Abstract
Background
Blunt and penetrating liver trauma is common and often presents major diagnostic and management problems.
Methods
A literature review was undertaken to determine the current consensus on investigation and management strategies.
Results
The liver is the most frequently injured organ following abdominal trauma. Immediate assessment with ultrasound has replaced diagnostic peritoneal lavage in the resuscitation room, but computerised tomography remains the gold standard investigation. Nonoperative management is preferred in stable patients but laparotomy is indicated in unstable patients. Damage control techniques such as perihepatic packing, hepatotomy plus direct suture, and resectional debridement are recommended. Major complex surgical procedures such as anatomical resection or atriocaval shunting are now thought to be redundant in the emergency setting. Packing is also recommended for the inexperienced surgeon to allow control and stabilisation prior to transfer to a tertiary centre. Interventional radiological techniques are becoming more widely used, particularly in patients who are being managed nonoperatively or have been stabilised by perihepatic packing.
Conclusions
Management of liver injuries has evolved significantly throughout the last two decades. In the absence of other abdominal injuries, operative management can usually be avoided. Patients with more complex injuries or subsequent complications should be transferred to a specialist centre to optimise final outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The liver is the most frequently injured abdominal organ, despite its relatively protected location [1–5]. Management of liver injuries has changed significantly over the last two decades, with significant improvement in outcomes. There is now a broad consensus regarding most aspects of management, with the introduction of standard protocols, but in other areas considerable controversy persists. This literature review considers the diagnosis, investigation, and recommendations for the management of trauma to the liver.
Mechanisms of injury
Road traffic accidents and antisocial, violent behaviour account for the majority of liver injuries. Industrial and farming accidents also account for a significant number. There is an interesting difference in incidence throughout the world, with penetrating injuries (gunshot and stab wounds) accounting for the majority in North America and South Africa and blunt injuries representing the majority in Europe and Australasia [6–11].
The liver consists of a relatively fragile parenchyma contained within the Glisson’s capsule, which is thin and does not afford it great protection. Hence, the parenchyma and its vasculature are very susceptible to blunt and penetrating trauma. The vasculature consists of wide-bore, thin-walled vessels with a high blood flow, and injury is usually associated with significant blood loss.
Blunt trauma in a road traffic accident, or fall from a height, may result in a deceleration injury as the liver continues to move on impact. This leads to tears at sites of fixation to the diaphragm and abdominal wall. A well-recognised deceleration injury involves a fracture between the posterior sector (segments VI and VII) and the anterior sector (segments V and VIII) of the right lobe (Fig. 1). This type of injury may be associated with a significant vascular injury due to a tear of the right hepatic vein. In contrast, a direct blow to the abdomen may produce a central crush injury, with an extensive stellate-type laceration involving segments IV, V, and VIII (Fig. 2). With this pattern of injury, an associated major vascular injury may be present, with disruption of the hepatic arteries, portal veins, or the major hepatic veins. This type of injury may also be associated with bleeding from the caudate lobe (segment I). Such an injury may occur with a blow from a fist or weapon, or a central crush in a road traffic, industrial, or farming accident. Overall, blunt trauma more commonly affects the right hepatic lobe, particularly the posterior sector, with the caudate lobe rarely affected [12, 13].
Penetrating injuries may be associated with a significant vascular injury. For example, a stab injury may cause major bleeding from one of the three hepatic veins or the vena cava and also from the portal vein or hepatic artery if it involves the hilum. Gunshots may similarly disrupt these major vessels; this disruption may be much more marked than with stab wounds due to the cavitation effect, particularly with bullets from high-velocity weapons.
The connection between the thin-walled hepatic veins and the inferior vena cava (IVC), at the site where the ligamentous mechanism anchors the liver to the diaphragm and posterior abdominal wall, represents a vulnerable area, particularly to shearing forces during blunt injury. Disruption here leads to the “juxtahepatic” venous injuries, which are usually associated with major blood loss and present a particularly challenging management problem.
Grading of liver injuries
The severity of liver injuries ranges from the relatively inconsequential minor capsular tear to extensive disruption of both lobes with associated hepatic vein, portal vein, or vena caval injury. While several classification systems have been devised, the most widely used is that of The American Association for the Surgery of Trauma (Table 1) [14]. Grade I or II injuries are generally considered minor and usually do not require operative treatment, while grade III–V injuries are severe and often require operative management. Significant vascular injuries can be associated with a haematoma or parenchymal laceration (grade III) but usually occur with major parenchymal disruption (grades IV and V). High-grade hepatic injuries are associated with a higher surgical intervention rate and a poorer prognosis, thus emphasising the importance of adherence to an appropriate management strategy [2, 7, 15–17].
Patient assessment and initial investigation
It is generally accepted that initial resuscitation and management is the same as for any patient with major trauma and should follow the Advanced Trauma Life Support (ATLS) principles of aggressive fluid resuscitation, guided by monitoring of central venous pressure and urinary output [18]. Management should also be directed toward avoidance of any of the sinister triad of hypothermia, coagulopathy, and acidosis, which are associated with significantly increased mortality. Mechanisms to avoid hypothermia are standard now in major centres and include the use of rewarming blankets and heat exchanger pumps for rapid infusion of resuscitation fluids and blood [19]. Avoidance of coagulopathy and acidosis depends on initial good resuscitation and prompt decision making in the next phases.
The next management phase depends largely on the response to resuscitation and the stability of the patient. Liver injury should be suspected in all patients with blunt or penetrating thoracoabdominal trauma but particularly in shocked patients with blunt or penetrating trauma to the right side. If such a patient remains unstable (systolic pressure <90 mmHg) despite adequate resuscitation (2 L of intravenous fluids), immediate operation is indicated to stop bleeding [18]. This message must be very strongly emphasised as it has been well established that delay in surgery and control of bleeding in the unstable patient are associated with a significantly higher mortality [20].
In stable patients, however, surgery is not the immediate priority and appropriate investigation, perhaps leading ultimately to nonoperative management, can be instituted. The main investigative and therapeutic modalities include ultrasonography, CT scanning, and interventional vascular radiological techniques.
Ultrasonography
Ultrasound has become a major investigative modality for abdominal injury. It is particularly useful for detecting injury to parenchymal organs and the presence of free intraperitoneal fluid or blood. Rapid diagnostic information is facilitated by the fact that it is noninvasive, easily accessible, and less costly than other investigations [21–23]. The particular relevance to major liver injury is the focused assessment by ultrasound for trauma (FAST), often performed in the emergency department, which involves a rapid examination of several areas, namely, the pericardial region, right upper quadrant (including Morrison’s pouch), left upper quadrant, and the pelvis, specifically looking for free fluid [24]. This rapid assessment is excellent for evaluation of the unstable patient in the acute setting in the emergency department. A more definitive ultrasound scan to assess the integrity of the liver and other abdominal organs would require a more prolonged period of sonographic assessment by an experienced radiologist and therefore is unsuitable for the unstable patient.
Ultrasound scanning is very accurate for blunt and penetrating abdominal injuries, with specificity reported between 95 and 100% and sensitivity between 63 and 100%. Ultrasound has largely replaced diagnostic peritoneal lavage (DPL) in the initial assessment of blunt truncal injuries [21, 24–44]. DPL is not of value in isolated organ injuries or retroperitoneal injuries and can also result in positive results for intra-abdominal injuries in up to 30% of patients that do not necessarily require surgery [27, 45, 46]. Although FAST provides a rapid assessment of liver disruption and intraperitoneal bleeding, it is a limited scan that is highly operator dependent. It is very important to note that a negative FAST scan does not safely rule out injury [30, 47]. Due to the operator dependence of the modality, different end points, and inconsistent comparative gold standards in the studies, the reported specificities, sensitivities, and overall accuracies are variable [48–52]. It has been demonstrated that up to a quarter of hepatic and splenic injuries, as well as renal, bladder, pancreatic, mesenteric, and gut injuries, can be missed if ultrasound is used as the primary investigative modality in the stable patient. However, while the possibility of false negatives is ever present, the combination of a negative ultrasound scan and normal clinical examination and observations almost excludes liver injury in the event of significant blunt trauma [21, 47, 53–55]. In patients with significant hepatic injury, ultrasound cannot accurately determine the extent of hepatic parenchymal or vascular injury and therefore should not be a substitute for CT scanning if the patient is stable [25, 49, 53].
