no significant difference existed between the two groups (P = 0.098). The postoperative serum total bilirubin and prothrombin time on day 3, which were related to hepatic ischemia–reperfusion injury, were not significantly different between the two groups.
Surgical complications including hemorrhage, bile leakage, intra-abdominal collection, and pleural effusion were similar in the two groups of patients (Table 3). There were no in-hospital deaths. No patients developed liver failure or postsurgical ascites/encephalopathy. The morbidity rates were similar in the two groups of patients (P = 0.161). On the basis of the Clavien classification of surgical complications, there were no significant differences between the two groups (P = 0.227). All patients with pleural effusion and bile leakage recovered after percutaneous drainage.
The changes in perioperative serum AST and total bilirubin are shown in Figs. 4 and 5, respectively. The postoperative serum AST level rose rapidly to a peak on day 1 and then decreased gradually (Fig. 4). The curves of the perioperative serum AST showed no marked difference in the two groups of patients (P = 0.086). The perioperative serum total bilirubin rose gradually after operation; then decreased in 2 or 3 days (Fig. 5). There were no significant differences in the perioperative total bilirubin levels between the two groups of patients (P = 0.829).
The number of patients with cirrhotic liver in this study was too small for a meaningful analysis of the impact of continuous occlusion of hepatic artery proper on the cirrhotic and the non-cirrhotic livers. However, no patient in this study suffered from severe liver failure after the operation.
Discussion
Enucleation is the procedure of choice for the treatment of liver hemangioma. Most surgeons have advocated enucleation since it was first described in 1988. Enucleation is safer, quicker, and is associated with less morbidity than partial hepatectomy. However, massive blood loss remains a problem for large hemangiomas of more than 10 cm in diameter and for centrally located liver hemangiomas [5, 12, 13]. The present study analyzed the surgical outcomes of 115 patients who underwent enucleation of liver hemangiomas under intermittent Pringle maneuver with or without continuous occlusion of the hepatic artery proper. Our results showed that enucleation with continuous occlusion of the hepatic artery had the advantage of significant reduction of intraoperative blood loss, and its safety was verified by this study.
Resection of a hepatic hemangioma was first reported by Hermann Pfannenstiel in 1898, and surgery remains the only consistently effective method of treatment [6]. In 1988, Alper et al. described a new technique of enucleation of hemangiomas by dissecting in a fibrous cleavage plane between the capsule of the hemangioma and surrounding normal liver tissue [14]. Enucleation can be performed for any size of hemangioma, and it has the advantage of not removing any liver parenchyma [15–18]. Nevertheless, massive blood loss remained a main problem. Data from Blumgart’s team showed the median blood loss for enucleation for 11 lesions in 10 patients was 800 ml, which compared favorably with that the findings reported by Schwartz (1,750 ml) [6] and Starzl et al. (1,680 ml) [5]. There are three reports on the use of transarterial embolization (TAE) of the feeding artery to treat giant hemangioma prior to surgical resection. The idea was to decrease the size of the hemangioma by blocking its arterial supply which facilitated subsequent mobilization of the liver, and consequently decreased intraoperative hemorrhage [8–10]. Our experience showed that giant hemangioma shrank rapidly in size within several minutes of occlusion of the hepatic artery. This resulted in an increase in space in the abdominal cavity and facilitated the operation. Also, there was a decrease in blood loss from the hemangioma during enucleation. To occlude the entire arterial blood supply to the liver and to the giant hemangioma, anomalies of the hepatic artery originating from the left gastric artery or the superior mesenteric artery should be sought.
Baer et al. reported ligating a branch of the hepatic artery before enucleation of liver hemangiomas to reduce blood loss. For a major lesion of the right or left liver, ligation of the corresponding right or left hepatic artery was advocated. For lesions of the quadrate lobe, they used a more selective dissection to control the relevant vessels within the umbilical fissure [5]. In their report, the median blood loss for enucleation of 11 lesions in 10 patients was 800 ml, but an intraoperative blood loss of 3,000 ml was reported in two patients. In addition, hepatic artery ligation can result in biliary ischemia and stricture.
The main concern over combining the Pringle maneuver with continuous occlusion of hepatic artery proper during enucleation of hemangioma is whether there is an increase in ischemic complications, especially when the occlusion is required for a long time. Our data show that there was no significant difference in the liver function tests perioperatively between two groups. The levels of postoperative serum AST and alanine transaminase (ALT) indicated liver damage caused by ischemia and reperfusion injury was mild.
