Abstract
Background
Cushing’s syndrome (CS), due to multiple etiologies, is a disorder associated with the ravages of cortisol excess. The purpose of this review article is to provide a historical synopsis of surgery for CS, review a recent 10-year period of operative management at a tertiary care facility, and to outline a practical approach to diagnosis and management.
Materials and Methods
From 1996 to 2005, 298 patients underwent 322 operative procedures for CS at Mayo Clinic, Rochester, Minnesota. A retrospective chart review was carried out. Data was gathered regarding demographics, preoperative assessment, procedures performed, and outcomes. Data are presented as counts and percentages. Five-year survival rates were calculated where applicable by the Kaplan-Meier method. Statistical analysis was carried out with SAS, version 9 (SAS Institute, Inc., Cary, NC).
Results
Two-hundred thirty-one patients (78%) had ACTH-dependent CS and 67 patients (22%) had ACTH-independent CS. One-hundred ninety-six patients (66%) had pituitary-dependent CS and 35 patients (12%) had ectopic ACTH syndrome. Fifty-four patients (18%) had cortisol-secreting adenomas, 10 patients (3%) had cortisol-producing adrenocortical carcinomas, and 1% had other causes. Cure rates for first time pituitary operations (transsphenoidal, sublabial, and endonasal) were 80% and 55% for reoperations. Most benign adrenal processes could be managed laparoscopically. Five-year survival rates (all causes) were 90%, 51%, and 23% for adrenocortical adenomas, ectopic ACTH syndrome, and adrenocortical carcinomas, respectively.
Conclusions
Surgery for CS is highly successful for pituitary-dependent CS and most ACTH-independent adrenal causes. Bilateral total adrenalectomy can also provide effective palliation from the ravages of hypercortisolism in patients with ectopic ACTH syndrome and for those who have failed transsphenoidal surgery. Unfortunately, to date, adrenocortical carcinomas are rarely cured. Future successes with this disease will likely depend on a better understanding of tumor biology, more effective adjuvant therapies and earlier detection. Clearly, IPSS, advances in cross-sectional imaging, along with developments in transsphenoidal and laparoscopic surgery, have had the greatest impact on today’s management of the complex patient with CS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
History of pituitary and adrenal surgery
The anatomist Bartolomeo Eustachius first described the “glandulae renibus incumbents” (glands lying on the kidneys) in 1552 [1,2]. Their function created much speculation in the nineteenth century when it was suggested that they might secrete a “peculiar matter into the blood” [1]. In 1845, the adrenal cortex and medulla were first described by Emil Huschke, an anatomist, at Jena [1]. Ten years later, Thomas Addison described 11 patients with clinical features attributable to adrenal insufficiency with anemia, debility, a weak heart, irritable stomach, and changes in skin color [3]. Autopsy findings demonstrated destruction of the adrenal glands by tuberculosis, metastatic cancer, or simply atrophy [3, 4]. It was Charles Edouard Brown-Séquard who concluded that the adrenal glands were essential for life, based on the fatal consequences of bilateral adrenalectomy when carried out in the animal model [5]. William Osler was the first to treat adrenal insufficiency in 1896, albeit unsuccessfully, in a human, using an extract of pig adrenal [6].
Abel isolated epinephrine in 1897 [7], which was used unsuccessfully at the Mayo Clinic in 1920 to treat a patient who developed Addison’s disease following radical nephrectomy [8]. It soon became clear that Addison’s disease was a disease of the adrenal cortex and not the medulla. In the early twentieth century, cortical extracts were used successfully in adrenalectomized animals [1]. In the 1940s, cortisone was isolated and synthesized as a result of work led by Kendall and Hench of the Mayo Clinic [9] and Tadeus Reichstein of Basle [10]. These men shared the Nobel Prize in Medicine in 1950 for describing the anti-inflammatory effects of cortisone in patients with rheumatoid arthritis. The discovery of aldosterone followed shortly thereafter by Tait and Simpson in London; its action found to exert influence over electrolyte fluxes [11].
The subsequent development of various techniques to measure urinary and plasma steroids paved the way for diagnosing CS, Addison’s disease, Conn’s syndrome, congenital adrenal hyperplasia, and virilizing/feminizing syndromes [1].
Adrenal imaging began with rather insensitive plain abdominal radiographs, followed by retroperitoneal gas insufflation [12], pyelography, caval venous sampling, phlebography, and early selective adrenal venous sampling [13, 14]. Scintigraphy developed by Beierwaltes and colleagues at the University of Michigan [15] employed radiolabeled cholesterol and was found to be useful to diagnose hyperplasia and functioning tumors of the adrenal cortex. Cross-sectional imaging with computed tomography (CT) was first used in 1975 at Mayo Clinic [16], and along with magnetic resonance imaging (MRI), remains, at present, the mainstay of adrenal imaging.
No adrenal tumor was accurately diagnosed preoperatively prior to 1905. Knowsley Thornton is credited with performing the first successful adrenalectomy in London in 1889 for a 20 lb, androgen-secreting, adrenocortical carcinoma [17]. Although Harvey Cushing had defined the fundamental role of the anterior pituitary basophil cell in Cushing’s disease [18], others soon recognized that the development of CS required the adrenal cortex.
Trans-sphenoidal approaches to pituitary tumors were first introduced by Schloffer in 1907 via a lateral rhinotomy [19]. Cushing modified this approach at the Johns Hopkins Hospital in 1909 [20, 21]. This patient (“my first one, HC”—as noted in the hospital record) with acromegaly had been referred to Cushing by Drs. Henry Plummer and Charles Mayo of the Mayo Clinic [21]. This began a long friendship between Cushing and the brothers Mayo, which ended with their deaths 30 years later. Cushing’s final publication was a tribute to the Mayo Brothers, published in Science, in 1939, shortly before his own death the same year [22].
After his first transsphenoidal operation, Cushing adopted a sublabial–trans-sphenoidal route to the sella, modified from that previously described by both Halstead and Kocher [21, 23]. By 1927, with his mastery of intracranial surgery, Cushing and all others abandoned the trans-sphenoidal approach for subfrontal or transfrontal routes to treat pituitary tumors. Most tumors during this era were large, and optic nerve decompression was more successful and complete via a transfrontal craniotomy [21]. The resurrection of trans-sphenoidal surgery awaited the development of the operating microscopic and the image intensifier, as well as the enthusiasm and skill of Norman Dott, Gerard Guiot, and Jules Hardy, who revitalized trans-sphenoidal adenomectomy during the latter half of the twentieth century, in the treatment of the more typical micro- and macroadenomas involving the anterior hypophysis [21]. The endoscopic endonasal approach to the sella gained widespread acceptance in the 1990s with advances in minimally invasive surgery and endoscopic sinus surgery.
Walters, in 1934, published early results of 10 adrenalectomies for CS. Even with subtotal adrenalectomy there was a 30% mortality rate [24]. Once cortisone became available the outlook for patients undergoing adrenalectomy for CS changed dramatically. The first reported perioperative use of cortisone for patients undergoing adrenalectomy for CS was in 1949, by Priestley, at Mayo Clinic [25]. The mortality rate fell to zero for the next 18 reported cases [25]. This practice had a profound effect on not only for the outcome of adrenalectomy for CS but also for pituitary adenomectomy in the ensuing years.
The first adrenalectomy was performed through a T-shaped subcostal incision, as described by Lagenbüch for cholecystectomy [26]. Subsequently, anterior transperitoneal, lateral, and retroperitoneal incisions were all used, and many of these approaches included resection of the 11th and 12th ribs [26]. In 1932, Lennox Broster of London devised a transpleural, transdiaphragmatic approach via a long, posterior, intercostal incision [27]. In 1927, Charles Mayo used a flank incision when performing the first adrenalectomy in the United States for a pheochromocytoma [28]. Anterior midline or bilateral subcostal incisions permitted full intra-abdominal exploration, and they remain valuable today for the management of large malignant adrenal tumors. Hugh Young, from Johns Hopkins, devised a posterior approach, removing the 12th rib (dorsal lumbotomy), permitting simultaneous exposure of both glands in the case of ACTH-dependent CS [29]. For years to come, this would be the standard approach for most small benign adrenocortical tumors, as well as for bilateral hyperplasia, as the complication rates were lower than had been reported with open anterior approaches (less blood loss, fewer pulmonary complications, and shorter period of ileus) [30]. This approach remained unchallenged until 1992, when Gagner described the transperitoneal laparoscopic approach for adrenalectomy [31]. This approach has been clearly shown to be associated with less pain, shorter hospitalization, quicker recovery and better cosmesis when compared to the open posterior approach [32–41]. In addition, long-term morbidity associated with subcostal nerve injury all but disappeared. More recently, Walz et al. [42–44] have demonstrated the safety and efficacy of the posterior retroperitoneoscopic route, thus avoiding the peritoneal cavity altogether in cases where intra-abdominal adhesions abound. In addition this approach avoids the need for repositioning in the case of bilateral hyperplasia. It is technically demanding, however, and it is sometimes impossible to carry out in the superobese patient.