Computerised tomography
Spiral computerised tomographic (CT) scanning has become the standard evaluation modality for stable patients with an abdominal injury [12, 56–58]. The trend toward nonsurgical management of liver trauma is largely due to the availability of this technique [59]. In addition to increasing the rate of detection of liver lesions following trauma, CT has also helped to improve the understanding of the course of liver injuries [60]. CT has particularly high sensitivity and specificity for detecting liver injuries, which improve with increasing time between injury and scanning, as lacerations and haematomas become better defined (Fig. 1). Hoff et al. [49] reported a sensitivity of 92–97% and a specificity of 98.7%. The development of multislice CT has improved sensitivity, and more rapid imaging allows visualisation of the major vascular structures in different phases following contrast enhancement. In addition, reconstruction can be performed in multiple planes without significant loss of image quality [61].
The type and extent of liver injury can be readily identified by CT, particularly subcapsular and intraparenchymal haematomas, lacerations, and vascular injuries. CT also gives an estimation of the volume of haemoperitoneum and an indication of ongoing haemorrhage and is an essential element in nonoperative management of liver injuries [56, 58, 62]. The measured attenuation on CT allows differentiation between clotted blood (45-70 HU) and active bleeding (30-45 HU) [59, 60]. The highest attenuating collection indicates the presence of the sentinel clot and may allow localisation of the source of bleeding [63]. Active ongoing haemorrhage is visible and can be demonstrated on CT as extravasation of contrast material and is a strong predictor of failure of nonsurgical management [30, 59, 64, 65]. The presence of ongoing haemorrhage on CT has been suggested as an indication for intervention, whether in the form of surgery or interventional radiology [59, 60]. Although detection of free fluid may point toward a possible laparotomy, it is essential to remember that the haemodynamic stability of the patient will dictate the course of treatment, as there can be significant discrepancies found between CT and operative findings [24, 49, 57, 66–69]. The additional information gleaned from a CT scan will help in appropriate discussion with a specialist unit at an early stage, even in the haemodynamically compromised patient [16, 68].
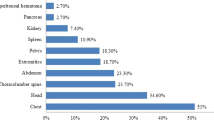
CT is also invaluable in the detection of associated intraperitoneal and retroperitoneal injuries [69, 70]. Concurrent injuries can exist, affecting the spleen in 21% of cases, kidney in 8.9%, bowel in 4%, chest in 53.9%, and associated pelvic fractures in 22.4% of cases [60]. CT can also be utilised in the follow-up of complications as a result of liver trauma. Delayed haemorrhage, bile leaks, and abscess formation can all be assessed with concomitant therapeutic options such as percutaneous drainage of an abscess or biloma [59, 60]. Follow-up by repeat CT scanning is recommended only when clinically indicated and particularly for grade IV or V liver injuries [60]. This is best performed 7-10 days post injury to detect complications [59, 70].
Although CT is the investigative gold standard, it is important to remember that it involves exposure to high levels of ionising radiation and the use of intravenous contrast may compromise renal function [30]. In the majority of hospitals the use of CT requires movement of the patient away from adequate resuscitation facilities to the X-ray department, highlighting the importance of haemodynamic stability in patients with abdominal trauma being considered for CT examination [30].
Interventional vascular radiological techniques
Interventional radiological techniques have become an integral part of the management of abdominal trauma and have added a new dimension to the management of hepatic vascular injuries (Fig. 3) [71–74]. This multidisciplinary approach to the management of complex hepatic injuries is becoming much more important as the role of interventional radiology expands. Denton et al. [75] reported successful use of a combination of arterial embolisation and transhepatic venous stenting in the management of a grade V injury involving the retrohepatic vena cava in a patient whose injury had been temporarily controlled by perihepatic packing. A similar combined surgical and radiological approach with stent placement in a ruptured hepatic vein was reported by Burch in 1997 [76]. Recent more extensive series of angiography for control of hepatic haemorrhage have reported increasing success, with identification and control of bleeding rates ranging from 68 to 87% [71, 77–80]. CT criteria, including grade of hepatic injury, evidence of arterial vascular injury, and the presence of hepatic venous injury, are being used increasingly in the selection of patients who should undergo hepatic angiography and possibly embolisation [56].
Angiography allows intervention at difficult-to-access locations [81]. This is important in both the pre- and postoperative stages of management [82]. Angioembolisation has been demonstrated to have a high success rate with low risk of rebleeding [77–79]. The increased role of angioembolisation has truncated any initial operative intervention and therefore stabilisation is reached sooner, with associated improved survival [79]. Further selective hepatic arterial embolisation following packing has been used effectively to control recurrent bleeding [75, 83, 84]. Sclafani et al. [84] reported a 93% success rate in the control of bleeding in a series of 60 patients by the use of coil embolisation, while others have demonstrated that the procedure reduces the volume of transfusion and the need for liver surgery [84–86].
In summary, angiography and embolisation or stenting is a very useful adjunctive technique in the stable patient who is being managed nonoperatively or in the patient who either has been stabilised by perihepatic packing or has rebled after a period of initial stability. It is likely that advances in interventional radiology will push the boundaries of nonsurgical management of liver trauma in the future.
Nonoperative management
Hogarth Pringle, in 1908, provided the first description of operative management of liver trauma [87]. Unfortunately, all eight patients died and Pringle recommended conservative nonoperative management of these patients. In the modern surgical literature, nonoperative management was first reported in 1972 and has been one of the most significant changes in the treatment of liver injuries over the last two decades [88–91]. This paradigm shift developed as a result of several factors: (1) the recognition that 50–80% of liver injuries stop bleeding spontaneously, (2) the precedent of successful nonoperative management in children, and (3) the significant development of liver imaging with CT scanning [69, 91–93]. While nonoperative management was initially introduced for minor injuries, it was soon in vogue for more severe injuries (grades III–V) [8, 9, 70, 94–98].
The mechanism of injury influences the management decision-making process. Evidence for the efficacy of nonoperative management of liver trauma accumulated throughout the 1990 s, with success rates ranging from 80 to 100% and documentation of significant reduction in blood transfusion requirements and reduced hospital stay [4, 10, 99–112]. Nonoperative management is usually recommended for stable patients following a stab injury [96, 98, 113–124]. There is increasing experience for the use of nonoperative management of gunshot wounds (which previously would have mandated exploration), and several authors report that these may be treated conservatively, provided there are no other significant injuries [115, 116]. Traditional fears relating to nonoperative management, such as increased sepsis rates due to infection of bile and blood collections, have been proved inaccurate [104]. Nonoperative management is not always successful, as demonstrated by a multicentre study that revealed that fewer than half the patients sustaining a blunt liver injury are suitable for nonoperative management [70].
The selection criteria for nonoperative management are always evolving and conservative approaches are increasingly adopted in the more severely injured. In particular, the threshold for volume of haemoperitoneum demonstrated on CT scan has increased [98, 117, 125]. Important assessment criteria for nonoperative management include (1) haemodynamic stability after resuscitation, (2) absence of signs of other visceral or retroperitoneal injuries that require surgery, and (3) the availability of an effective multidisciplinary team providing good-quality CT imaging, intensive care facilities, and suitably experienced surgeons [8, 9, 16, 70, 88, 96–98, 117]. While there has been considerable debate about the grade of liver injury and the acceptable volume of haemoperitoneum, it is now generally accepted that the ultimate decisive factor in favour of nonoperative management is the haemodynamic stability of the patient, irrespective of the grade of injury or the volume of haemoperitoneum. It is also essential that appropriate clinical and radiological follow-up is arranged [78, 96–98, 102, 118, 121, 124–127].
