Abstract
Background
In patients with primary hyperaldosteronism, solitary adrenal adenomas are an indication for surgical intervention. In contrast, adrenal hyperplasia is almost exclusively treated by drugs.
Patients and methods
In a prospective clinical study 183 patients (81 men, 102 women; age 49.6 ± 12.8 years) with Conn’s syndrome were operated on using the posterior retroperitoneoscopic approach. Tumor size ranged from 0.2 to 5.0 cm (mean 1.5 ± 0.8 cm). Final histology described a solitary adenoma in 127 patients and adrenal hyperplasia in 56 patients. Partial adrenalectomies were performed in 47 operations.
Results
The perioperative complication rate was 4%, mortality zero. In none of the cases was conversion to open surgery necessary. The mean operating time was 58 ± 32 minutes (range 20–230 minutes) and was associated with sex (p < 0.001) but not with the extent of resection (partial vs. total, p = 0.51) or with tumor size (≤1.5 vs. >1.5 cm; p = 0.43) or tumor site (p = 0.77). Median blood loss was 15 ml. Median duration of postoperative hospitalization was 4 days. After a mean follow-up of nearly 5 years, 96% of patients are normokalemic, 30% of patients are cured (normotensive without medication), and 87% showed an improvement of hypertension (normotensive without or with reduced medication). Cure of hypertension depended on the patient’s age (p < 0.001) and sex (p < 0.001), duration of hypertension (p < 0.05), and histomorphology (p < 0.001). Improvement of hypertension was not associated with any of these factors.
Conclusions
Retroperitoneoscopic removal of adrenal glands in patients with Conn’s syndrome is a safe, rapidly performed surgical procedure and can thus be considered as first choice option for treatment of both solitary adrenal adenomas and hyperplasia presenting with a clinically predominating nodule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary hyperaldosteronism (Conn’s syndrome) is caused by hyperfunctioning aldosterone-secreting adrenocortical tissue; it presents morphologically as either a unilateral solitary adenoma or (bilateral) hyperplasia with multiple nodules. It has generally been accepted that surgical removal is the treatment of choice for solitary adenomas. Thereby, hypokalemia is almost always normalized and arterial hypertension is cured or at least diminished in most cases [1]. In contrast, patients with bilateral hyperplasia require lifelong treatment with drugs as cure of Conn’s syndrome can be principally achieved only by bilateral adrenalectomy. In clinical practice, the distinction between adenoma and hyperplasia is occasionally impossible. Therefore, in some cases unilateral surgery for Conn’s syndrome incidentally removes hyperplastic tissue. In the present article, we summarize our experience gained in 183 patients with Conn’s syndrome (all adrenals were removed by the posterior retroperitoneoscopic approach) caused by either adrenocortical adenomas or asymmetrical nodular hyperplasia.
Patients and methods
In the setting of a prospective study, 183 patients (81 men, 102 women; aged 49.6 ± 12.8 years, range 19–78 years) with Conn’s syndrome were operated on endoscopically between August 1994 and January 2007. From 1994 to August 1999 the operations (n = 46) were performed at the Department of General Surgery, University Hospital of Essen and since September 1999 at the Department of Surgery and Center of Minimally Invasive Surgery, Kliniken Essen-Mitte (n = 137). There was no conventional open surgery for Conn’s syndrome during the whole period. The operations were accomplished by five surgeons assisted overall by 42 surgeons controlling the camera. Indications for adrenal surgery were based on clinical parameters (elevated blood pressure), laboratory tests (serum potassium, increased ratio of aldosterone-concentration and renin activity/concentration, plasma aldosterone >200 ng/L [2]) and imaging methods (computed tomography and/or magnetic resonance imaging). Surgery was performed when unilateral disease was expected. Venous sampling was used to confirm lateralization in 57 selected cases. All operations were performed after obtaining informed consent from the patients acknowledging that they were taking part in a prospective study.
Preoperatively, patients received antihypertensive medication with or without application of a mineralocorticoid receptor antagonist and/or potassium according to the physician’s preference. After surgery, patients were sent to the intensive care unit (ICU) or a normal ward depending on the current postoperative strategy and on patients’ concomitants. During the first years (up to 1998) patients with Conn’s syndrome were routinely treated on the ICU for one night; later, the ICU was only used for patients who had special cardiac risks. Oral intake and mobilization were initiated on the day of surgery. Postoperatively, mineralocorticoid receptor antagonists and potassium medication were generally abandoned, with other antihypertensive drugs being maintained or reduced during the hospital stay.