Mayo clinic experience (1996–2005)
Methods
We reviewed 298 consecutive patients undergoing 322 operations for CS at Mayo Clinic, Rochester, Minnesota, from 1996 to 2005. This study was undertaken in accordance with the regulations of the Institutional Review Board of the Mayo Foundation, and the anonymous data of patients who wished to be excluded from research studies were not included. Data were gathered regarding demographics, preoperative assessment, procedures performed, and outcomes. Long-term follow-up was obtained via mailed questionnaire. Data are presented as counts and percentages, unless otherwise specified. For patients with adrenocortical carcinoma, ectopic ACTH syndrome, and adrenocortical adenomas, 5-year survival rates were calculated by the Kaplan-Meier method. Statistical analysis was carried out with SAS, version 9. (SAS Institute, Inc. Cary, NC).
Results
Overview
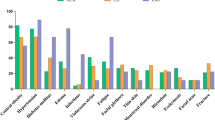
From 1996 to 2005, 298 patients underwent 322 operative procedures at Mayo Clinic for CS. The additional 24 operations include patients that had a second pituitary operation or bilateral adrenalectomy for persistent/recurrent pituitary CS. Two-hundred thirty-one patients (78%) had ACTH-dependent CS, and 67 patients (22%) had ACTH-independent CS. One-hundred ninety-six patients (66%) had pituitary-dependent CS, and 35 patients (12%) had ectopic ACTH syndrome. Fifty-four patients (18%) had solitary cortisol-secreting adenomas, 10 patients (3%) had cortisol-producing adrenocortical carcinomas, and 1% had ACTH-independent macronodular adrenal hyperplasia (AIMAH), primary pigmented nodular adrenal disease (PPNAD), and bilateral cortisol-secreting adenomas (Fig. 1). Females predominated among patients with pituitary-dependent CS (79%), adrenocortical adenomas (81%), and adrenocortical carcinomas (90%). Among patients with ectopic ACTH syndrome, males were seen more frequently (54%).
Cushing’s disease
One-hundred seventy-two patients underwent pituitary surgery for Cushing’s disease: 147 (86%) first-time operations, 23 (13%) reoperations, and 2 patients (1%) underwent trans-sphenoidal surgery after pituitary irradiation. Two patients (1%) underwent craniotomy (both reoperations). The remainder underwent either endoscopic endonasal (69%) or sublabial trans-sphenoidal (30%) pituitary surgery. Eighty percent of patients undergoing first-time operations were cured, whereas only 55% of reoperations were successful (Fig. 2).
The 30-day operative mortality was zero. Complications occurred in 20% of the endoscopic endonasal group, compared to 24% of the sublabial trans-sphenoidal group. The most common complications included transient diabetes insipidus, the syndrome of inappropriate antidiuretic hormone, and cerebrospinal fluid leak (most of which were transient, not requiring reoperation).
Ectopic ACTH syndrome
Thirty-five patients underwent bilateral adrenalectomy (74% laparoscopic, 26% open) for ectopic ACTH syndrome. In 21 patients (60%), the primary site could be identified but was either unresectable or associated with metastatic disease. In 14 patients (40%), the primary site could not be found. When identified, the most common primary sites included lung carcinoids and islet cell carcinomas. Five-year survival following bilateral (laparoscopic) adrenalectomy was 51.3% (95% CI: 32.6,80.8).
Cortisol-secreting adenomas, AIMAH and PPNAD
Fifty-four patients underwent adrenalectomy for single cortisol-secreting adenomas (91% laparoscopic, 9% open). Seventeen percent were cortical-sparing procedures. No deaths occurred, and transient complications were noted in 8%. Five-year survival (all causes) was 90% (95% CI: 79.3,100).
Patients with ACTH-independent macronodular adrenal hyperplasia (AIMAH), primary pigmented nodular adrenocortical disease (PPNAD), and bilateral cortisol-secreting adenomas were treated successfully with bilateral total adrenalectomy (AIMAH, PPNAD) or bilateral cortical-sparing adrenalectomy.
Ten patients were treated during this time period for cortisol-secreting adrenocortical carcinoma (nine women, one man) at a mean age of 46 years. Eight operations were performed in an open, anterior fashion. Two tumors, presenting as well-circumscribed incidentalomas, were removed laparoscopically. Five-year survival was 23% (95% CI: 6.8,76.8).
Cushing’s syndrome overview
Hypothalamic–pituitary–adrenal (HPA) axis
Glucocorticoid synthesis and secretion by way of the adrenal cortex (zona fasciculata) is stimulated by ACTH from anterior pituitary corticotroph cells. ACTH secretion is regulated by multiple hypothalamic factors. Corticotropin-releasing hormone (CRH), in conjunction with vasopressin and oxytocin from the neurohypophysis, stimulate ACTH production. Corticotropin-releasing hormone neurons are controlled by afferent pathways conveying both sensory and cognitive information. Hypothalamic–pituitary–adrenal activity increases in response to both stress and circadian regulation. Glucocorticoids ultimately regulate their own secretion by feedback inhibition at multiple levels within the central nervous system (HPA axis). Other immune and metabolic signals also provide additional feedback effecting glucocorticoid secretion [45].
ACTH biosynthesis
ACTH is the principal hormone stimulating adrenal glucocorticoid synthesis and secretion. ACTH has 39 amino acids but is synthesized in the anterior pituitary from the larger 241-amino acid precursor, pro-opiomelanocortin (POMC), which is cleaved to form β-lipoprotein and pro-ACTH. Pro-ACTH is further cleaved to form the bioactive molecule, ACTH. Melanocyte-stimulating hormones (MSH α, β, γ) are also cleaved products from POMC. The increased pigmentation characteristic of Addison’s disease, once thought to be related to increased α-MSH levels, is most likely due to accelerated binding of ACTH to the melanocortin-2 receptor at increased concentrations [46, 47].
Cortisol biosynthesis
Glucocorticoids are secreted daily in relatively high amounts (cortisol: 10–20 mg/day) from the zona fasciculate, where the enzymes specific for cortisol synthesis are expressed. Biosynthesis and secretion are under the control of ACTH [46]. The process begins with the steroidogenic acute regulatory (StAR) protein-mediated uptake of cholesterol into the mitochondria of adrenocortical cells and ends with 11β-hydroxylase conversion of 11-deoxycortisol to cortisol. Over 90% of circulating cortisol is bound to the α2-globulin, cortisol-binding globulin (CBG). The major route of cortisol metabolism results from the conversion of cortisol to cortisone via the enzymatic activity of 11β-hydroxysteroid dehydrogenase in the distal nephrons. The free cortisol excreted via the kidneys represents only 1% of the total cortisol secretion rate [46,47].
Effects of glucocorticoids in health and disease
Glucocorticoids carry out their activities through a series of events that follow nuclear receptor binding throughout the body. Glucocorticoids increase blood glucose concentrations through their action on glycogen, protein, and lipid metabolism. They stimulate adipocyte differentiation and visceral fat deposition. They induce insulin resistance in muscle and result in catabolic changes in muscle, skin, and connective tissue. Muscle protein synthesis is reduced. Osteoblast function is inhibited by glucocorticoids; thus the osteoporosis and osteopenia associated with glucocorticoid excess. Glucocorticoids increase blood pressure through a variety of mechanisms involving the kidney and peripheral vasculature. They increase the sensitivity of vascular smooth muscle to both catecholamines and angiotensin II, while reducing nitric-oxide-mediated endothelial dilatation. In the kidney, excess glucocorticoids can act in the distal nephron by stimulating the mineralocorticoid receptor, resulting in sodium retention and potassium loss. Glucocorticoids also stimulate angiotensinogen synthesis. Glucocorticoids suppress immunologic responses; thus they serve important therapeutic roles as anti-inflammatory and immunosuppressive agents. They inhibit actions of both T and B-cell lymphocytes. The brain is an important target for glucocorticoids, as is the eye, where they increase intraocular pressure. Glucocorticoid excess is associated with depression, psychosis, and euphoria. Chronic administration can lead to peptic ulcer disease and pancreatitis. Glucocorticoids inhibit linear skeletal growth in children. They also suppress luteinizing hormone (LH), follicle-stimulating hormone, thyroid-stimulating hormone release, and growth hormone secretion [46, 47].
Classification of endogenous hypercortisolism
The most common cause of CS is exogenous administration of glucocorticoids for the myriad of anti-inflammatory, antineoplastic, and immunosuppressive roles, for which they are often prescribed. Exogenous CS will not be discussed further.
The modern-day classification of endogenous CS can be divided into ACTH-dependent and ACTH-independent causes (Fig. 3) [46, 48, 49]. ACTH-dependent causes account for 80% of all cases of endogenous CS; 20% are due to ACTH-independent CS. Seventy percent of all cases of CS are due to pituitary-dependent CS, and 10% are due to the ectopic ACTH syndrome. Less than 1% of cases are due to ectopic CRH syndrome. Adrenal adenomas account for 10% of all causes of CS. Adrenocortical carcinomas causing CS account for 5%–8%; AIMAH and PPNAD account for less than 1%.