The failure rate of nonoperative management leading to the necessity to resort to open surgery is significantly higher in grade IV and V injuries compared to grade I–III injuries [70, 104]. However, the necessity to resort to surgical intervention is rarely due to liver-related complications [89]. The most common reason for surgical intervention in patients initially managed nonoperatively is coexisting abdominal injury such as delayed bleeding from the spleen or kidney [102]. Failure of nonoperative management due to delayed liver bleeding is rare (0–3.5%) [70, 97, 101, 102, 104]. Other factors identified as predictors of nonoperative management failure include age, haemoglobin, blood pressure, active extravasation on CT, and the need for blood transfusion [78, 89, 96, 104, 128].
As experience with nonoperative management has increased, it has become apparent that serial follow-up CT scanning is not necessary for patients with grade I–II injuries, provided the patient remains haemodynamically stable [129, 130]. For other patients, it is essential that appropriate clinical and radiological follow-up is arranged. Also, in those with large haematomas or significant vascular injuries (grades IV and V), it is still recommended to look for signs of further bleeding which would merit further investigation and management, e.g., angiography and embolisation or stenting [131].
Nonoperative management has become the standard of care in patients with blunt liver trauma, with a 23.5% reduction in mortality in grade III and grade IV patients [96, 99, 110, 132, 133]. The same technique has also emerged as effective management in appropriately selected patients with liver gunshot injuries [134, 135].
Operative management
Incision and initial control of bleeding
When nonoperative management is not possible, or fails, the surgeon must be prepared to conduct a resuscitative laparotomy. The most widely adopted incision for the patient with liver trauma is a long midline laparotomy, which can be extended to the right chest if a posterior right lobe injury, major hepatic venous injury, or vena caval injury is encountered. Historically, incisions were extended using an oblique incision to open the right thorax and diaphragm, but a median sternotomy may be used to access the chest. An effective alternative, which gives good exposure and avoids a thoracotomy, is a right subcostal extension (Fig. 4). A bilateral subcostal incision is sometimes favoured by hepatobiliary surgeons if there is an obvious penetrating through-and-through liver injury. This allows excellent exposure of the right lobe of the liver, the hepatic veins, and vena cava without having to open the chest or diaphragm; however, it does compromise access to the lower abdomen (Fig. 4).
If a major liver injury is encountered, immediate control of bleeding is an absolute priority because the greatest threat to the patient’s life at this juncture is exsanguination [136, 137]. The liver should immediately be manually closed and compressed (Fig. 5). Tamponade can then be maintained by packs, which can also be manually compressed if bleeding continues. If this still does not control the bleeding, pedicle occlusion (Pringle manoeuvre) should be applied using an atraumatic vascular clamp or noncrushing bowel clamp (Fig. 6). This manoeuvre can be both therapeutic and diagnostic. If a Pringle manoeuvre controls the bleeding it is unlikely that a major hepatic venous or vena caval injury has occurred. Although the recommended occlusion time is controversial, it is generally agreed that up to 1 h can be tolerated. If bleeding continues despite pedicle occlusion, a major vascular injury (hepatic venous or vena caval) is likely and further packing and manual compression should be used [138].
These measures for rapid control of bleeding are important and should be maintained to help the anaesthetist achieve restoration of the blood volume and effective intraoperative resuscitation [139]. Attempts to identify and repair hepatic vascular injuries before effective resuscitation has been achieved should be avoided as they will invariably lead to further exsanguination, hypotension, acidosis, and coagulopathy [11, 139].
Damage control surgery
The concept of damage control surgery involves three principle phases [140]. Phase 1 involves initial control of haemorrhage and contamination followed by packing and rapid wound closure. This is followed by further resuscitation and stabilisation in the intensive care unit for a 24–48-h period until normal physiological parameters have been restored (phase 2). Phase 3 consists of re-exploration and definitive repair. The major aim initially, therefore, is to minimise the metabolic insult (particularly hypothermia, coagulopathy, and acidosis) without immediate concern for restoration of anatomical integrity. Implicit in this concept is limiting the operating time (abbreviated laparotomy) and avoidance of prolonged or complex attempts at anatomical reconstruction [79, 140–142]. This obviously includes avoidance of opening body cavities that have not been traumatised (e.g., thoracic cavity) as this increases operating time and increases the burden of injured tissue in an already unstable patient.
The damage control concept is very appropriate for the management of major liver injuries and, in fact, was initially described by Halsted in 1908 for the control of liver bleeding by packing. It was repopularised in the early 1980 s, particularly for patients who had developed a coagulopathy (nonsurgical bleeding), but was more widely adopted throughout the next two decades. Damage control approaches are associated with a significant survival advantage compared to traditional prolonged surgical techniques [79, 143].
The three key factors that interact to produce a deteriorating metabolic situation are hypothermia, coagulopathy, and acidosis. Patients in this condition are at the limit of their physiological reserve and persistence with prolonged and complex surgical repair attempts will cause exceptionally high mortality [140]. Early recognition of hypothermia, coagulopathy, and acidosis is the key to the damage control approach. It is recommended that definitive surgery should cease and a damage control approach be adopted when hypothermia is deteriorating or a temperature of 34°C is reached, when coagulopathy has developed (nonsurgical oozing or prothrombin time greater than 50% above normal), or when acidosis exists (pH <7.2 despite adequate volume resuscitation) [140].
Perihepatic packing
Elder in 1887 suggested that a liver injury causing haemorrhage would invariably be fatal [144]. Pringle’s landmark paper offered an operative strategy by which this blood loss could be temporarily stanched [87]. After the Second World War, Madding et al. [145] showed a reduced mortality using early laparotomy, drainage, and asepsis. Operative strategies then included parenchymal approximation with large stitches, vessel ligation, and resection. Due to mortality falling from 62.5 to 27.7% by these means, more aggressive strategies were adopted, rather than perihepatic packing [145, 146]. This has since been replaced by a return to favour of the art of perihepatic packing, with associated success.
Perihepatic packing will control profuse haemorrhaging in up to 80% of patients undergoing laparotomy and will allow intraoperative resuscitation (resuscitative packing) [138–143, 147–149]. In the management of severe injuries of the liver, packing has emerged as the key to effective damage control [147, 148]. However, more definitive “therapeutic” packing is also a very effective technique, particularly when used judiciously to prevent the cascade of hypothermia, coagulopathy, and acidosis [91, 150–152]. Although perihepatic packing was somewhat discouraged in the era when definitive surgical repair was popular, packing has become increasingly adopted during the last two decades [140, 153]. Packing is particularly useful for more extensive injuries (grade III–IV) but has also been shown to be effective for even the more major vascular injuries (grade IV–V) [15, 153, 154]. This technique is also extremely useful for the general surgeon in a district hospital as it can be life-saving until major surgery can be performed following transfer to a major trauma or hepatobiliary unit [139]. This on-going role of stabilising the patient to ensure safe transfer to another surgical institution is well recognised and its importance should not be underestimated.
The technique of perihepatic packing is straightforward. Following manual closure or approximation of the liver parenchyma, large, folded laparotomy packs are inserted over the diaphragmatic surfaces of the liver to produce a tamponade effect between the liver and the abdominal wall and thoracic cage. Krige et al. [11] recommend a “six pack” technique (Fig. 7). Packs should not be forced deep into liver fractures as this can extend the injury and cause venous tears and increased bleeding (Fig. 7).
Care must be taken to avoid excessive packing as this can cause vena caval and renal vein compression, leading to abdominal compartment syndrome (ACS). This is a particular risk with infrahepatic packs and these should be avoided if possible. To reduce the risk of ACS, some advocate closing the upper part of the wound to enhance the tamponade effect but leaving the lower two-thirds open and temporarily covered with a silastic sheet sutured to the skin edges [155, 156]. Appropriate perihepatic packing is essential since the efficacy of the procedure significantly impacts patient outcome [157].
Following resuscitation and stabilisation with correction of coagulation and metabolic deficits, packs are removed after approximately 36–48 h [153]. During this period broad-spectrum antibiotics should be given to reduce the risk of sepsis which occurs in 10–30% of cases [9, 156–161]. The exact timing of the removal of packs is controversial, but they should not be removed before 24 h as this is related to rebleeding and leaving them in place for 24 h or more does improve outcome [138, 149, 160–166]. Delayed removal (up to 1 week after injury) is not associated with an increased incidence of organ-specific or systemic complications [167–169]. Due to the risk of rebleeding during pack removal, some authors recommend insertion of plastic sheets between the liver and packs or insertion of an omental pedicle covered by a plastic sterile drape [161]. Alternatively, an absorbable mesh pack in children or a nonstick bowel bag in cases of extensive hepatic capsular disruption has been suggested to prevent rebleeding [170, 171].