The posterior retroperitoneoscopic approach was performed without exception for all 183 operations, resulting in 136 total adrenalectomies and 47 partially removed adrenal glands. One patient with bilateral hyperplasia was operated on twice in our institution: right-sided partial adrenalectomy was performed 5 years after total left-sided adrenalectomy. A second patient with hyperplasia had undergone left-sided adrenalectomy at another hospital 14 years prior to the right-sided partial adrenalectomy for recurrent primary hyperaldosteronism. In the other 45 cases, partial adrenalectomy was chosen if the anatomic localization (typically lateral) of the tumor allowed safe dissection from the normal tissue. In cases of doubt, total adrenalectomy was performed, especially when more than one nodule was visualized or assumed.
The surgical technique of the posterior retroperitoneoscopic adrenalectomy has been described elsewhere [3–5]. With increasing experience, multiple modifications have been introduced during the study period. Essential changes in the surgical technique applied were the use of increased carbon dioxide pressure of ≥20 mmHg (beginning with the 50th procedure), starting the adrenal dissection at the lower adrenal pole (from procedure 56), the use of ultrasonic dissection (from procedure 119), and more recently the use of bipolar scissors (from procedure 150).
The currently applied technique is described as follows: Anesthesia is induced intravenously via peripheral venous lines and maintained as balanced anesthesia with isoflurane and semifentanil. Central venous catheter and arterial lines are usually not used for patients with hyperaldosteronism. The procedure is performed with the patient in the prone position lying on a rectangular support that allows the ventral abdominal wall to hang through. Initially, a 1.5 cm transverse incision just below the tip of the 12th rib is performed. The retroperitoneal space is reached by blunt and sharp dissection of the abdominal wall. A small cavity is prepared digitally for insertion of one 5 mm trocar 4 to 5 cm laterally underneath the 11th rib. This trocar is placed under finger guidance. In the same way, a 10 mm trocar is inserted 4 to 5 cm medial to the initial wound with a skin incision about 4 cm below the 12th rib, placing the trocar at an angle of 45° cranially. Thereby, the retroperitoneum is entered just below the 12th rib. A blunt trocar with an inflatable balloon and an adjustable sleeve is introduced into the initial incision site and blocked. The capnoretroperitoneum is created by maintaining a carbon dioxide pressure of 20 to 28 mmHg. Retroperitoneoscopy is usually performed by a 10 mm 30° endoscope, which is initially introduced via the middle trocar and after creation of the retroperitoneal space placed via the trocar nearest the spine.
Following creation of the retroperitoneal space underneath the diaphragm by pushing down the fatty tissue, the upper pole of the kidney is mobilized, thereby exposing the area of the adrenal gland. The upper pole of the kidney is retracted by one of the instruments in the medial or lateral trocar. Mobilization of the adrenal gland begins from lateral to medial on the backside of the peritoneum, identifying the lower pole of the adrenal gland.
On the right side, the adrenal gland arteries cross the vena cava medioposteriorly. These vessels are separated with a bipolar scissor. By lifting up the adrenal gland, the inferior vena cava is visualized posteriorly in its retroperitoneal-cranial segment. The short suprarenal vein thus becomes clearly visible running posterolaterally. This vessel is followed to a length of 1 cm and is divided by bipolar scissors. During the last year of the study, clips were no longer used before dissection of main adrenal veins. Preparation of the right adrenal gland is completed by lateral and cranial dissection. For left-sided retroperitoneoscopic adrenalectomy, the adrenal gland vein must be prepared in the space between the adrenal gland and the diaphragmatic branch medial to the upper pole of the kidney. After bipolar dissection of the main vein lateral of the conjunction with the diaphragmatic vein, preparation of the adrenal gland is continued medially, laterally, and cranially by lifting the gland at its venous stump. All adrenal gland manipulations are performed carefully using blunt palpation probes to prevent injury of the adrenal capsule. The completely mobilized adrenal gland is placed in a retrieval bag and pulled through the initial subcostal incision. The surgical technique for partial adrenalectomy has also been described previously [6, 7]. An endoscopic ultrasound probe has been extremely useful for identifying the margin of normal and tumor tissue. A monoplar hook or bipolar scissors is used to transect the adrenal tissue. Usually a suctioning drain is not necessary. Finally, the wounds are closed with absorbable sutures.