To the best of our knowledge, this is the first report on using this technique for enucleation of liver hemangioma [19–23]. Our results show this technique to be safe, and it significantly reduced intraoperative blood loss. We now routinely use this technique for enucleation of liver hemangiomas.
References
Fu XH, Lai ECH, Yao XP et al (2009) Enucleation of liver hemangiomas: is there a difference in surgical outcomes for centrally or peripherally located lesions? Am J Surg 198:184–187
Erdogan D, Busch OR, van Delden OM et al (2007) Management of liver hemangiomas according to size and symptoms. J Gastroenterol Hepatol 22:1953–1958
Lerner SM, Hiatt JR, Salamandra J et al (2004) Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg 139:818–821
Blumgart LH (2000) Liver resection for benign disease and for liver and biliary tumors. In: Blumgart LH, Fong Y (eds) Surgery of the liver and biliary tract, 3rd edn. W.B. Saunders, Philadelphia, pp 1639–1713
Baer HU, Dennison AR, Mouton W et al (1992) Enucleation of giant hemangiomas of the liver. Technical and pathologic aspects of a neglected procedure. Ann Surg 216:673–676
Schwartz SI (1987) Cavernous hemangioma of the liver: a single institution report of 16 resections. Ann Surg 205:456–465
Starzl TE, Koep LJ, Weil R et al (1980) Excisional treatment ofcavernous hemangioma of the liver. Ann Surg 192:25–27
Giavroglou C, Economou H, Ioannidis I (2003) Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol 26:92–96
Srivastava DN, Gandhi D, Seith A et al (2001) Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: a prospective study. Abdom Imaging 26:510–514
Vassiou K, Rountas H, Liakou P et al (2007) Embolization of a giant hepatic hemangioma prior to urgent liver resection. Case report and review of the literature. Cardiovasc Intervent Radiol 30:800–802
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Gedaly R, Pomposelli JJ, Pomfret EA et al (1999) Cavernous hemangioma of the liver: anatomic resection vs. enucleation. Arch Surg 134:407–411
Hamaloglu E, Altun H, Ozdemir A et al (2005) Giant liver hemangioma: therapy by enucleation or liver resection. World J Surg 29:890–893
Alper A, Ariogul O, Emre A et al (1988) Treatment of liver hemangiomas by enucleation. Arch Surg 123:660–661
Singh RK, Kapoor S, Sahni P et al (2007) Giant haemangioma of the liver: is enucleation better than resection? Ann R Coll Surg Engl 89:490–493
Yoon SS, Charny CK, Fong Y et al (2003) Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg 197:392–402
Terkivatan T, Vrijland WW, Den Hoed PT et al (2002) Size of lesion is not a criterion for resection during management of giant liver haemangioma. Br J Surg 89:1240–1244
Tsai HP, Jeng LB, Lee WC et al (2003) Clinical experience of hepatic hemangioma undergoing hepatic resection. Dig Dis Sci 48:916–920
Ozden I, Emre A, Alper A et al (2000) Long-term results of surgery for liver hemangiomas. Arch Surg 135:978–981
Popescu I, Ciurea S, Brasoveanu V et al (2001) Liver hemangioma revisited: current surgical indications, technical aspects, results. Hepatogastroenterology 48:770–776
Brouwers MA, Peeters PM, de Jong KP et al (1997) Surgical treatment of giant haemangioma of the liver. Br J Surg 84:314–316
Duron JJ, Keilani K, Jost JL et al (1995) Giant cavernous hepatic hemangiomas in adults: enucleation under selective blood inflow control. Am Surg 61:1019–1022
Demiryurek H, Alabaz O, Agdemir D et al (1997) Symptomatic giant cavernous haemangioma of the liver: Is enucleation a safe method? A single institution report. HPB Surg 10:299–304
Acknowledgments
This work was supported by National S&T Major Project (No. 2008ZX10002-026). The authors are grateful to our research assistants Zhongfang Jie and Hua Li for their help with data collection and statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, F., Lau, WY., Qian, C. et al. Surgical Treatment of Giant Liver Hemangiomas: Enucleation with Continuous Occlusion of Hepatic Artery Proper and Intermittent Pringle Maneuver. World J Surg 34, 2162–2167 (2010). https://doi.org/10.1007/s00268-010-0592-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-010-0592-3