Definitive surgical procedures for liver injuries
Following initial control of bleeding by manual compression, Pringle manoeuvre, and perihepatic packing to allow adequate resuscitation, the surgeon must decide on the next phase of management. If bleeding has stopped after careful pack removal, no further intervention is necessary. If bleeding persists, it is important to obtain adequate exposure and visualisation of the injury. Intermittent release of the Pringle clamp combined with effective suction may allow identification of deep bleeding sites and control by direct suture. If the bleeding is too profuse, experienced judgment is invaluable for deciding whether to continue with exploration and attempted repair or whether to opt for definitive perihepatic packing. This decision depends on the stability of the patient and the presence of adverse factors such as coagulopathy. In general, persistent bleeding at this stage will be due to major parenchymal disruption or a major vascular injury and it may be prudent to opt for definitive packing, particularly in an unstable patient with developing coagulopathy. If, however, the patient remains relatively stable with no signs of coagulopathy, a spectrum of operative techniques is available. No single technique is superior and applicable to every patient; therefore, the one selected will depend on the nature of the injury.
Hepatotomy and selective vascular suture or ligation
Combined hepatotomy and selective vascular ligation has emerged as the preferred method of management for major hepatic venous, portal venous, and arterial injuries in many centres [11, 153, 172]. It is performed under Pringle control and involves finger fracture or Kelly clamp extension of the laceration to allow suture or ligation of the bleeding vessels (Fig. 8). Intermittent release of the Pringle clamp allows detection of ruptured hepatic arterial or portal venous branches and their direct suture or clipping.
The hepatotomy and ligation technique has been used extensively for control of bleeding and avoidance of packing with good results [11, 139, 153, 173]. For control of major vascular injuries, Pachter et al. [153] recommend a rapid and extensive finger fracture, often through normal parenchyma, to reach the site of injury. However, it is important to emphasise that with a major hepatic venous injury, significant haemorrhage may occur while attempting to extend a deep liver laceration and that this bleeding will not be controlled by a Pringle clamp and increased morbidity may be incurred. In such cases, or for a mashed liver following a high-velocity road traffic accident, hepatotomy should be abandoned and an alternative such as total vascular exclusion or manual closure and definitive packing should be adopted [139].
Hepatorrhaphy
Hepatorrhaphy was one of the earliest and most widely practised techniques for control of major haemorrhage from liver vascular injuries. It involved wide placement of large sutures (liver sutures) in the parenchyma to compress it and tamponade bleeding vessels. However, the compression necessary to stop the bleeding results in a significant risk of parenchymal ischaemia and necrosis and the technique is no longer recommended [174–177].
Selective hepatic artery ligation
Selective hepatic artery ligation was once widely used in liver trauma but has largely been replaced by more effective alternatives, e.g., hepatotomy and suture or manual closure and packing. In dire emergencies, large sutures encompassing the vascular structures of the right lobe can be used [163, 173]. One reason for not using this technique may be when the right or left hepatic artery has been significantly disrupted in a portal hepatic injury. In this situation it can simply be ligated without any risk of ischaemia of the liver lobe, provided the ipsilateral portal vein is intact. If the right hepatic artery is ligated, a cholecystectomy should be performed to avoid ischaemic necrosis [62, 178–181].
Nonanatomical resection (resectional debridement)
This refers to removal of devitalised parenchyma using the line of injury as the boundary of the resection rather than standard anatomical planes [107, 182]. It may be performed at the initial laparotomy or at relaparotomy following packing. It is essential that the patient is haemodynamically stable and not have a coagulopathy. The principle is to limit the extent of parenchymal dissection so that operating time is short and new tissue planes with further potential for bleeding are not created [139, 183]. Ideally, it should be achieved with minimal additional liver resection, but if this is necessary, finger fracture, a Kelly clamp, or ultrasonic dissection may be used [153]. Extensive dissection through uninjured parenchyma should be avoided if possible. In some cases simple completion of an extensive parenchymal avulsion may suffice, e.g., when there has been an avulsion of the posterior sector of the right lobe (segments VI and VII). This type of injury is often associated with a right hepatic vein laceration and completion of the “resection” will allow control and suture of this. In such situations, vascular stapling devices are extremely useful for rapid and secure division of major veins.
Anatomical resection
This involves resection along standard anatomical planes (usually right hepatectomy) after identification and control of the relevant inflow and outflow vessels. This was performed extensively in the 1960s and during the war in Vietnam, where a rapid right hepatectomy with no hilar control was introduced. Almost universally, extensive anatomical resection for trauma was associated with a very high mortality rate [101, 173]. This, plus the fact that the time and magnitude of the surgery goes against the later principles of conservative surgery and damage control, has resulted in anatomical resection being practised rarely and it is now performed in only approximately 2–4% major liver trauma cases [164].
However, anatomical resection does have significant merit as it removes the source of bleeding and sepsis. This is particularly so when a lobe is shattered or there is proximal ductal injury and devascularisation and repair attempts will inevitably fail. In addition, the traditional poor results and lack of enthusiasm for this technique have been contradicted by the results of some recent series, particularly that from Strong et al. [172] who achieved excellent results in a series of 37 patients, 11 of whom (33%) had grade V juxtahepatic venous injuries [172, 184]. These results probably reflect the fact that this procedure was performed in a specialist liver resection and transplantation unit, and while the majority of liver injuries continue to be managed initially in trauma centres or district hospitals, it is likely that more conservative and damage control procedures will remain the most widely practised techniques.
Total vascular exclusion
Total vascular exclusion was initially introduced for elective liver resection and was later used to manage major retrohepatic venous injuries. The technique involves clamping of the portal triad and infra- and suprahepatic IVC and therefore requires experience with mobilisation of the liver as done in liver resection and transplantation (Fig. 9). Excellent results were reported for this technique by Khaneja et al. [185] who used it to manage grade V penetrating injuries with 90% of patients surviving the operation and an overall survival rate of 70%. One major drawback of this technique is the effect of caval clamping which results in decreased venous return, leading to severe hypotension and circulatory collapse in an already hypovolaemic patient. As a result of this and the experience required to perform it, it is unlikely that this technique will become more widespread and perihepatic packing is likely to be preferable.
Venovenous bypass
Total vascular exclusion can create physiological derangement in venous return that is not compatible with maintaining a cardiac output. This may be overcome by extracorporeal bypass, which involves shunting of blood, via a vortex pump, from the common femoral and mesenteric veins to the axillary or internal jugular veins, as used in liver transplantation [186, 187]. Experience with this is limited and its use is usually restricted to units with specialist transplantation experience.
Atriocaval shunt
The principle of the atriocaval shunt is that caval control is obtained above and below the liver while venous return from the IVC to the right atrium is maintained. Essentially, it was designed to achieve what can now be achieved by total vascular exclusion and venovenous bypass. The technique involves opening the chest via a median sternotomy and passing a shunt (chest drain) down into the IVC via the right atrial appendage (Fig. 10). The supra- and infrahepatic IVC are controlled by tapes and a Pringle clamp is applied, thus producing vascular isolation of the liver. In an attempt to avoid a sternotomy, a balloon shunt, which can be introduced via the saphenofemoral junction, has been developed [188].
The atriocaval shunt, although logical in principle and initially widely adopted in trauma centres in the U.S., has been associated with very poor survival figures: “more authors than survivors”! [9, 139, 153, 173, 189–193]. Buechter et al. [189] reported a 90% mortality rate for ten patients with juxtahepatic venous injuries who were managed with a shunt, compared to 60% mortality for those managed with total vascular exclusion. Similarly, Burch et al. [194] reported a 67% mortality rate when an atriocaval shunt was used compared to a 47% mortality rate with nonshunting alternatives. In addition, the necessity for a thoracotomy adds further injury to an already severely injured patient and goes directly against the later concept of damage control surgery. For these reasons, atriocaval shunting has largely been replaced by the alternatives described earlier [189, 195, 196].