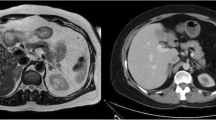
Prospective documentation included age, sex, tumor size and side, operating time (defined as skin incision to skin closure), intraoperative blood loss, intraoperative and postoperative complications, and duration of hospitalization. Retrospectively, duration and type of preoperative and postoperative medication, preoperative diagnostic tools, and adrenal morphology were evaluated. The latter included histopathologic analysis of the adrenals removed during the present study. Adrenal glands with only a singular tumor were defined as “solitary adenomas”. If more than one lesion could be identified, “nodular hyperplasia” was assumed (Fig. 1).
For recruiting long-term results, the patients, their general practitioners, and in most cases continuously caring endocrinologists were contacted by phone, or the patients were personally seen for clinical follow-up. If possible, data were recorded as mean values ± standard deviation. For group comparison, the Mann-Whitney U-test and Fisher’s exact test were performed as indicated. Significance was accepted at p < 0.05. Statistical analyses were performed by a commercially available program for personal computers (StatView 5.0; Abacus Concepts, Berkeley, CA, USA).
Results
None of the patients of the present series died from the surgical procedure, nor were there any appreciable complications intraoperatively. All retroperitoneoscopic procedures were completed by endoscopy without the need for conversion to open or laparoscopic approaches. Postoperative complications were as follows: four segmental relaxations of the abdominal wall, two cases of hypoesthesia of the abdominal wall, one retroperitoneal hematoma, and one myocardial infarction. The mean intraoperative blood loss was 15 ml (range 0–100 ml) without requirement of blood transfusions. Any aftereffects of a subcostal nerve lesion (relaxation, hypoesthesia) disappeared within 6 months with one exception.
The mean operating time for posterior retroperitoneoscopic adrenal surgery (n = 183) was 58 ± 32 minutes (range 20–230 minutes) with a reduction from 74 ± 34 minutes for the first half of the study to 42 ± 18 minutes for the second half (p < 0.001). The learning curve is demonstrated in Figure 2. There were no major differences between right (n = 71) and left (n = 112) adrenalectomies (58 ± 34 vs. 59 ± 31 minutes, p = 0.77), between partial (n = 47) and total (n = 136) adrenalectomies (59 ± 41 vs. 58 ± 28 minutes, p = 0.51), or for small and large tumors [≤1.5 cm; (n = 116) vs. >1.5 cm (n = 67): 57 ± 33 vs. 55 ± 29 minutes; p = 0.43]. In contrast, the patients’ sex [male (n = 81) vs. female (n = 102): 67 ± 36 vs. 51 ± 27 minutes; p < 0.001] affected the operating time. The mean tumor size was statistically smaller in men (1.2 ± 0.6 cm; n = 82) than in women (1.8 ± 0.9; n = 101) (p < 0.001); the mean blood loss did not differ significantly, with slightly higher values in men (20 ± 38 vs. 10 ± 17 ml; p = 0.08). The median duration of hospitalization was 4 days (range 1–13 days). Observation in the ICU was needed after 28 of 183 procedures (15%). Following the last 100 operations, only one patient had to be treated under ICU conditions.
Histologic examination revealed solitary adenomas in 127 patients and nodular hyperplasia in 56. Patients with an adenoma were significantly younger than those with nodular hyperplasia (47.8 ± 13.1 vs. 53.8 ± 10.9 years, p < 0.05). Hyperplastic tissue was more often removed on the left side (n = 44) than on the right side (n = 12). The size of tumors ranged from 0.2 to 5.0 cm (mean 1.5 ± 0.8 cm); 28 adrenal lesions were >2 cm (15%). The mean size of solitary adenomas (n = 127) was 1.6 ± 0.8 cm, thus exceeding the largest nodules in nodular hyperplasia (n = 56; 1.3 ± 0.8 cm; p < 0.01).