In 1912, Harvey Cushing described a 23-year-old woman (Minnie G) with obesity, amenorrhea, and hirsutism [50]. Twenty years later, he postulated that this “polyglandular syndrome” was due to a primary pituitary abnormality causing adrenal hyperplasia [18]. Although some cases of CS were recognized as due to primary adrenal tumors, most were and still are due to corticotroph adenomas of the pituitary. Ectopic ACTH syndrome was not recognized until the 1960s [51]. The term “Cushing’s syndrome” is used for all causes, an the term “Cushing’s disease” is reserved specifically for those cases of pituitary-dependent CS.
Clinical features of cushing’s syndrome
Cushing’s original description of “Minnie G” included obesity, moon facies, hirsutism, and plethora [50]. This clinical picture is not always present, and one must maintain a high index of suspicion when this condition is suspected. Weight gain and centripetal obesity are the most common signs in adults; with generalized obesity seen more frequently in children [46]. Fat deposition occurs over the thoracocervical spine (“buffalo hump”), in the supraclavicular regions, and over the cheeks and temporal regions (moon-like facies). Gonadal dysfunction with menstrual irregularity and loss of libido occur frequently [46]. Hirsutism (vellus hypertrichosis) is common in females, as is acne, a phenomenon secondary to excessive ACTH stimulation of the zona reticularis. Hypogonadism occurs because of the inhibitory effect of cortisol on pituitary gonadtropin secretion. Fifty percent of patients with CS have psychiatric disturbances. Agitated depression and lethargy are most common, but paranoia and psychosis are well recognized. Cognitive function may be impaired. Irritability and insomnia may be early features. Poor linear growth and weight gain are common in children. Loss of height in adults is often due to vertebral compression fractures. Rib fractures occur and are frequently painless. Aseptic necrosis of femoral and humeral heads can also be seen. Cortisol excess results in skin thinning with separation and exposure of underlying subcutaneous vascular tissue, giving rise to so-called plethora. Minimal trauma results in “easy bruising.” Acne can occur over the face, chest, and back. The typical red–purple striae, greater than 1 cm in width, are most frequently found on the abdomen but can be found on the upper thighs, breasts and arms (nearly pathognomonic) [46, 48]. Increased skin pigmentation is common in ectopic ACTH syndrome where very high ACTH levels stimulate skin melanocyte receptors. Along with bruising, myopathy is a major discriminatory feature of CS [46, 48]. Proximal myopathy of the lower extremities and shoulder girdle are common. Inability to rise from a crouching position is often revealing. Hypertension occurs in up to 75% of patients with CS. This, coupled with recognized metabolic consequences, contributes to the increased cardiovascular mortality seen in untreated cases [46, 48]. Thromboembolic events and infections are also more common in CS. Fungal nail and skin infections (tinea versicolor), systemic fungal infections, as well as other opportunistic infections (e.g., pneumocystis carinii) can be seen in those with glucocorticoid excess [46, 48]. Bowel perforations and poor wound healing are also more common. Glucose intolerance and overt diabetes are seen in up to one-third of patients with CS. Hypokalemic alkalosis, seen in 10%–15% of patients with pituitary CS, is present in over 95% of those with ectopic ACTH syndrome, due to marked increases in cortisol levels and its effect on the mineralocorticoid receptor. In addition to suppressing pituitary gonadotropins, glucocorticoids directly inhibit Leydig cell function. Excess cortisol can increase fat deposition in the retro-orbital regions, leading to exophthalmos, and in the epidural space, leading to neurologic sequelae from cord or cauda equina compression. Excess cortisol also increases intraocular pressure, resulting in glaucoma [46, 48].
ACTH-dependent cushing’s syndrome
Pituitary-dependent cushing’s or sushing’s disease
Cushing’s disease or pituitary-dependent CS accounts for nearly 70% of all cases of endogenous hypercortisolism [52]. The adrenal glands show bilateral adrenocortical hyperplasia with diffuse expansion of the zonae fasciculata and reticularis due to uncontrolled ACTH stimulation from a primary pituitary source. Occasionally, nodular hyperplasia is intermingled with diffuse internodular hyperplasia, especially in older adults and those with longstanding Cushing’s disease. In 85%–90% of cases, a pituitary adenoma, of monoclonal origin, can be found [46]. Basophil hyperplasia is much less common and may be primary, or much less likely secondary, to a hypothalamic or ectopic source of CRH. In pituitary CS, there is relative resistance of ACTH secretion to glucocorticoid feedback inhibition. In Cushing’s disease, the predominant finding is a mild increase in serum ACTH concentrations with loss of normal circadian regulation [46, 48, 52].
Ectopic ACTH syndrome
Pro-opiomelanocortin is expressed by several extrapituitary tissues. In 10% of cases, CS may be associated with autonomous ACTH (rarely CRH) production by non-pituitary tumors [46, 48, 49, 52–55]. Small-cell lung cancers account for nearly half the cases reported and are often associated with rapid tumor progression. More indolent courses can be seen with lung carcinoids, islet cell tumors, thymic carcinoids, medullary thyroid carcinoma, and pheochromocytomas, as well as other carcinoid tumors. Exceedingly rare causes include non-small-cell lung cancers and cancers of the prostate, breast, ovary, gallbladder, and colon. Lung carcinoids may remain radiographically occult for many years [48, 58, 52, 53, 55]. Circulating ACTH and cortisol levels can become exceedingly high in a short period (less than 3 months). These patients often display marked skin pigmentation. Metabolic abnormalities predominate, including glucose intolerance, hypokalemia, and alkalosis. Weight loss, myopathy, and peripheral edema are more common features compared to the centripetal obesity, moon facies, and plethora seen more often in patients with the more indolent forms of pituitary-dependent CS. Pituitary-dependent CS is far more common in women, whereas ectopic ACTH syndrome is more common in men. Much less common are cases of ectopic CRH production associated with bronchial carcinoids, medullary thyroid carcinoma, prostatic adenocarcinoma, or pheochromocytoma [46].
ACTH-independent cushing’s syndrome
Adrenal adenomas and carcinomas
Ten percent of patients with endogenous CS harbor adrenal adenomas. Primary adrenocortical carcinoma accounts for less than 8% of all patients with endogenous CS. In children with endogenous CS, 65% have an adrenal etiology and most are malignant [46, 48, 52]. The onset of clinical features is often subtle and indolent with adenomas but frequently quite rapid with adrenocortical carcinomas. In addition to the stigmata of CS, patients with adrenocortical carcinoma may present with flank/abdominal pain, weight loss, fever, or a palpable abdominal mass [56]. Most reported series of adrenocortical carcinoma find that approximately half are functional, with cortisol (67%) or mixed hormonal (15%) patterns (cortisol, androgens, and mineralocorticoids) prevailing [56]. In females, there may be features of virilization that include hirsutism, clitoromegaly, deepening of the voice, temporal wasting, and severe acne [46, 48, 52]. Adrenocortical carcinomas and adenomas associated with autonomous cortisol secretion will demonstrate atrophy of the ipsilateral nontumoral cortex and contralateral gland on cross-sectional imaging and pathologic examination. On average, adrenocortical carcinomas are very large (mean diameter > 12 cm), with areas of hemorrhage and necrosis. Nearly 20% will demonstrate adrenal vein, renal vein, or inferior vena caval tumor thrombus [57]. Nearly half have metastasized prior to their discovery. Adrenocortical carcinomas may also be associated with cancer predisposition syndromes (Li-Fraumeni and Beckwith-Wiedemann syndromes) [58].
The prevalence of cortisol-secreting adenomas may actually be higher than previously thought. Adrenal incidentalomas are being detected with increasing frequency as a result of the widespread use of cross-sectional imaging studies. These adenomas (and rarely carcinomas) autonomously secrete cortisol and lead to suppression of the normal circadian secretion of ACTH by the pituitary gland. Subclinical CS [58–63] has been reported in 5%–20% of patients with adrenal incidentalomas [58–63]. It is unclear what percentage of patients with subclinical CS will develop overt CS over time. Atherosclerosis and cardiovascular complications, so prevalent in these patients, have favored an aggressive surgical approach (unilateral adrenalectomy) but long-term health benefits remain to be clarified [59–65].
ACTH-independent macronodular adrenal hyperplasia (AIMAH) and primary pigmented nodular adrenocortical disease (PPNAD)
Fifteen to twenty percent of patients with endogenous CS have ACTH-independent hypercortisolism, which in well over 90% of cases is due to unilateral tumors. Rarely do adrenals harbor solitary, bilateral, cortisol-secreting tumors [46, 58]. The remaining cases (< 2%) are due to two fascinating (bilateral) entities: AIMAH and PPNAD [58, 66]. Because these two forms of CS are not caused by a pituitary tumor, the Nelson-Salassa syndrome (growth of a corticotroph neoplasm and skin hyperpigmentation) does not occur after bilateral adrenalectomy for these entities. AIMAH typically presents with mild overt CS, but it can on occasion be associated with subclinical CS. The combined adrenal weight ranges from 60 to 400 g, with discrete nodules up to 5 cm in size [46, 58]. This contrasts with the mean weight of adrenals from patients with Cushing’s disease of 20–25 g (normal: less than 10 g total). The intervening cortex is often hypertrophied but may be atrophic in some cases. The nodules are yellow, lacking the dark pigment so often interspersed in solitary, cortisol-secreting adenomas.