Liver transplantation
Transplantation in a small number of patients with massive liver damage or grade VI avulsion injuries has been reported [197, 198]. The key obstacle to this approach is how to keep the patient alive while waiting for a suitable graft. Several techniques have been introduced for maintaining venous return and splanchnic decompression during the anhepatic phase. These include venovenous bypass and construction of a temporary end-to-side portocaval shunt [199, 200]. However, while liver transplantation may be life-saving for major liver trauma, the logistical problems will mean that it remains a limited option, available only in specialist centres.
Summary
This literature review has shown that the management of injuries of the liver has evolved significantly throughout the last two decades. Nonoperative techniques for the management of grade IV–V injuries in stable patients have been established, although there is a higher failure rate for these injuries compared with grade I–III injuries. Interventional radiological techniques have become more widely used in patients who are being managed nonoperatively or who have been stabilised by perihepatic packing. In unstable patients immediate control of bleeding is critical and the recommended techniques are manual compression, Pringle manoeuvre, and perihepatic packing. In terms of surgical management there has been a definite move away from major, time-consuming procedures toward conservative surgery and damage control. The preferred surgical technique for inaccessible bleeding within a laceration is rapid finger fracture hepatotomy and direct suture or ligation. Prolonged attempts at surgical control and repair should be avoided, and definitive perihepatic packing should be employed at an early stage in the persistently unstable patient or at the first signs of coagulopathy. Packing is also the recommended technique for the inexperienced surgeon, to allow control and stabilisation prior to transfer. Nonanatomical resection (resectional debridement) is recommended when there is unviable parenchyma. Anatomical resection is generally reserved for a devascularised lobe with a major ductal injury. Hepatorrhaphy and selective arterial ligation are no longer recommended. As a result of the high mortality associated with atriocaval shunting, this technique is also no longer recommended and has been replaced largely by perihepatic packing or total vascular exclusion, with or without venovenous bypass.
References
Feliciano DV (1989) Surgery for liver trauma. Surg Clin North Am 69:273–284
Matthes G, Stengel D, Seifert J et al (2003) Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole-body helical computed tomography. World J Surg 27:1124–1130
Clancy TV, Maxwell GJ, Covington DL et al (2001) A statewide analysis of level I and II trauma centers for patients with major injuries. J Trauma 51:346–351
Miller PR, Croce MA, Bee TK et al (2002) Associated injuries in blunt solid organ trauma: the implications for missed injury in non-operative management. J Trauma 53:238–242
Shanmuganathan K, Mirvis SE, Chiu WC (2004) Penetrating torso trauma: triple-contrast helical CT in peritoneal violation and organ injury. A prospective study in 200 patients. Radiology 231:775–784
Lucas CE, Ledgerwood AM (2000) Changing times and the treatment of liver injury. Am Surg 66:337–341
Sikhondze WL, Madiba TE, Naidoo NM et al (2007) Predictors of outcome in patients requiring surgery for liver trauma. Injury 38:65–70
Feliciano DV, Mattox KL, Jordan GL et al (1986) Management of 1000 consecutive cases of hepatic trauma (1979–1984). Ann Surg 204:438–445
Cogbill TH, Moore EE, Jurkovich GJ et al (1988) Severe hepatic trauma: a multi-center experience with 1335 liver injuries. J Trauma 28:1433–1438
Schweizer W, Tanner S, Baer HU et al (1993) Management of traumatic liver injuries. Br J Surg 80:86–88
Krige JEJ, Bornman PC, Terblanche J (1997) Liver trauma in 446 patients. South Afr J Surg 1:10–15
Becker CD, Mentha G, Terrier F (1998) Blunt abdominal trauma in adults: role of CT in the diagnosis and management of visceral injuries. Eur Radiol 8:553–562
Becker CD, Gal I, Baer HU et al (1996) Blunt hepatic trauma in adults: correlation of CT injury grading with outcome. Radiology 201:215–222
Moore EE, Cogbill TH, Jurkovich GJ et al (1995) Organ injury scaling: spleen and liver (1994 revision). J Trauma 38:323–324
Gao JM, Du DY, Zhao XJ et al (2003) Liver trauma: Experience in 348 cases. World J Surg 27:703
Brammer RD, Bramhall SR, Mirza DF et al (2002) A 10-year experience of complex liver trauma. Br J Surg 89:1532–1537
Gur S, Orsel A, Atahan K et al (2003) Surgical treatment of liver trauma. Hepatogastroenterology 50:2109–2111
American College of Surgeons (1997) Advanced Trauma Life Support manual. American College of Surgeons, Chicago, IL
Peng RY, Bongard FS (1999) Hypothermia in trauma patients. J Am Coll Surg 188:688–696
Hoyt DB, Bulger EM, Knudson MM et al (1994) Death in the operating room: analysis of a multicenter experience. J Trauma 37:426–432
Nural MS, Yardan T, Guven H et al (2005) Diagnostic value of ultrasonography in the evaluation of blunt abdominal trauma. Diagn Interv Radiol 11:41–44
Kirby RM, Braithwaite M (2000) Management of liver trauma. Br J Surg 87:1732
Kimura A, Otsuka T (1991) Emergency center ultrasonography in the evaluation of hemoperitoneum: a prospective study. J Trauma 31:20–23
Scalea T, Rodriguez A, Chiu W et al (1999) Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma 46:466–472
Royal College of Radiologists (2007) Making the best use of clinical radiology services: referral guidelines. RCR, London
Boulanger BR, Kearney PA, Brenneman FD et al (2000) Utilisation of FAST (focused assessment with sonography for trauma) in 1999: results of a survey of North American trauma centres. Am Surg 65:1049–1055
Richards JR, Schleper NH, Woo BD et al (2002) Sonographic assessment of blunt abdominal trauma: a 4-year prospective study. J Clin Ultrasound 30:59–67
Melanson SW, Heller M (1998) The emerging role of bedside ultrasonography in trauma care. Emerg Med Clin North Am 16:165–189
Bode PJ, Edwards MJ, Kruit MC et al (1999) Sonography in a clinical algorithm for early evaluation of 1671 patients with blunt abdominal trauma. AJR Am J Roentgenol 172:905–911
Jansen JO, Yule SR, Loudon MA (2008) Investigation of blunt abdominal trauma. BMJ 336:938–942
Pearl WS, Todd KH (1996) Ultrasonography for the initial evaluation of blunt abdominal trauma: A review of prospective trials. Ann Emerg Med 27:353–361
Rozycki GS, Shackford SR (1996) Ultrasound, what every trauma surgeon should know. J Trauma 40:1–4
McGahan JP, Richards JR (1999) Blunt abdominal trauma: the role of emergent sonography and a review of the literature. AJR Am J Roentgenol 172:897–903
Yoshii H, Sato M, Yamamoto S et al (1998) Usefulness and limitations of ultrasonography in the initial evaluation of blunt abdominal trauma. J Trauma 45:45–51
Hoffmann R, Nerlich M, Muggia-Sullan M et al (1992) Blunt abdominal trauma in cases of multiple trauma evaluated by ultrasonography: a prospective analysis of 291 patients. J Trauma 32:452–458
Rothlin MA, Naf R, Amgwerd M et al (1993) Ultrasound in blunt abdominal and thoracic trauma. J Trauma 34:488–495
McGahan JP, Rose J, Coates TL et al (1997) Use of ultrasonography in the patient with acute abdominal trauma. J Ultrasound Med 16:653–662