During follow-up, four patients died (two patients from myocardial infarction and two from gastric and bronchial carcinoma). A total of 19 patients were lost to follow-up owing to a change of address. Therefore, follow-up (mean 59 ± 40 months; range 6–159 months) was completed in 160 patients (87%). In this group (75 men, 85 women), the duration of preoperative hypertension was 10.2 ± 8.9 years (range 1–40 years). Local tumor control has been achieved in all these patients, including those who underwent partial adrenalectomy. Normokalemia (without specific mediation) was found in 155 patients (96%). Cure of hypertension (defined as systolic pressure ≤140 mmHg without medication) was seen in 48 patients (30%) and a partial response (defined as systolic pressure ≤140 mmHg with reduced medication) in 91 patients (57%). Hypertension persisted in 21 patients (Table 1). The duration of preoperative hypertension was significantly shorter in cured patients (7.3 ± 7.1 vs. 11.6 ± 9.4 years; p < 0.01). In an univariate analysis, cure (normotension without medication) depended on the duration of disease [≤5 years (n = 62) vs. >5 years (n = 89): 40% vs. 24%; p < 0.05], patients’ age at surgery [≤50 years (n = 88) vs. >50 years (n = 72): 42% vs. 15%; p < 0.001], patients’ sex [male (n = 75) vs. female (n = 85): 16% vs. 42%; p < 0.001], and histomorphology [solitary adenoma (n = 111) vs. nodular hyperplasia (n = 49): 39% vs. 10%; p < 0.001]. In the subgroup of patients older than 50 years at the time of operation, cure was not related to duration of disease [(≤5 years (n = 11) vs. ≥5 years (n = 56; p < 0.27)] or histomorphology [solitary adenoma (n = 41) vs. nodular hyperplasia (n = 31): 24% vs. 7%; p = 0.06];it was related to sex [male (n = 38) vs. female (n = 34): 5% vs. 29%; p < 0.01]. The cure rate was significantly higher following partial adrenalectomy (n = 37) than after total adrenalectomy (n = 123): 57% vs. 22% (p < 0.001).
Improvement of blood pressure—defined as the “cure group” and “partial response group” combined (n = 139)—did not depend on the patients’ age at surgery [≤50 years (n = 88) vs. >50 years (n = 72): 88% vs. 86%, p = 0.82], duration of disease [(≤5 years (n = 62) vs. ≥5 years (n = 89), p = 0.46)], or histomorphology [solitary adenoma (n = 111) vs. nodular hyperplasia (n = 49): 90% vs. 80%, p = 0.08]. In the subgroup of patients older than 50 years, improvement of blood pressure by adrenal surgery marginally depended on the duration of disease [(≤5 years (n = 19) vs. ≥5 years (n = 48; p = 0.05)] and not on sex [male (n = 38) vs. female (n = 34): 84% vs. 88%, p = 0.74] or histomorphology [solitary adenoma (n = 41) vs. nodular hyperplasia (n = 31): 90% vs. 81%, p = 0.31]. No difference in the rate of blood pressure improvement was found in patients with partial adrenalectomy (n = 37) versus those with total adrenalectomy (92% vs. 85%, p = 0.41).
In one patient with concomitant adrenal hyperplasia and a predominant adrenal tumor, clinical symptoms disappeared after removal of the left adrenal gland. Five years later she redeveloped hypokalemia and elevated blood pressure. Computed tomography revealed a tumor in the right adrenal gland, and the tumor was removed by partial adrenalectomy. After the second operation, stable normokalemia and normal blood pressure with medication have been achieved and remain so as of this writing (follow-up >3.5 years). In the second patient contralateral partial adrenalectomy 14 years after the first operation resulted in normokalemia and normal blood pressure with medication (follow-up >1 year).
Discussion
This study describes our experience with minimally invasive surgical treatment in patients with primary hyperaldosteronism. Our surgical approach was, without exception, the posterior retroperitoneoscopic method, which we have routinely applied for all types of benign adrenal tumors since 1994. As outlined in previous publications [3–5], this method can be applied with maximum safety. Similar results have been presented by Bonjer et al. [8], Sasagawa et al. [9], and Zhang et al. [10], who also use retroperitoneoscopic approaches, as well as by Gagner et al. and Henry et al., who prefer the lateral laparoscopic technique [11, 12]. Overall, these data clearly demonstrate that in experienced hands the laparoscopic and retroperitoneoscopic techniques are safe options for removal of adrenal tumors. Comparative studies have suggested that retroperitoneoscopic methods may be more rapid and less painful, but the only prospective randomized study, by Fernandez-Cruz et al. [13], comparing lateral laparoscopic and retroperitoneoscopic approaches did not demonstrate any significant differences. Nevertheless, the mean operating time of our series using the retroperitoneoscopic route exclusively proved to be definitively shorter than that in all other comparable studies using the laparoscopic approach routinely [11, 14–16]. This emphasizes the major advantage of the retroperitoneoscopic technique, which allows direct access to the adrenal glands, a fact that has recently been stressed by others [17].