The mechanism(s) by which cortisol production is stimulated in AIMAH, in the face of suppressed ACTH levels, is thought to be regulated via the ectopic expression or the overactive eutopic expression of several membrane-bound hormone receptors [58, 67–69]. Most of these receptors belong to the superfamily of G protein–coupled receptors that have become aberrantly coupled to steroidogenesis. These include receptors for gastric inhibitory polypeptide, vasopressin, β-adrenergic, LH, human chorionic gonadotropin, serotonin, and angiotensin [58,67–69].
Fifty percent of patients with PPNAD appear to be sporadic, while the remaining are familial, usually associated with Carney complex (CNC) [46, 58, 66, 70, 71]. Approximately one-half of the cases of CNC are familial. Forty percent harbor identifiable mutations in the PRKAR1A gene. Putative genetic loci have been identified for CNC by linkage analysis at chromosome 2p16 and 17q22–24 [46, 58]. Carney complex is characterized by CS caused by PPNAD, cardiac and skin myxomas, spotty skin pigmentation, schwannomas, and a variety of endocrine and non-endocrine tumors. PPNAD is the principal endocrine manifestation of CNC. Growth hormone-secreting pituitary tumors arise in 10%. Testicular tumors can be found in 30%. Large-cell calcifying Sertoli-cell tumors (causing estrogen production with associated gynecomastia and precocious puberty), adrenocortical rests, and Leydig cell tumors have all been described. Follicular cell-derived, benign and malignant thyroid neoplasms are also more frequent. Other manifestations include psammomatous melanotic schwannomas, epithelioid blue nevi, and ductal adenomas of the breast. Centrofacial spotty pigmentation, as well as conjunctival and mucosal pigmentation are common. Blue nevi, compound nevi, and café-au-lait spots can also be found. Cardiac myxomas are responsible for significant morbidity and mortality in CNC, necessitating screening and follow-up echocardiograms [46, 58, 66, 70, 71]. Cutaneous myxomas can be found on the external eyelids and within the external auditory canals.
In PPNAD, unlike AIMAH, the overall gland size is not increased. Several small black and brown nodules (containing lipofuscin) are spread out through an otherwise atrophic cortex.
Bilateral, nodular, adrenocortical disease causing CS in an ACTH-independent fashion can also be seen, albeit rarely, in the multiple endocrine neoplasia type 1 and McCune-Albright syndromes [58].
Case finding for Cushing’s syndrome
The diagnosis of CS is one of the most challenging problems in endocrinology (Fig. 4). The incidence of CS is approximately two cases, per one million persons, per year [48]. Patients not cured by surgical intervention or with malignant disease have a poor prognosis, with a standard mortality ratio of 3.8–5.0 compared to normal controls. The onset is often insidious with a usual duration of illness before clinical diagnosis ranging from 3 to 5 years [48]. Findling and Raff [48] have outlined who should be screened for CS (Table 1). Old photographs are particularly helpful in differentiating cases of CS from normal aging and simple weight gain.
The clinical features of hypercortisolism are common, while endogenous CS is uncommon. It is the clinician’s role to recognize possible CS patients, confirm with appropriate, cost-effective laboratory testing, determine a precise etiology, and provide for, when possible, a definitive cure. Many of the signs and symptoms of CS are common findings in the general population, including hypertension, obesity, abnormal glucose tolerance, and menstrual dysfunction. However, if the patient has centripetal obesity with prominent dorsocervical and supraclavicular fat pads, peripheral muscle wasting, thinning skin with ecchymoses, proximal muscle weakness, and/or fungal skin infections, the suspicion is raised considerably [46, 48]. The prevalence of CS may be as high as 4% among obese type 2 diabetics who have poor glycemic control [72]. Case finding and subtype determination are critical to the application of appropriate intervention.
Case finding for Cushing’s syndrome
Clinical assessment is critical (Fig. 4) [46, 48, 73–84]. If the clinical assessment does not support the diagnosis, do not test for CS, as no case finding methodology has perfect sensitivity. When hypercortisolism is suspected, measurement of urinary free cortisol (UFC) in a 24-h collection (measure urine creatinine for complete collection quality control), by high performance liquid chromatography (HPLC)-tandem mass spectrometry, is our first test of choice at Mayo Clinic. The suspicion for CS is very high when 24-h UFC is greater than twice the upper limit of normal (normal range: 3.5–45 μg or 9.7–124 nmol/day). Loss of diurnal variation in serum cortisol concentrations raises suspicion, but diurnal variations in serum cortisol can be perturbed under many circumstances, including stress and hospitalization. Urinary free cortisol excretion can be quite variable within individual patients with CS; indeed, with serial measurements, 10%–15% of patients will have one of four serial 24-h UFCs in the normal range. All etiologies of CS can produce cortisol in an episodic fashion. Therefore, if suspicion is high based on clinical assessment, repeated (multiple) collections are indicated. Baseline 24-h UFC measurements may be elevated by drugs (e.g., carbamazepine–when measured by HPLC, but not by HPLC-tandem mass spectrometry), high urine volumes (> 4 l per 24 h), severe illness, alcoholism, depression, and obstructive sleep apnea (OSA).
An overnight 1-mg dexamethasone suppression test (DST) is most useful for testing for adrenal autonomy in patients with adrenal incidentalomas [59]. The 8 a.m. plasma cortisol level in normals will suppress to below 5 μg/dl (138 nmol/l) with this test. There are, however, many causes for cortisol non-suppression with the overnight 1-mg DST. These include patient error, increased CBG due to estrogen therapy or pregnancy, alcoholism, OSA, depression, panic attacks, obsessive compulsive disorder, obesity, drugs that accelerate dexamethasone metabolism (anticonvulsants, primidone, and rifampin), renal failure, stress, cortisol assay insensitivity, or lab error. Failure to suppress is a positive case finding test and warrants a 2-day, low-dose DST.
When the clinical picture is consistent with CS and the baseline 24-h UFC exceeds five times the upper limit of normal, 300 μg (> 828 nmol/day), no additional studies to confirm CS are needed.
With equivocal findings and a 24-h UFC < 300 μg (< 828 nmol/day), hypercortisolism should be confirmed with a 2-day, low-dose DST. Dexamethasone 0.5 mg orally, every 6 h, for 48 h, is given. A 24-h UFC, collected during the second day of suppression, ≥ 3.5 μg (≥ 9.7 nmol/day) is consistent with the diagnosis of CS (exceptions include those situations listed above for the overnight 1-mg DST). This test is far from perfect, with a sensitivity of 79%, specificity of 74%, and overall accuracy of 71%. It is most efficacious for ruling out CS in those patients where the index of suspicion for CS is low. In addition, some patients with mild pituitary-dependent CS may suppress with a low-dose DST.
The dexamethasone–CRH test was developed as a way to correct the suppression with a low-dose DST observed in some patients with pituitary CS. A serum cortisol concentration ≥1.4 μg/dl (>37.6 nmol/l) at 15 min following CRH administration is consistent with CS. Although purported to be highly accurate, false positive results have been seen in our clinic.
Late night plasma or salivary cortisol can also be measured. A midnight, sleeping, serum cortisol concentration >1.8 μg/dl (>50 nmol/l) is 100% sensitive in patients with CS [48]. Precision, however, usually requires hospitalization. A practical alternative is to measure salivary cortisol, which correlates well with serum cortisol. It is done by having the patient chew on a special cotton swab for 2 min, after which the swab is placed in a specially designed plastic container. A salivary cortisol level obtained at 11 p.m. that is >100 ng/dl (>2.6 nmol/l) is highly sensitive for CS, and this test is becoming increasingly more popular in many centers. There remain, however, problems with the sensitivity and precision of the assay. Reproducibility has been difficult to achieve in some laboratories.
As can be seen, no single test is satisfactory for the case finding and confirmation of CS. However, the clinican should be certain of the diagnosis of CS prior to proceeding on to subtype evaluation and localization. Patients whose clinical assessment suggests a possible diagnosis of CS (when exogenous sources have been excluded) should be evaluated with multiple tests for diagnostic confirmation. These would include: 24-h UFC, 11 p.m. salivary cortisol, and, less often, the 2-day low-dose DST or dexamethasone-CRH test. The patient with clinically evident CS and a 24-h UFC > 300 μg (>828 nmol/day) needs no further confirmatory studies. The patient with an incidentally discovered adrenal mass and an 8 a.m. plasma cortisol concentration below 5 μg/dl (138 nmol/l) after an overnight 1-mg DST, also needs no further testing for cortisol autonomy.
Subtype evaluation
Once CS has been confirmed, the next step is subtype evaluation (Fig. 5). One must always keep in mind that 70% of patients with endogenous hypercortisolism have pituitary-dependent Cushing’s syndrome, and that there is no fixed algorithm that can be applied to all patients.