Rozycki GS, Ochsner MG, Schmidt JA et al (1995) A prospective study of surgeon performed ultrasound as the primary adjuvant modality for injured patient assessment. J Trauma 39:492–500
McKenney MG, Martim L, Lentz K et al (1996) 1000 consecutive ultrasounds for blunt abdominal trauma. J Trauma 40:607–612
Sirlin CB, Casola G, Brown MA et al (2001) US of blunt abdominal trauma: importance of free pelvic fluid in women of reproductive age. Radiology 219:229–235
Fernandez L, McKenney MG, McKenney KL et al (1998) Ultrasound in blunt abdominal trauma. J Trauma 45:841–848
McKenney M, Lentz K, Nunez D et al (1994) Can ultrasound replace diagnostic peritoneal lavage in the assessment of blunt trauma? J Trauma 37:439–441
Brown MA, Casola G, Sirlin CB et al (2001) Blunt abdominal trauma: screening US in 2693 patients. Radiology 218:352–358
McKenney KL, Nunez DB, McKenney MG et al (1998) Sonography as the primary screening technique for blunt abdominal trauma: experience with 899 patients. AJR Am J Roentgenol 170:979–985
Nagy KK, Roberts RR, Joseph KT et al (2000) Experience with over 2500 diagnostic peritoneal lavage for suspected intra-abdominal injury following blunt trauma. Injury 31:479–482
Bain IM, Kirby RM, Tiwari P et al (1998) Survey of abdominal ultrasound and diagnostic peritoneal lavage for suspected intra-abdominal injury following blunt trauma. Injury 29:65–71
Sirlin CB, Brown MA, Andrade-Barreto OA et al (2004) Blunt abdominal trauma: clinical value of negative screening US scans. Radiology 230:661–668
Healey M, Simons RK, Winchell RJ et al (1996) A prospective evaluation of abdominal ultrasound in trauma: is it useful? J Trauma 40:875–885
Hoff WS, Holevar M, Nagy KK et al (2002) Practice management guidelines for the evaluation of blunt abdominal trauma: the EAST practice management guidelines work group. J Trauma 53:602–615
Rozycki GS, Ballard RB, Feliciano DV et al (1998) Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg 228:557–567
Boulanger BR, McLellan BA, Brenneman FD et al (1996) Emergent abdominal sonography as a screening test in a new diagnostic algorithm for blunt trauma. J Trauma 40:867–874
Rozycki GS, Ochsner MG, Jaffin JH et al (1993) Prospective evaluation of surgeons’ use of ultrasound in the evaluation of trauma patients. J Trauma 34:516–527
Shuman WP, Holtzman SR, Bree RL et al. (2005) American College of Radiology appropriateness criteria. Blunt abdominal trauma. Available at http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalimaging.aspx
Stengel D, Bauwens K, Sehouli J et al (2001) Systematic review and meta-analysis of emergency ultrasonography for blunt abdominal trauma. Br J Surg 88:901–912
Stengel D, Bauwens K, Sehouli J et al. (2005) Emergency ultrasound based algorithms for diagnosing blunt abdominal trauma. Cochrane Database Syst Rev (2):CD004446
Poletti PA, Mirvis SE, Shanmuganathan K et al (2000) CT criteria for management of blunt liver trauma: correlation with angiographic and surgical findings. Radiology 216:418–427
Delgado Millan MA, Deballon PO (2001) Computed tomography, angiography, and endoscopic retrograde cholangiopancreatography in the nonoperative management of hepatic and spenic trauma. World J Surg 25:1397–1402
Shanmuganathan K, Mirvis SE (1998) CT scan evaluation of blunt hepatic trauma. Radiol Clin N Am 36:399–411
Yoon W, Jeong YY, Kim JK et al (2005) CT in blunt liver trauma. Radiographics 25:87–104
Taourel P, Vernhet H, Suau A et al (2007) Vascular emergencies in liver trauma. Eur J Radiol 64:73–82
Shanmuganathan K (2004) Multi-detector row CT imaging of blunt abdominal trauma. Semin Ultrasound CT MR 25:180–224
Shuman WP (1997) CT of blunt abdominal trauma in adults. Radiology 205:297–306
Lubner M, Menias C, Rucker C et al (2007) Blood in the belly: CT findings of hemoperitoneum. Radiographics 27:109–125
Fang JF, Chen RJ, Wong YC et al (1998) Pooling of contrast material on computed tomography mandates aggressive management of blunt hepatic injury. Am J Surg 176:315–319
Wong YC, Wang LJ, See LC et al (2003) Contrast material extravasation on contrast-enhanced helical computed tomographic scan of blunt abdominal trauma: its significance on the choice, time, and outcome of treatment. J Trauma 54:164–170
JL Isenhour, Marx J (2007) Advances in abdominal trauma. Emerg Med Clin North Am 25:713–733
Poletti PA, Wintermark M, Schmyer P et al (2002) Traumatic injuries: role of imaging in the management of the polytrauma victim. Eur Radiol 12:969–978
Sriussadaporn S (1993) CT scan in blunt abdominal trauma. Injury 24:541–544
Pachter HL, Hofstetter SR (1995) The current status of nonoperative management of adult blunt hepatic injuries. Am J Surg 169:442–454
Pachter HL, Knudson MM, Esrig B et al (1996) Status of non-operative management of blunt hepatic injuries in 1995: a multicenter experience in 404 patients. J Trauma 40:31–38
Carrillo EH, Spain DA, Wohltmann CD et al (2000) Interventional techniques are useful adjuncts in nonoperative management of hepatic injuries. J Trauma 46:619–624
Corr P, Beningfield SJ, Krige JEJ (1992) Selective hepatic artery embolization in complex liver trauma. Injury 23:347–349
Hagiwara A, Yukioka T, Ohta S et al (1997) Nonsurgical management of patients with blunt hepatic injury: efficacy of transcatheter arterial embolisation. AJR Am J Roentgenol 169:1151–1156
Ciraulo DL, Luk S, Palter M et al (1998) Selective hepatic arterial embolisation of Grade IV and V blunt hepatic injuries: An extension of resuscitation in the nonoperative management of traumatic hepatic injuries. J Trauma 45:353–358
Denton JR, Moore EE, Coldwell DM (1997) Multimodality treatment for grade 5 hepatic injuries: ‘perihepatic packing’, arterial embolisation and venous stenting. J Trauma 42:964–968
Burch JM (1997) New concepts in trauma. Am J Surg 173:44–46
Asensio JA, Demetriades D, Chahwan S et al (2000) Approach to the management of complex hepatic injuries. J Trauma 48:66–72
Richardson JD, Franklin GA, Lukan JK et al (2000) Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg 232:324–330
Johnson JW, Gracias VH, Schwab CW et al (2001) Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma 51:261–269
Bochicchio GV (2002) The management of complex liver injuries. Trauma Q 15:55–76
Hoffer EK, Borsa JJ, Bloch RD et al (1999) Endovascular techniques in the damage control setting. Imaging Symp 19:1340–1348
Johnston JW, Gracias VH, Reilly PM et al (2001) Hepatic angiography in the damage control population. J Trauma 50:176
Wagner WH, Lundell CJ, Donovan AJ (1985) Percutaneous angiographic embolisation for hepatic arterial haemorrhage. Arch Surg 120:1241–1249
Sclafani SJ, Shaftan GW, McAuley J et al (1984) Interventional radiology in the management of hepatic trauma. J Trauma 24:256
Wahl WL, Ahrns KS, Brandt MM et al (2002) The need for early angiographic embolisation in blunt liver injuries. J Trauma 52:1097–1101
Mohr AM, Lavery RF, Barone A et al (2003) Angiographic embolisation for liver injuries: low mortality, high morbidity. J Trauma 55:1077–1081
Pringle JH (1908) Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 48:541–549
Ritchie JP, Fonkalsrud EW (1972) Subcapsular haematoma of the liver: nonoperative management. Arch Surg 104:781–784
Velmahos GC, Toutouzas KG, Radin R et al (2003) Nonoperative treatment of blunt injury to solid abdominal organs: a prospective study. Arch Surg 138:844–851