Over the last 13 years, our surgical technique of retroperitoneoscopic adrenalectomy has been substantially improved stepwise. Particularly the use of increased carbon dioxide pressures and in more recent years the dissection with modern devices, such as the harmonic scalpel or bipolar scissors, proved to be relevant components of the technical evolution. High gas pressure in the retroperitoneum does not influence the stability of the circulation in patients in prone position [18]. Therefore, we generously inflate carbon dioxide up to values of 28 mmHg with the desirable side effect of prohibiting venous bleeding. Even in the case of injury to large vessels (e.g., adrenal or caval vein), no relevant bleeding occurs. This certainly contributes to the negligible blood loss in our series (<20 ml). For 2 years we have dissected the adrenal vessels, including the main adrenal veins, without using vascular clips, thereby avoiding the risk of bleeding by unintentional removal of clips. The new devices also allow safe transection of adrenal tissue during partial adrenalectomy. Concerning the operating time, we have achieved with the posterior retroperitoneoscopic adrenalectomy the same levels as have been achieved for laparoscopic cholecystectomy or appendectomy in our institution. Therefore, it can be categorized as a relatively easy minimally invasive procedure especially in patients with Conn’s syndrome. Patients suffering from Conn’s syndrome are usually slim (compared to those with Cushing’s disease or syndrome) and present with small tumors. Additionally, complications are rare (the rate in our series was 4%, with only one major postoperative complication in 183 patients), providing a low threshold for indicating surgery.
Partial adrenalectomy has become an important option in endoscopic adrenal surgery. After our initial description in 1995 [3] and following our midterm analysis of 22 patients [6], various groups have adopted this concept [9, 15, 19–22]. With one exception [23], the authors reported on “biochemically” successful operations. Ishidoya et al., however, noted two cases of persistent hypertension with high aldosterone plasma levels among 29 partial adrenalectomies, whereas recovery of hypertension was achieved in all 63 patients with total adrenalectomy [23]. In contrast, our study demonstrates that clinical cure and improvement rates of blood pressure are at least as high after partial adrenalectomy as after total adrenalectomy. The finding of a statistically higher cure rate after partial adrenalectomy seems to be biased by the selection criteria, as only distinct unilocular nodules were removed by partial adrenalectomy. The principal precondition for successful adrenal resection in patients with Conn’s syndrome is the clear differentiation of neoplastic from normal adrenal tissue. This is substantially guaranteed by the magnification offered by the endoscopic equipment (recently of high-definition quality) including laparoscopic ultrasound probes, allowing manipulation with increased precision compared to that during conventional adrenal procedures.
In contrast to other functioning adrenal tumors, such as adenomas in Cushing’s syndrome or pheochromocytomas, cure rates for clinical symptoms are lower in patients with primary hyperaldosteronism. Although hypokalemia can be normalized in 96% to 100% of patients [8, 14–16, 24–26], hypertension remains in approximately 50% of cases. Owing to a broad range of definitions of “cure” for elevated blood pressure (<101 mmHg diastolic [27] to <140/90 mmHg [28] with or without medication), cure rates range from 19% to 88% [8, 14–16, 24–31]. In our present study, 48 of 160 patients (30%) with a mean long-term follow-up of 5 years were cured of hypertension. Overall, however, blood pressure could be improved in 87% of patients. As already demonstrated by others [28, 30, 31], risk factors for cure failure are patient’s age and sex as well as the preoperative duration of the hypertension. Persistent hypertension may also be influenced by the family history [28, 30] and response to spironolactone treatment [30, 32, 33].