Algorithm for subtype evaluation of Cushing’s syndrome. ACC, adrenocortical carcinoma; ACTH, corticotropin; AVS, adrenal venous sampling; BLA, bilateral laparoscopic adrenalectomy; CT, computed tomography; IPSS, inferior petrosal sinus sampling; MRI, magnetic resonance imaging; TSS, transsphenoidal surgery. (* TSS in appropriate clinical setting, † Chest, abdomen, ‡ Octreoscan, 123-I-metaiodobenzylguanidine scintigraphy, positron emission tomography scanning with 18F-fluorodeoxyglucose, § Calcitonin, 5-hydroxyindoleacetic acid, gastrin, plasma fractionated metanephrines)
The plasma ACTH concentration classifies the subtype of CS as either ACTH-dependent or ACTH-independent. When measured with an IRMA assay (normal 10–60 pg/ml or 2.2–13.3 pmol/l) plasma ACTH levels < 5 pg/ml indicate primary adrenal disease. ACTH levels in the 20–200 pg/ml range generally indicate pituitary-dependent disease, and ACTH levels in the 50 to > 200 pg/ml range, are found in patients with ectopic ACTH syndrome.
When ACTH levels are less than 5 pg/ml, the next step in subtype evaluation is computerized cross-sectional imaging (computerized tomography [CT]; less often, magnetic resonance imaging [ MRI]) of the adrenal glands, which will most often delineate the type of adrenal disease (Fig. 6A–E) [83, 85, 86]. One must keep in mind, however, that 5%–10% of the population harbor adrenal nodules > 1 cm [59]. Benign cortisol-secreting adrenal adenomas are usually 2–6 cm in diameter. Rarely, bilateral cortisol-secreting adenoma may be found. Adrenocortical carcinomas are rarely seen below 5 cm in size, but when present, they usually display a worrisome radiographic phenotype, consisting of a heterogeneous appearance that may include areas of necrosis, hemorrhage, calcification, delayed or incomplete washout of intravenous contrast, or bright areas on T2-weighted MRI. Adrenocortical carcinomas may also demonstrate distant metastases (lung, bone, liver), regional lymphadenopathy, venous tumor thrombi, or direct invasion of contiguous structures. Magnetic resonance angiography or venography can be particularly helpful in delineating the presence and extent or vascular involvement [84]. Korobkin et al. demonstrated, with a specificity of 85% and a sensitivity of 100%, a cut-off point of 18 Hounsfield units (HU) on unenhanced CT scans below which an adrenal lesion may be designated as a lipid-rich benign adenoma [86].
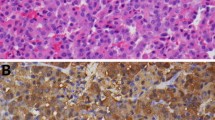
A) Axial CT image showing a small adrenal cortical adenoma (arrow). B) Axial CT image showing a large left adrenocortical carcinoma (arrow). C) Axial CT image from a patient with AIMAH showing massively enlarged adrenal glands (arrows) bilaterally. D) Gross pathology photograph of an ACTH-secreting adrenal pheochromocytoma with associated cortical hyperplasia. E) Coronal MRI showing an ACTH-secreting pituitary microadenoma (arrow) in the superior right location in the sella and adjacent to the right cavernous carotid artery
Axial, in-phase and out-of-phase, fast, multiplanar spoiled, gradient-echo (FMPSPGR) T1-weighted, MR images displaying classic signal dropout suggest the presence of intracellular lipid, a characteristic of benign adenoma. An axial fast spin-echo, T2-weighted MR image of a benign adenoma is isotense relative to the liver [58, 84].
In PPNAD, CT may demonstrate bilateral micronodularity (string of beads). Adrenal gland size is usually normal [58, 84]. The diagnosis is suspected in the appropriate clinical setting. When diagnosed, the patient and family members must be screened for cardiac myxomas, a common cause of sudden death in PPNAD patients with Carney complex. Genetic testing is available for the PRKAR1A mutations seen in 40% of patients with CNC.
In AIMAH, CT [58, 84] will demonstrate massive bilateral adrenal enlargement (occasionally asymmetric), with nodules reaching 5 cm in size. Research protocols are available to screen for abnormal adrenal expression and/or function of receptors that include gastrointestinal polypeptide, interleukin-1, LH, vasopressin, and β-adrenergic agonists. Unfortunately, to date, directed medical therapy (octreotide, V1a receptor antagonists, β-blockers, gonadotropin-releasing hormone agonists, 5HT4, and AT-1 antagonists) has generally proven unsuccessful [58], necessitating bilateral total adrenalectomy to resolve hypercortisolemia in most patients.
When ACTH levels are normal to moderately elevated, ACTH-dependent CS is confirmed. Again, the clinical presentation is key. For example, a 45-year-old woman with slow onset of CS, over many years, with classic signs and symptoms, almost certainly has pituitary-dependent CS (>95% probablility). A 50-year-old man with a 3-month history of weight loss, marked skin pigmentation, hypokalemic alkalosis, glucose intolerance, an ACTH level >100 pg/ml, and 24-h UFC> 500–1000 μg almost certainly has ectopic ACTH syndrome.
A pituitary-dedicated MRI, with gadolinium enhancement, is indicated in all patients with ACTH-dependent CS (85% will have Cushing’s disease) [52, 83, 85]. If a definite pituitary tumor is present (hypodense, non-enhancing, ≥ 5 mm) and the clinical scenario is consistent with pituitary disease (female, indolent disease, UFC less than fivefold elevated but more than twofold elevated), additional studies are usually not required before definitive treatment. Smaller (< 5 mm) pituitary lesions are common in as many as 10%–20% of normal individuals and are being detected with increasing frequency with each new generation MR scanner [52, 83, 85]. Thus, a small (<5 mm) lesion should be considered non-specific, and inferior petrosal sinus sampling (IPSS) should be performed. This is also true if the pituitary MRI is normal. Fifty percent of patients with Cushing’s disease have a normal pituitary MRI [83].
Inferior petrosal sinus sampling with CRH stimulation is considered the most important advance in subtype evaluation of CS in the past quarter century [87–96]. Both inferior petrosal sinuses are sampled simultaneously because of pulsatile ACTH secretion. Sampling of the cavernous sinuses or internal jugular veins can also be performed, but this is either more cumbersome and dangerous (cavernous sinuses) or less accurate (internal jugular veins) than IPSS. Also, IPSS that demonstrates a central to peripheral vein gradient of >2:1 at baseline and >3:1 after CRH administration is diagnostic of Cushing’s disease (95% sensitivity, 100% specificity). Further, IPSS may provide useful lateralization information for the neurosurgeon if the side-to-side ACTH ratio is >1.5:1. Factors that may cause misleading IPSS results include episodic disease (cyclical CS), incorrect catheter placement, anomalous venous drainage, and the rare ectopic CRH-secreting tumor. There are significant risks associated with IPSS, including venous thrombosis, pulmonary embolism, cranial nerve palsy, and brain stem infarct, thus mandating the availability of an experienced interventional neuroradiologist [90–93]. The IPSS procedure is technically successful 85%–99% of the time when performed in experienced centers.
In the past, the CRH stimulation test and the high-dose 2-day DST were employed to aid subtype evaluation, but both have fallen by the wayside. In the former, the percent increase in serum ACTH and cortisol concentrations after administration of CRH in normal persons overlaps with values found in patients with Cushing’s disease and in patients with ectopic ACTH syndrome. The high-dose DST has not been helpful in clearly differentiating ectopic ACTH syndrome from pituitary CS, in our experience.
In ACTH-dependent CS, patients with a negative pituitary MRI and lack of a central-to-peripheral gradient on IPSS, a search for an ectopic ACTH-secreting tumor should be carried out, along with adrenal cross-sectional CT imaging. In the appropriate clinical setting (male, rapid onset of CS, predominance of metabolic abnormalities, and very high cortisol/ACTH levels), IPSS can be avoided if the pituitary MRI is negative. Computerized tomography and/or MRI of the neck, chest, abdomen, and pelvis should be carried out [83]. Unfortunately, somatostatin receptor imaging with 111In-DTPA-pentetreotide [97–99] has not proven terribly accurate in localizing ectopic ACTH-secreting tumors, many of which can remain occult for many years. Diagnostic sensitivities ranging from 30%–80% have been reported. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) has added little to the diagnostic armamentarium for ectopic ACTH-secreting tumors [99]. It is hoped that diagnostic accuracy of PET will improve with the use of additional isotopes ([11C]5-HTP, [11C]2-DOPA), but this has yet to be proven [100]. In rare situations, biochemical markers may be of assistance, such as calcitonin (medullary thyroid cancer), plasma and urinary fractionated metanephrines (pheochromocytoma), gastrin (gastrinoma), and urinary 5-hydroxyindoleacetic acid (carcinoid tumors). However, when these markers are elevated, the source is usually already apparent by conventional imaging. 123I-metaiodobenzylguanidine (MIBG) scintigraphy can be helpful, on occasion, in localizing an ACTH-secreting (or CRH-secreting) pheochromocytoma and its metastases, if present.
Treatment
Treatment of patients with CS includes resolving the hypercortisolemic state, management of complications of CS (hypertension, osteoporosis, diabetes mellitus, myopathy), and the restoration of the normal HPA axis, all of which may take months to years to accomplish. In some patients, full recovery never takes place, despite normalization of plasma cortisol levels and the HPA axis.