Haan JM, Bocchicchio GV, Kramer N et al (2005) Nonoperative management of blunt splenic injury: a 5-year experience. J Trauma 58:492–498
Stein DM, Scalea TM (2006) Nonoperative management of spleen and liver injuries. J Intensive Care Med 21:296–304
Stylianos S (2000) Evidence-based guidelines for resource utilisation in children with isolated spleen or liver injury. The APSA Trauma Committee. J Pediatr Surg 35:164–167
Losty PD, Okoye BO, Walter DP et al (1997) Management of blunt liver trauma in children. Br J Surg 84:1006–1008
Kozar R, Moore JB, Niles SE et al (2005) Complications of nonoperative management of high-grade blunt hepatic injuries. J Trauma 59:1066–1071
Moore EE, Shackford SR, Pachter HL et al (1989) Organ injury scaling: spleen liver and kidney. J Trauma 29:1664–1666
Meredith JW, Young JS, Bowling J (1994) Nonoperative management of blunt hepatic trauma: the exception or the rule? J Trauma 36:529–535
Knudson MM, Lim RC Jr, Oakes DD et al (1990) Nonoperative management of blunt liver injuries in adults: the need for continued surveillance. J Trauma 30:1494–1500
Meyer AA, Crass RA, Lim RC Jr et al (1985) Selective nonoperative management of blunt liver injury using computed tomography. Arch Surg 120:550–554
Sherman HF, Savage BA, Jones LM et al (1994) Nonoperative management of blunt hepatic injuries: safe at any grade? J Trauma 37:616–621
Croce MA, Fabian TC, Menke PG et al (1995) Nonoperative management of blunt hepatic trauma is the treatment of choice for haemodynamically stable patients. Results of a prospective trial. Ann Surg 221:744–755
Pachter HL, Feliciano DV (1996) Complex hepatic injuries. Surg Clin N Am 76:763–782
Velmahos GC, Toutouzas K, Radin R et al (2003) High success with nonoperative management of blunt hepatic trauma: the liver is a sturdy organ. Arch Surg 138:475–485
Coughlin PA, Stringer MD, Lodge JP et al (2004) Management of blunt liver trauma in a tertiary referral centre. Br J Surg 91:317–321
Malhotra AK, Fabian TC, Croce MA et al (2000) Blunt hepatic injury: a paradigm shift from operative to nonoperative management in the 1990 s. Ann Surg 231:804–813
Hollands MJ, Little LM (1991) Non-operative management of blunt liver injuries. Br J Surg 78:968–972
Bynoe RP, Bell RM, Miles WS et al (1992) Complications of non-operative management of blunt hepatic injuries. J Trauma 32:308–315
Fang JF, Chen RJ, Lin BC et al (2000) Blunt hepatic injury: minimal intervention in the policy of treatment. J Trauma 49:722–728
Knudson MM, Maull KI (1999) Nonoperative management of solid organ injuries: past, present and future. Surg Clin North Am 79:1357–1371
Maull KI (2001) Current status of nonoperative management of liver injuries. World J Surg 25:1403–1404
Goan YG, Huang MS, Lin JM (1998) Nonoperative management for extensive hepatic and splenic injuries with significant hemoperitoneum in adults. J Trauma 45:360–364
Brasel KJ, DeLise CM, Oslen CJ et al (1997) Trends in the management of hepatic injury. Am J Surg 174:674–677
Ochsner MG (2001) Factors of failure for nonoperative management of blunt liver and splenic injuries. World J Surg 25:1393–1396
Nanace FC, Cohn I (1969) Surgical judgment in the management of stab injuries of the abdomen: a retrospective and prospective analysis based on a study of 600 stabbed patients. Ann Surg 170:569–580
Demetriades D, Rabinowitz C, Sofianos C (1986) Non-operative management of penetrating liver injuries: a prospective study. Br J Surg 73:736–737
Demetriades D, Charalambides D, Lakhoo M et al (1991) Gunshot wounds of the abdomen: role of selective conservative management. Br J Surg 78:220–222
Muckart DJJ, Abdool-Carrim ATO, King B (1990) Selective conservative management of abdominal gunshot wounds: a prospective study. Br J Surg 77:652–655
Farnell MB, Spencer QP, Thompson E et al (1988) Nonoperative management of blunt hepatic trauma in adults. Surgery 104:748–756
Feliciano DV (1992) Continuing evolution in the approach to severe liver trauma. Ann Surg 216:521–523
Durham RM, Buckley J, Keegan M et al (1992) Management of blunt hepatic injuries. Am J Surg 164:477–481
Federico JA, Horner WR, Clark DE et al (1990) Blunt hepatic trauma. Nonoperative management in adults. Arch Surg 125:905–909
Carillo EH, Platz A, Miller FB et al (1998) Nonoperative management of blunt liver trauma. Br J Surg 85:461–468
Renz BM, Feliciano DV (1994) Gunshot wounds to the right thoracoabdomen: a prospective study of nonoperative management. J Trauma 37:737–744
Chmielewski GW, Nicholas JM, Dulchavsky SA et al (1995) Nonoperative management of gunshot of the abdomen. Am Surg 61:665–668
Demetriades D, Gomez H, Chahwan S et al (1999) Gunshot injuries to the liver: the role of selective nonoperative management. J Am Coll Surg 188:343–348
Beckingham IJ, Krige JE (2001) ABC of diseases of liver, pancreas and bilary system. Liver and pancreatic trauma. BMJ 322:783–785
Yaman I, Nazli O, Tugrul T et al (2007) Surgical treatment of hepatic injury: morbidity and mortality analysis of 109 cases. Hepatogastroenterology 54:1507–1511
Shapiro MB, Nance ML, Schiller HJ et al (2001) Nonoperative management of solid abdominal organ injuries from blunt trauma: impact of neurologic impairment. Am Surg 67:793–796
Schroeppel TJ, Croce MA (2007) Diagnosis and management of blunt abdominal solid organ injury. Curr Opin Crit Care 13:399–404
Davis KA, Brody JM, Cioffi WG (1996) Computed tomography in blunt hepatic trauma. Arch Surg 131:255–260
Allins A, Ho T, Nguyen TH et al (1996) Limited value of routine follow-up CT scans in nonoperative management of blunt liver and splenic injuries. Am Surg 62:883
Cuff RF, Cogbill TH, Lambert PJ (2000) Nonoperative management of blunt liver trauma: the value of follow-up abdominal computed tomography scans. Am Surg 66:332–336
Demetriades D, Hadkizacharia P, Constantinou C et al (2006) Selective nonoperative management of penetrating abdominal solid organ injuries. Ann Surg 244:620–628
Coimbra R, Hoyt DB, Engelhart S et al (2006) Nonoperative management reduces the overall mortality of grades 3 and 4 blunt liver injuries. Int Surg 91:251–257
Omoshoro-Jones JAO, Nicol AJ, Navsaria PH et al (2005) Selective non-operative management of liver gunshot injuries. Br J Surg 92:890–895
DuBose J, Inaba K, Teixeira PG et al (2007) Selective non-operative management of solid organ injury following abdominal gunshot wounds. Injury 38:1084–1090
Marr JDF, Krige JEJ, Terblanche J (2000) Analysis of 153 gunshot wounds of the liver. Br J Surg 87:1030–1034
Krige JEJ, Bornman PC, Terblance J (1992) Therapeutic perihepatic packing in complex liver trauma. Br J Surg 79:43–46
Nicol AJ, Hommes M, Primrose R et al (2007) Packing for control of hemorrhage in major liver trauma. World J Surg 31:569–574
Krige JEJ (2000) Liver fracture and bleeding. Br J Surg 87:1615–1616
Hoey BA, Schwab CW (2002) Damage control surgery. Scand J Surg 91:92–103
Neuhaus SJ, Bessell JR (2004) Damage control laparotomy in the Australian military. ANZ J Surg 74:18–22
Tugnoli G, Casali M, Villani S et al (2003) The “damage-control” in severe hepatic injuries: our experience. Ann Ital Chir 74:529–533
Rotondo MF, Schwab CW, McGonigal MD et al (1993) ‘Damage control’: An approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 35:375–383
Tilton BT (1905) Some considerations regarding wounds of the liver. Ann Surg 41:20–30
Madding GF, Peniston WH (1957) Liver haemostasis. Surg Gynaecol Obstet 104:417–424