Primary hyperaldosteronism can be caused by a variety of morphologically different adrenocortical lesions. In addition to “pure” solitary adenoma or diffuse micronodular hyperplasia, these two conditions can be found admixed (Fig. 1). In this study, we made a clear-cut distinction between solitary adenoma and multifocal adrenal lesions. The latter were pragmatically named “hyperplasia”, although we cannot rule out the existence of more than one adenoma. Surprisingly few studies include histopathologic data in the analysis of clinical long-term outcomes in patients with Conn’s syndrome. Proye and coworkers found no relation between outcome and the histology of the adrenal specimen [30]. In contrast, Obara et al. [34] demonstrated that persistent hypertension developed more frequently in patients with multiple adenomas or an adenoma associated with additional nodules than in patients with a solitary adenoma. Celen et al. [33] associated nodular hyperplasia with no cure, whereas this relation was not demonstrable in cases of persistent hypertension caused by adenomas combined with additional nodules. In the presented series, cure of hypertension (normotension without medication: blood pressure ≤140/90 mmHg) was significantly associated with solitary adenomas. Nevertheless, improvement of hypertension (normotension without or with reduced medication: blood pressure ≤140/90 mmHg) was achieved in 80% of patients with multiple adrenal nodules. Because the latter group of patients presented with a predominant adrenal tumor visible on imaging, we concluded that patients with Conn’s syndrome caused by hyperplastic adrenal glands with a predominant nodule commonly benefit from adrenal surgery.
Based on our experience, it remains obscure whether Conn’s syndrome can be caused by unilateral hyperplasia. Although we could show that cure of hypertension and normokalemia occurred in patients with hyperplastic lesions, it cannot be presumed that the contralateral adrenal gland is lacking hyperplastic lesions. The two patients described in this study with proven bilateral hyperplasia underwent contralateral adrenal surgery after 5 and 14 years, respectively, indicating the need for long-term follow-up. Therefore, the recently published study by Goh et al. [35] seems critical. The authors collected data from 30 Conn patients in the literature with assumed “unilateral hyperplasia.” The mean follow-up of this group was only 12 months, with two patients having been followed more than 5 years. From our point of view, these data are not sufficient to allow any conclusion concerning the clinical course of those adrenal lesions.
Conclusions
We present one of the largest series of patients with surgical treated primary hyperaldosteronism. Following removal by a safe, rapid posterior retroperitoneoscopic approach, long-term cure or improvement of hypertension was achieved in 30% and 87%, respectively. Apparently, the subgroup of patients with adrenal hyperplasia causing Conn’s syndrome also benefits from adrenal surgery; thus the disease does not represent a contraindication to surgery.
References
Young WF (2007) Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) 66:607–618
Unger N, Lopez Schmidt I, Pitt C et al (2004) Comparison of active renin concentration and plasma renin activity for the diagnosis of primary hyperaldosteronism in patients with an adrenal mass. Eur J Endocrinol 150:517–523
Walz MK, Peitgen K, Hoermann R et al (1996) Posterior retroperitoneoscopy as a new minimally invasive approach for adrenalectomy: results of 30 adrenalectomies in 27 patients. World J Surg 20:769–774
Walz MK, Peitgen K, Walz MV et al (2001) Posterior retroperitoneoscopic adrenalectomy: lessons learned within five years. World J Surg 25:728–734
Walz MK, Alesina PF, Wenger FA et al (2006) Posterior retroperitoneoscopic adrenalectomy—results of 560 procedures in 520 patients. Surgery 140:943–948; discussion 948–950
Walz MK, Peitgen K, Saller B et al (1998) Subtotal adrenalectomy by the posterior retroperitoneoscopic approach. World J Surg 22:621–626
Walz MK, Peitgen K, Diesing D et al (2004) Partial versus total adrenalectomy by the posterior retroperitoneoscopic approach: early and long-term results of 325 consecutive procedures in primary adrenal neoplasias. World J Surg 28:1323–1329
Bonjer HJ, Sorm V, Berends FJ et al (2000) Endoscopic retroperitoneal adrenalectomy: lessons learned from 111 consecutive cases. Ann Surg 232:796–803