Treatment—pituitary cushing’s syndrome
Selective pituitary adenomectomy by trans-sphenoidal surgery (TSS), either sublabial, or preferably today, endoscopic transnasal, is the treatment of choice for patients with Cushing’s disease [101–110]. The endonasal route, in experienced hands, has lower morbidity, eliminating the painful gingival incision and dental paresthesias associated with the sublabial approach to the sphenoid sinus. The short-term cure rate for ACTH-secreting microadenomas is 80%–85%. Success rates decrease appreciably for larger macroadenomas, locally invasive tumors, and treatment at less experienced centers. Trans-sphenoidal surgery is safe and effective in experienced hands. Perioperative mortality rates range from 0% to 2%. Morbidity includes diabetes insipidus, syndrome of inappropriate antidiuretic hormone secretion, cerebrospinal fluid rhinorrhea, meningitis, optic nerve injury, other cranial nerve injuries, vascular accident, nasal septal perforations, epistaxis, sinusitis, and graft site dehiscence. Most morbidities are uncommon and transient.
Postoperative cortisol levels can initially remain normal to elevated in some patients; thus patients with readily measurable cortisol levels immediately postoperatively may still be cured. In our experience, however, when plasma cortisol levels exceed 20 μg/dl (550 nmol/l) more than 4 days after TSS, the patient has not been cured. Cure cannot be predicted on the basis of pathologic findings (adenoma versus no adenoma versus hyperplasia). Peculiar delays in the development of secondary adrenal insufficiency occur such that careful postoperative steroid management and withdrawal should be performed under the guidance of an experienced endocrinologist. Full HPA axis recovery may require 6–24 months, and long-term physical rehabilitation may be necessary.
In the setting of failed TSS, a second operation is considered but is rarely curative. Total hypophysectomy is an option, but concerns for future reproductive function in young women and panhypopituitarism make this a less than desirable option. In the case of severe CS, prompt bilateral (laparoscopic) adrenalectomy can be life saving [41, 111, 112]. In those patients hospitalized with severe metabolic derangements, institution of medical therapy (see below), antibiotic coverage for opportunistic infections, and ulcer prophylaxis with proton-pump inhibitors can reduce perioperative morbidity; however, long delays should be avoided.
There are two principal issues of concern following bilateral total adrenalectomy for Cushing’s disease: Addisonian crises and Nelson-Salassa syndrome. Following bilateral laparoscopic adrenalectomy, oral glucocorticoid and mineralocorticoid replacement are required lifelong. A certain degree of patient sophistication is necessary to minimize episodes of Addisonian crises. Wells et al. reported a 23% incidence of crises requiring hospitalization and one death following bilateral adrenalectomy [113]. It is suspected that most retrospective studies underestimate its true incidence. Patient education is key to minimizing these occurrences. All patients should wear a medicine-alert bracelet and carry with them a prefilled glucocorticoid syringe for intramuscular injection when illness supervenes and oral augmentation is not an option.
The routine use of prophylactic radiation following bilateral adrenalectomy for pituitary-dependent CS remains controversial [114–116]. Nelson-Salassa syndrome is characterized by the unrestrained growth of an untreated corticotroph pituitary tumor and its associated mass effects following bilateral adrenalectomy in pituitary-dependent CS. Eleven percent of such patients at Mayo Clinic developed Nelson-Salassa syndrome, but most had clear evidence of an invasive pituitary disease at the outset [114]. Therefore, most patients undergoing bilateral adrenalectomy never develop Nelson-Salassa syndrome; but rather, their ACTH-secreting pituitary microadenomas remain <10 mm and do not require tumor-directed therapy. Three percent of radiated patients in our series developed secondary central nervous system neoplasms [114], and pituitary radiotherapy is associated with other morbidity as well (see below). We therefore reserve (stereotactic) radiotherapy only for those patients with large invasive pituitary tumors or residual pituitary tumors that demonstrate clinically important growth on serial MRI.
Radiotherapy
Radiotherapy is an alternative treatment for recurrent or persistent Cushing’s disease [117–119]. Options include conventional fractionated radiation, but more often, stereotactic radiosurgery with protons or photons (gamma knife, linear accelerator). Advantages of radiotherapy include efficacy in controlling hypercortisolism and its noninvasive nature. A major disadvantage, however, is the significant delay between radiation delivery and subsequent decline in cortisol levels. This delay often requires the use of cortisol-lowering medications (see below). The risk of delayed hypopituitarism is also significant. Other less common side effects include optic nerve atrophy with visual impairment, secondary tumor development, and cognitive dysfunction.
Treatment— ectopic ACTH syndrome
Optimal treatment would ideally involve resecting the primary tumor for cure. This, however, is often not possible. In 35% of cases, the tumor cannot be found at the time of diagnosis of CS. Eighty-five percent of patients have metastatic disease or incompletely resectable disease when tumor is present at the time of diagnosis of CS. In addition, tumors may be discovered up to 20 years before or after the diagnosis of CS. In a recent series from Mayo Clinic, the most frequent etiologies were as follows: bronchial carcinoid 25%, islet cell carcinoma 16%, small-cell lung cancer 8%, medullary thyroid carcinoma 7%, thymic carcinoid 5%, metastatic neuroendocrine tumor of unknown primary 5%, pheochromocytoma 3%, and no tumor found in 16% [53]. In patients in whom we are unable to either find the tumor or completely resect all disease, bilateral (laparoscopic) adrenalectomy has become the treatment of choice [53, 111, 120]. In the setting of occult or indolent metastatic disease, bilateral laparoscopic adrenalectomy effectively addresses the ravages of extreme hypercortisolism that often pose a greater immediate risk to the patient than the malignancy itself. These patients, often sicker than patients with persistent or recurrent Cushing’s disease, may benefit from similar medical preparation, as described under the medical treatment of patients with Cushing’s disease.
Treatment—adrenal adenoma(s)
Cortisol-secreting adrenal adenomas are best treated in 2007 with unilateral, laparoscopic or posterior retroperitoneoscopic adrenalectomy, depending on the experience and preference of the operating surgeon [32–44]. Both have well-defined benefits over open approaches, particularly with regard to wound healing in human and animal models with CS [121]. The rare, bilateral functioning adenomas can be managed with cortical-sparing adrenalectomies in a laparoscopic or open fashion, using intraoperative ultrasound as a guide for complete tumor resection [122, 123].
Most adrenal incidentalomas are benign, but the risk of malignancy increases with increasing size and the presence of worrisome radiographic phenotypes. Therefore, most surgeons would favor removal of all adrenal incidentalomas over 4 cm in greatest diameter, those that are functioning, and those with concerning radiographic features. Obvious malignant tumors are best excised in an open fashion, but smaller, potentially malignant incidentalomas, 4–7 cm in size, can be managed laparoscopically by an experienced surgeon when localized and clearly confined to the adrenal gland. The risk of capsular disruption in this setting is low and no greater than that with open surgery. Lymph nodes can be examined and removed laparoscopically [59, 124, 125].
Treatment—PPNAD and AIMAH
Definitive treatment for PPNAD or AIMAH is bilateral total adrenalectomy [58]. Rare cures following unilateral adrenalectomy are suspect, and persistent glucocorticoid autonomy is likely. Medical therapy in AIMAH, directed at abnormal receptor function/expression, has been, for the most part, disappointing [58]. PPNAD is ideally suited to bilateral laparoscopic adrenalectomy. Because of the benign, ACTH-independent nature of macronodular adrenal hyperplasia, select cases may be amenable to a laparoscopic approach as well.
Treatment—adrenocortical carcinoma
Adrenocortical carcinoma remains an enigma for endocrinologists, oncologists, and endocrine surgeons alike. It remains one of the most lethal solid tumors known, often striking individuals in the prime of life. Fortunately, its occurrence is a rarity. In a recent review from Mayo Clinic (1980–1996), 58 patients underwent primary operative management of adrenocortical carcinoma [56]. One-half were functional (>two-thirds with cortisol excess). Resection with intent to cure was carried out in 71%. Perioperative mortality was 5%, with a 73% recurrence rate at a median time to recurrence of 17 months. Five-year survival by the Kaplan-Meier method was 37%. Most series that include nonoperated patients portend a 10%–20%, 5-year survival rate. Prognostic factors favor lower TNM stage, functioning versus nonfunctioning tumors, and the use of chemotherapy (typically mitotane) in stage III/IV patients.
Renal vein or caval tumor thrombus in and of itself does not preclude surgical resection for intent to cure and may not alter an already dismal prognosis, when compared to stage I or II disease, if completely resected [126, 127]. Recent data support the use of mitotane for both resected and incompletely resected tumors or metastatic disease [128, 129]. Palliative debulking in the setting of stage IV disease is questionable at best. Such patients are better managed with adrenolytic agents or agents that block steroidogenesis, along with palliative radiotherapy for painful bone metastases. Most patients today with advanced or disseminated disease (stage III/IV) should be considered for protocol-based therapy. A number of regimens alone or combining systemic chemotherapy, mitotane, and/or novel therapies are available [130–135].
Trials of dendritic cell therapy have begun, and different strategies of immunotherapy, including DNA vaccination, are under investigation in animal models. The adrenals are a preferred target for adenovirus, and the results of gene therapy in preclinical studies are promising. There is evidence that histone deacetylase inhibitors can further enhance the rate of adenoviral infectivity in human adrenal cancer cell lines. The testing of retroviral vectors, non-viral vectors, small, interfering RNA technology, and combined technologies are all on the horizon. Anti-angiogenic substances are being applied in preclinical settings. According to a recent consensus conference, the future treatment of adrenocortical cancer should be hypothesis driven and based on a thorough analysis of tumor biology [129].