Madding GF (1958) Wounds of the liver. Surg Clin North Am 38:1619–1629
Calne RY, Wells FC, Forty J (1982) Twenty-six cases of liver trauma. Br J Surg 69:365–368
Lucas CE, Ledgerwood AM (1976) Prospective evaluation of hemostatic techniques for liver injuries. J Trauma 16:442–451
Baracco-Gandolfo VB, Vidarte O, Baracco-Miller V et al (1986) Prolonged closed liver packing in severe hepatic trauma: experience with 36 patients. J Trauma 26:754–756
Moore FA, Moore EE, Seagraves A (1985) Non-resectional management of major hepatic trauma. Am J Surg 150:725–729
Walt AJ (1986) Discussion: packing for control of hepatic haemorrhage. J Trauma 26:741–756
Little JM, Fernandes A, Tait N (1986) Liver trauma. Aust N Z J Surg 56:613–619
Pachter HL, Spencer FC, Hofstetter SR et al (1992) Significant trends in the treatment of hepatic injuries. Experience with 411 injuries. Ann Surg 215:492–500
Caruso DM, Battistella FD, Owings JT et al (1999) Perihepatic packing of major liver injuries: complications and mortality. Arch Surg 134:958–964
Sheridan R, Driscoll D, Felsen R (1997) Packing and temporary closure in liver injury. Injury 28:711–712
Cue JI, Cryer HG, Miller FB et al (1990) Packing and planned re-exploration for hepatic and retroperitoneal haemorrhage: critical refinements of a useful technique. J Trauma 30:1007–1013
Aydin U, Yazici P, Zeytunlu M et al (2008) Is it more dangerous to perform inadequate packing? World J Emerg Surg 3:1
Calne RY, McMaster P, Pentlow BD (1979) The treatment of major liver trauma by primary packing with transfer of the patients for definitive treatment. Br J Surg 66:338–339
Carmona RH, Peck DZ, Lim RC (1984) The role of packing and planned reoperation in severe hepatic trauma. J Trauma 24:779–784
Svoboda JA, Peter ET, Dang CV et al (1982) Severe liver trauma in the face of coagulopathy. A case for temporary packing and early exploration. Am J Surg 144:717–721
Feliciano DV, Mattox KL, Burch JM et al (1986) Packing for control of hepatic haemorrhage. J Trauma 26:738–743
Knudson MM, Lim RC, Olcott EW (1994) Morbidity and mortality following major penetrating liver injuries. Arch Surg 129:256–261
Sharp KW, Locicero RJ (1992) Abdominal packing for surgically uncontrollable haemorrhage. Ann Surg 215:467–475
Parks RW, Chrysos M, Diamond T (1999) Management of liver trauma. Br J Surg 86:1121–1135
Feliciano DV, Mattox KL, Jordan GL Jr (1981) Intra-abdominal packing for control of hepatic haemorrhage: a re-appraisal. J Trauma 21:285–290
Ivatury RR, Nallathambi M, Gunduz Y et al (1986) Liver packing for uncontrolled haemorrhage: a re-appraisal. J Trauma 26:744
Balogh Z, McKinley BA, Cox CS et al (2003) Abdominal compartment syndrome: the cause or the effect of multiple organ failure? Shock 20:483–492
Meldrum DR, Moore FA, Moore EE et al (1995) Cardiopulmonary hazards of perihepatic packing for major liver injuries. Am J Surg 170:537–542
Meldrum DR, Moore FA, Moore EE et al (1997) Prospective characterisation and selective management of the abdominal compartment syndrome. Am J Surg 174:667–673
Iuchtman M, Alfici R, Sternberg A et al (2004) Mesh wrap in severe pediatric liver trauma. J Pediatr Surg 39:1485–1489
Sitzmann JV, Spector SA, Jin X et al (2005) A technique for emergency liver packing. J Gastrointest Surg 9:284–287
Strong RW, Lynch SV, Wall DR et al (1998) Anatomic resection for severe liver trauma. Surgery 123:251–257
Beal SL (1990) Fatal hepatic haemorrhage: an unresolved problem in the management of complex liver injuries. J Trauma 30:163–169
Ochsner MG, Jaffin JH, Golocovosky M et al (1993) Major hepatic trauma. Surg Clin North Am 73:337–352
Watson CJE, Calne RY, Padhani AR, Dixon AK (1991) Surgical restraint in the management of liver trauma. Br J Surg 78:1071–1075
Mays ET, Conti S, Fallahzadeh H et al (1979) Hepatic artery ligation. Surgery 86:536–543
Flint LM, Polk HC Jr (1979) Selective hepatic artery ligation: limitations and failures. J Trauma 19:319–323
Wilson RH, Moorehead RJ (1991) Hepatic trauma and its management. Injury 22:439–445
Mays ET (1976) The hazards of suturing certain wounds of the liver. Surg Gynecol Obstet 143:201–204
Lim RC Jr, Guiliano AE, Trunkey DD (1997) Postoperative treatment of patients after liver resection for trauma: a follow-up study. Arch Surg 112:429–435
Ringe B, Pichlmayr R, Ziegler H et al (1991) Management of severe hepatic trauma by two-stage total hepatectomy and subsequent liver transplantation. Surgery 109:792–795
Duane TM, Como JJ, Bochichio GV et al (2004) Re-evaluating the management and outcomes of severe blunt liver injury. J Trauma 57:494–500
Pachter HL, Spencer FC, Hofstetter SR (1983) Experience with the finger fracture technique to achieve intra-hepatic hemostasis in 75 patients with severe injuries to the liver. Ann Surg 197:771–777
Menegaux F, Langlois P, Chigot JP (1993) Severe blunt trauma of the liver: a study of mortality factors. J Trauma 35:865–869
Khaneja SC, Pizzi WF, Barie PS et al (1997) Management of penetrating juxtahepatic inferior vena cava injuries under total vascular exclusion. J Am Coll Surg 184:469–474
Baumgartner F, Scudamore C, Nair C et al (1995) Venovenous bypass for major hepatic and caval trauma. J Trauma 39:671–673
Rogers FB, Reese J, Shackford SR et al (1997) The use of venovenous bypass and total vascular isolation of the liver in the surgical management of juxtahepatic venous injuries in blunt hepatic trauma. J Trauma 43:530–533
McAnena OJ, Moore EE, Moore FA (1989) Insertion of a retrohepatic vena cava balloon shunt through the saphenofemoral junction. Am J Surg 158:463–466
Buechter KJ, Sereda D, Gomez G et al (1989) Retrohepatic vein injuries: experience with 20 cases. J Trauma 29:1698–1704
Elerding SC, Moore EE Jr (1980) Recent experience with trauma of the liver. Surg Gynecol Obstet 150:853–855
Rovito PF (1987) Atrial caval shunting in blunt hepatic vascular injury. Ann Surg 205:318–321
Beal SL, Ward RE (1989) Successful atrial caval shunting in the management of retrohepatic venous injuries. Am J Surg 158:409–413
Hollands MJ, Little JM (1990) Hepatic venous injury after blunt abdominal trauma. Surgery 197:149–152
Burch JM, Feliciano DV, Mattox KL (1988) The atriocaval shunt. Facts and fiction. Ann Surg 207:555–568
Pachter HL, Spencer FC, Hofstetter SR (1986) The management of juxtaheptatic venous injuries without an atrial-caval shunt: preliminary clinical observations. Surgery 99:569
Coln D, Creighton J, Schorn L (1980) Successful management of hepatic vein injury from blunt trauma in children. Am J Surg 140:858
Jeng LB, Hsu CH, Wang CS et al (1993) Emergent liver transplantation to salvage a hepatic avulsion injury with a disrupted suprahepatic vena cava. Arch Surg 128:1075–1077
Ginzburg E, Shatz D, Lynn M et al (1998) The role of liver transplantation in subacute trauma patients. Am Surg 64:363–364
Ringe B, Pichlmayr R (1995) Total hepatectomy and liver transplantation: a life saving procedure in patients with severe hepatic trauma. Br J Surg 82:837–839
Demirbas A, Fragulidis GP, Karatzas T et al (1997) Role of liver transplantation in the management of liver trauma. Transplant Proc 29:2848
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badger, S.A., Barclay, R., Campbell, P. et al. Management of Liver Trauma. World J Surg 33, 2522–2537 (2009). https://doi.org/10.1007/s00268-009-0215-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0215-z