Sasagawa I, Suzuki Y, Itoh K et al (2003) Posterior retroperitoneoscopic partial adrenalectomy: clinical experience in 47 procedures. Eur Urol 43:381–385
Zhang X, He H, Chen Z et al (2004) [Retroperitoneal laparoscopic management of primary aldosteronism with report of 130 cases]. Zhonghua Wai Ke Za Zhi 42:1093–1095
Gagner M, Pomp A, Heniford BT et al (1997) Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg 226:238–246
Henry JF, Sebag F, Iacobone M et al (2002) [Lessons learned from 274 laparoscopic adrenalectomies]. Ann Chir 127:512–519
Fernandez-Cruz L, Saenz A, Benarroch G et al (1996) Laparoscopic unilateral and bilateral adrenalectomy for Cushing’s syndrome: transperitoneal and retroperitoneal approaches. Ann Surg 224:727–734; discussion 734–726
Rossi H, Kim A, Prinz RA (2002) Primary hyperaldosteronism in the era of laparoscopic adrenalectomy. Am Surg 68:253–256; discussion 256–257
Meria P, Kempf BF, Hermieu JF et al (2003) Laparoscopic management of primary hyperaldosteronism: clinical experience with 212 cases. J Urol 169:32–35
Goh BK, Tan YH, Yip SK et al (2004) Outcome of patients undergoing laparoscopic adrenalectomy for primary hyperaldosteronism. JSLS 8:320–325
Zhang X, Fu B, Lang B et al (2007) Technique of anatomical retroperitoneoscopic adrenalectomy with report of 800 cases. J Urol 177:1254–1257
Giebler RM, Walz MK, Peitgen K et al (1996) Hemodynamic changes after retroperitoneal CO2 insufflation for posterior retroperitoneoscopic adrenalectomy. Anesth Analg 82:827–831
Imai T, Tanaka Y, Kikumori T et al (1999) Laparoscopic partial adrenalectomy. Surg Endosc 13:343–345
Kok KY, Yapp SK (2002) Laparoscopic adrenal-sparing surgery for primary hyperaldosteronism due to aldosterone-producing adenoma. Surg Endosc 16:108–111
Ikeda Y, Takami H, Sasaki Y et al (2003) Is laparoscopic partial or cortical-sparing adrenalectomy worthwile? Eur Surg 35:89–92
Jeschke K, Janetschek G, Peschel R et al (2003) Laparoscopic partial adrenalectomy in patients with aldosterone-producing adenomas: indications, technique, and results. Urology 61:69–72
Ishidoya S, Ito A, Sakai K et al (2005) Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol 174:40–43
Shen WT, Lim RC, Siperstein AE et al (1999) Laparoscopic vs open adrenalectomy for the treatment of primary hyperaldosteronism. Arch Surg 134:628–631; discussion 631–622
Brunt LM, Moley JF, Doherty GM et al (2001) Outcomes analysis in patients undergoing laparoscopic adrenalectomy for hormonally active adrenal tumors. Surgery 130:629–635
Nwariaku FE, Miller BS, Auchus R et al (2006) Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg 141:497–502; discussion 502–493
Favia G, Lumachi F, Scarpa V et al (1992) Adrenalectomy in primary aldosteronism: a long-term follow-up study in 52 patients. World J Surg 16:680–683
Sawka AM, Young WF, Thompson GB et al (2001) Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 135:258–261
Stowasser M, Klemm SA, Tunny TJ et al (1994) Response to unilateral adrenalectomy for aldosterone-producing adenoma: effect of potassium levels and angiotensin responsiveness. Clin Exp Pharmacol Physiol 21:319–322
Proye CA, Mulliez EA, Carnaille BM et al (1998) Essential hypertension: first reason for persistent hypertension after unilateral adrenalectomy for primary aldosteronism? Surgery 124:1128–1133
Gockel I, Heintz A, Polta M et al (2007) Long-term results of endoscopic adrenalectomy for Conn’s syndrome. Am Surg 73:174–180
Lo CY, Tam PC, Kung AW et al (1996) Primary aldosteronism: results of surgical treatment. Ann Surg 224:125–130
Celen O, O’Brien MJ, Melby JC et al (1996) Factors influencing outcome of surgery for primary aldosteronism. Arch Surg 131:646–650
Obara T, Ito Y, Okamoto T et al (1992) Risk factors associated with postoperative persistent hypertension in patients with primary aldosteronism. Surgery 112:987–993
Goh BK, Tan YH, Chang KT et al (2007) Primary hyperaldosteronism secondary to unilateral adrenal hyperplasia: an unusual cause of surgically correctable hypertension: a review of 30 cases. World J Surg 31:72–79
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walz, M.K., Gwosdz, R., Levin, S.L. et al. Retroperitoneoscopic Adrenalectomy in Conn’s Syndrome Caused by Adrenal Adenomas or Nodular Hyperplasia. World J Surg 32, 847–853 (2008). https://doi.org/10.1007/s00268-008-9513-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-008-9513-0