Replacement therapy following surgery for ACTH-independent cushing’s syndrome
All patients undergoing surgery for CS require a standard perioperative steroid preparation. Successful surgery will ultimately render all patients hypocortisolemic postoperatively, although there may, on occasion, be delays following TSS. In general, patients leave the hospital on replacement doses or, more often, higher than replacement doses, depending on their clinical status. For patients who have undergone trans-sphenoidal adenomectomy or unilateral adrenalectomy, replacement doses of glucocorticoids are given and tapered over time until return of function of the HPA axis is documented. Oftentimes this can be accomplished without the need of an ACTH-stimulation test. The process, both physiologic and morphologic, may take weeks to months or beyond to complete.
Patients undergoing bilateral total adrenalectomy will need lifelong glucocorticoid and mineralocorticoid replacement after the normal perioperative steroid taper.
Medical therapy for cushing’s syndrome
When definitive surgical treatment (or radiotherapy) is not, or is no longer, an option, or when optimizing a patient’s condition is necessary for a brief period of time prior to definitive surgical therapy (persistent or recurrent Cushing’s disease or ectopic ACTH syndrome), medical therapy has a role in the management of patients with endogenous hypercortisolism. Medical therapy also plays a role in the management of the pregnant female presenting with CS (see the following section).
-
Metyrapone inhibits 11β-hydroxylase and is given to lower cortisol concentrations before definitive therapy is begun. Nausea and hypokalemia are the principal side effects [46].
-
Aminoglutethimide is more toxic than metyrapone and blocks enzymes earlier in steroidogenesis. It is often used in conjunction with metyrapone and causes nausea, marked lethargy, and skin rash [46].
-
Trilostan, a 3β-HSD inhibitor, has been shown to be effective in patients with adrenal adenomas. It is ineffective in Cushing’s disease because the block in steroidogenesis can be overcome by increasing ACTH levels [46].
-
Ketoconazole, an antifungal agent, blocks a variety of steroidogenic cytochrome P450-dependent enzymes, thus lowering plasma cortisol levels. Daily doses of 400–1,200 mg are required, and usage is often limited by the development of abnormal liver function tests. Fluconazole may have similar action [46, 136].
-
Mitotane (o,p’-DDD), a derivative of DDT, is an adrenolytic agent that is taken up by normal and malignant adrenal tissue, resulting in adrenal necrosis and atrophy. Doses up to 10–20 g/day may be required, and such doses may be impossible to achieve because of nausea and other side effects (fatigue and skin rash). Both glucocorticoid and mineralocorticoid replacement may be required [46].
-
PPAR-γ ligands have been shown in in vitro and in vivo Cushing’s disease models to reduce ACTH levels in Cushing’s disease [137].
-
Etomidate, an intravenous anesthetic agent, is a member of the imidazole group, that includes ketoconazole and fluconazole, agents with antifungal properties. Etomidate lowers plasma cortisol levels by a mechanism similar to that seen with ketoconazole. It can be used for patients unable to take oral ketoconazole or metyrapone or in conjunction with other forms of medical therapy [138].
Cushing’s syndrome during pregnancy
Because of the negative effect of cortisol excess on gonadotropins, pregnancy is very uncommon in the setting of CS. Cushing’s syndrome in pregnancy is associated with increased fetal morbidity and mortality [139]. The diagnosis can be challenging because of the profound effects of pregnancy on the HPA axis, normally leading to increased circulating cortisol and ACTH levels. In pregnancy, however, the proportion of adrenal causes is increased. Surgery, both pituitary and adrenal, can be a safe treatment option during the second trimester. Medical therapy, with metyrapone, is also an option to bring the patient to full-term delivery prior to definitive surgical intervention.
Conclusions
Surgery for CS is highly successful for pituitary-dependent CS and for most ACTH-independent adrenal causes. Bilateral total adrenalectomy can also provide effective palliation from the ravages of hypercortisolism in patients with ectopic ACTH syndrome and for those who have failed trans-sphenoidal surgery. Unfortunately, adrenocortical carcinomas are rarely cured. Future successes with this disease will likely depend on a better understanding of tumor biology, more effective adjuvant therapies, and earlier detection. Clearly, IPSS, advances in cross-sectional imaging, along with developments in trans-sphenoidal and laparoscopic surgery, have had the greatest impact on today’s management of the complex patient with CS.
References
Harris DA, Wheeler MH (2005) History of adrenal surgery. In: Linos D, van Heerden JA (eds) Adrenal Glands. Springer-Verlag, Heidelberg, p. 1–6
Eustachii B. Opuscula Anatomica. Quoted by Harrison TS, Gann DS, Edis AJ et al. (1975) Surgical disorders of the adrenal gland, New York, Grune & Stratton, 1–2
Addison T (1855) On the constitutional and local effects of disease of the suprarenal capsules. S. Highley, London
Trousseau A (1856) Bronze Addison’s disease. Arch Gen Med 8:478
Brown-Séquard E (1856) Recherches éxperimentales sur la physiologie et la pathologie des capsules surrenals. Arch Gen Med (Paris) 8:385–401
Osler W (1896) Six cases of Addison’s disease. Int Med Mag 5:3–11
Abel JJ, Crawford AC (1897) On the blood-pressure raising constituent of the suprarenal capsule. Johns Hopkins Hosp Bull 8:151–157
Rolleston HD (1936) The endocrine organs in health and disease, with a historical review. Oxford University Press, London, p. 355
Kendall EC, Mason HL, Myers CSA et al. (1936) A physiological and chemical investigation of the suprarenal cortex. J Biol Chem 114:57–58
Reichstein T (1936) Constituents of the adrenal cortex. Helv Chiim Acta 19:402–412
Simpson SA, Tait JF, Bush JE (1952) Secretion of a salt retaining hormone by the mammalian adrenal cortex. Lancet 2:226–2232
Cahill GF (1935) Air injections to demonstrate the adrenals by x-ray. J Urol 34:238–243
Melby JC, Spark FF, Dale SL et al. (1967) Diagnosis and localization of aldosterone producing adenomas by adrenal vein catheterization. N Engl J Med 277:1050–1056
Adamson U, Efendic S, Granberg PO et al. (1980) Preoperative localization of aldosterone-producing adenomas. An analysis of the efficiency of different diagnostic procedures made from 11 cases and from a review of the literature. Acta Med Scand 208:101–109
Thrall JH, Freitas JE, Beierwaltes WH (1978) Adrenal scintigraphy. Semin Nucl Med 23–41
Sheedy PF, Stephens DH, Hattery RR et al. (1976) Computed tomography of the body: initial clinical trials with the EMI prototype. Am J Roentgenol 127:23–51
Thornton JK (1890) Abdominal nephrectomy for large sarcoma of the left suprarenal capsule: recovery. Trans Clin Soc Lond 23:150–153
Cushing H (1932) The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Johns Hopkins Hosp Bull 50:137–195
Schloffer H (1907) Erfolgreiche operation eines hypophysentumors and nasalem wege. Wien Klin Wochenschr 20:621–624
Cushing H (1909) Partial hypophysectomy for acromegaly. With remarks on the function of the hypophysis. Ann Surg 50:1002–1017
Cohen-Gadol AA, Liu JK, Laws ER Jr (2005) Cushing’s first case of transsphenoidal surgery: the launch of the pituitary surgery era. J Neurosurg 103:570–574
Fulton JF (1946) Harvey Cushing: A Biography. Charles C Thomas, Springfield, IL
Halstead AE (1910) Remarks on the operative treatment of tumors of the hypophysis. With the report of two cases operated on by an oronasal method. Surg Gynecol Obstet 10:494–502
Walters W, Wilder RM, Kepler EJ (1934) The suprarenal cortical syndrome. Ann Surg 100:670–688
Priestley JT, Sprague RG, Walters W et al. (1951) Subtotal adrenalectomy for Cushing’s syndrome. Ann Surg 134:464–475
von Langenbüch C (1882) Ein fall von exstirpation der gallenblase. Berlin Klin Wochenschr 19:725–727. Quoted by Welbourn RB in The History of Endocrine Surgery, London, Praeger, 1990, p. 151
Broster LR, Hill HG, Greenfield JG (1932) Adreno-genital syndrome and unilateral adrenalectomy. Br J Surg 19:557–570
Mayo CH (1927) Paroxysmal hypertension with tumour of retroperitoneal nerve. JAMA 89:1047–1050
Young HH (1936) Technique for simultaneous exposure and operation on the adrenals. Surg Gynecol Obstet 63:179–188
Russell CF, Hamberger B, van Heerden JA et al. (1982) Adrenalectomy: anterior or posterior approach? Am J Surg 144:322–324
Gagner M, Lacrois A, Bolte E (1992) Laparoscopic adrenalectomy in Cushing’s syndrome and phaeochromocytoma. N Engl J Med 327:1033
Thompson GB, Grant CS, van Heerden JA et al. (1997) Laparoscopic versus open posterior adrenalectomy: a case-control study of 100 patients. Surgery 122:1132–1136
Lal G, Duh QY (2003) Laparoscopic adrenalectomy—indications and technique. Surg Oncol 12:105–123
Micali S, Peluso G, De Stefani S et al. (2005) Laparoscopic adrenal surgery: new frontiers. J Endourol 19:272–278
Porpiglia F, Fiori C, Bovio S et al. (2004) Bilateral adrenalectomy for Cushing’s syndrome: a comparison between laparoscopic and open surgery. J Endocrinol Invest 27:654–658
Saunders BD, Wainess RM, Dimick JB et al. (2004) Trends in utilization of adrenalectomy in the United States: have indications changed? World J Surg 28:1169–1175
Naya Y, Suzuki H, Komiya A et al. (2005) Laparoscopic adrenalectomy in patients with large adrenal tumors. Int J Urol 12:134–139
Chavez-Rodriguez J, Pasieka JL (2005) Adrenal lesions assessed in the era of laparoscopic adrenalectomy: a modern day series. Am J Surg 189:581–585
Hara I, Kawabata G, Hara S et al. (2005) Clinical outcomes of laparoscopic adrenalectomy according to tumor size. Int J Urol 12:1015–1021
Gumbs AA, Gagner M (2006) Laparoscopic adrenalectomy. Best Clin Pract Res Clin Endocrinol Metab 20:483–499
Young WF Jr, Thompson GB (2005) Laparoscopic adrenalectomy for patients who have Cushing’s syndrome. Endocrinol Metab Clin North Am 34:489–499
Mercan S, Seven R, Ozarmagan S et al. (1995) Endoscopic retroperitoneal adrenalectomy. Surgery 118:1071–1075
Walz MK, Alesina PF, Wenger FA et al. (2006) Posterior retroperitoneoscopic adrenalectomy—results of 560 procedures in 520 patients. Surgery 140:943–950
Barczyski M, Konturek A, Golkowski F et al. (2007) Posterior retroperitoneoscopic adrenalectomy: a comparison between the initial experience in the invention phase and introductory phase of the new surgical technique. World J Surg 31:65–71
Jacobson L (2005) Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am 34:271–292
Stewart PM (2003) The adrenal cortex. In: Larsen PR, Knonenberg HM, Melmed S, Polansky KS (eds) Williams Textbook of Endocrinology, Tenth Edition. Saunders, Philadelphia, pp. 491–548
Arlt W, Stewart PM (2005) Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am 34:293–313
Findling JW, Raff H (2005) Screening and diagnosis of Cushing’s syndrome. Endocrinol Metab Clin North Am 34:385–402
Carpenter PC (1998) Cushing’s syndrome. In: Rakel RE (ed) Conn’s Current Therapy. WB Saunders, Phildelphia, pp. 616–620
Cushing H (1912) The Pituitary Body and its Disorders: Clinical Status Produced by Disorders of the Hypophysis Cerebri. JB Lippincott, Philadelphia
Meador CK, Liddle GW, Island DP et al. (1962) Cause of Cushing’s syndrome in patients with tumors arising from “nonendocrine” tissue. J Clin Endocrinol Metab 22:693–703
Lindsay JR, Nieman LK (2005) Differential diagnosis and imaging in Cushing’s syndrome. Endocrinol Metab Clin North Am 34:403–421
Aniszewski JP, Young WF Jr, Thompson GB et al. (2001) Cushing syndrome due to ectopic adrenocorticotropic hormone secretion. World J Surg 25:934–940
Vrezas I, Willenberg HS, Mansmann G et al. (2003) Ectopic adrenocorticotropin (ACTH) and corticotropin-releasing hormone (CRH) production in the adrenal gland: basic and clinical aspects. Microsc Res Tech 61:308–314
Hérnandez I, Ispinosa-de-los-Monteros AL, Mendoza V et al. (2006) Ectopic ACTH-secreting syndrome: a single center experience report with a high prevalence of occult tumor. Arch Med Res 37:976–980
Kendrick ML, Lloyd R, Erickson L et al. (2001) Adrenocortical carcinoma: surgical progress or status quo? Arch Surg 136:543–549
Icard P, Chapuis Y, Andreassian B et al. (1992) Adrenocortical carcinoma in surgically treated patients. A retrospective study on 156 cases by the French Association of Endocrine Surgery. Surgery 112:9792
Lacroix A, Bourdeau I (2005) Bilateral adrenal Cushing’s syndrome: macronodular adrenal hyperplasia and primary pigmented nodular adrenocortical disease. Endocrinol Metab Clin North Am 34:441–458
Young WF Jr (2007) The incidentally discovered adrenal mass. N Engl J Med 356:601–610
Barzon L, Sonino N, Fallo F et al. (2003) Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 149:273–285
Tsagarakis S, Vassiliadi D, Thalassinos N (2006) Endogenous subclinical hypercortisolism: diagnostic uncertainties and clinical implications. J Endocrinol Invest 29:471–482
Sippel RS, Chen H (2004) Subclinical Cushing’s syndrome in adrenal incidentaloma. Surg Clin North Am 84:875–885
Terzolo M, Bovio S, Reimondo G et al. (2005) Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am 34:423–439
Izaki H, Fukumori T, Takahashi M et al. (2006) Indications for laparoscopic adrenalectomy for non-functional adrenal tumor with hypertension: usefulness of adrenocortical scintigraphy. Int J Urol 13:677–681
Midorikawa S, Sanada H, Hashimoto S et al. (2001) The improvement of insulin resistance in patients with adrenal incidentaloma by surgical resection. Clin. Endocrinol. 54:797–804
Young WF Jr, Carney JA, Musa BU et al. (1989) Familial Cushing’s syndrome due to primary pigmented nodular adrenocortical disease: reinvestigation 50 years later. N Engl J Med 321:1659–1664
Lacroix A, Baldacchino V, Bourdeau I et al. Cushing’s syndrome variants secondary to aberrant hormone receptors. Trends Endocrinol Metab 15:375–382
Christopoulos S, Bourdeau I, Lacroix A (2004) Aberrant expression of hormone receptors in adrenal Cushing’s syndrome. Pituitary 7:231–241
Swain JM, Grant CS, Schlinkert RT et al. (1998) Corticotropin-independent macronodular adrenal hyperplasia. A clinicopathologic correlation. Arch Surg 133:541–546
Storr HL, Mitchell H, Swords FM et al. (2004) Clinical features, diagnosis, treatment, and molecular studies in paediatric Cushing’s syndrome due to primary nodular adrenocortical hyperplasia. Clin Endocrinol 61:553–559
Gunther DF, Bourdeau I, Matyakhina L et al. (2004) Cyclical Cushing’s syndrome presenting in infancy: an early form of primary pigmented nodular adrenocortical disease, or a new entity? J Clin Endocrinol Metab 89:3173–3182
Contreras LN, Cardosa E, Lozano MP et al. (2000) Detection of preclinical Cushing’s syndrome in overweight type 2 diabetic patients. Medicina 60:326–330
Davies JS, Ogunko A, Smith J et al. (2000) Diagnostic dilemmas in Cushing’s syndrome. Ann Clin Biochem 37:85–89
Newell-Price J, Bertagna X, Grossman AB et al. (2006) Cushing’s syndrome. Lancet 367:1605–1617
Lin DD, Loughlin KR (2005) Diagnosis and management of surgical adrenal disease. Urology 66:476–483
Nieman LK, Ilias I (2005) Evaluation and treatment of Cushing’s syndrome. Am J Med 118:1340–1346
Arnaldi G, Angeli A, Atkinson AB et al. (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602
Raff H, Findling JW (2003) A physiologic approach to diagnosis of the Cushing syndrome. Ann Intern Med 138:980–991
Simard M (2004) The biochemical investigation of Cushing syndrome. Neurosurg Focus 16:1–5
Nieman LK, Cutler GB Jr (1999) The sensitivity of the urine-free cortisol measurement as a screening test for Cushing’s syndrome. In Program and Abstracts of the 72nd Annual Meeting of the Endocrine Society, Atlanta, Georgia, June, p. 230
Puig J, Wagner A, Caballero A et al. (1999) Cost-effectiveness and accuracy of the tests used in the differential diagnosis of Cushing’s. Pituitary 1:125–132
Raff H, Raff JL, Findling JW (1998) Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab 83:2681–2686
Lindsay JR, Nieman LK (2005) Differential diagnosis and imaging in Cushing’s syndrome. Endocrinol. Metab Clin North Am 34:403–421
Yanovski JA, Cutler GB Jr, Chrousos GP et al. (1993) Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. JAMA 269:2232–2238
Rockall AG, Babar SA, Sohaib SA et al. (2004) CT and MR imaging of the adrenal glands in ACTH-independent Cushing’s syndrome. Radiographics 24:435–452
Korobkin M, Brodeur FJ, Yutzy GG et al. (1996) Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. Am J Roentgenol 166:531–536
Findling JW, Kehoe ME, Shaker JL et al. (1991) Routine inferior petrosal sinus sampling in the differential diagnosis of adrenocorticotropin (ACTH)-dependent Cushing’s syndrome: early recognition of the occult ectopic ACTH syndrome. J Clin Endocdrinol Metab 73:408–413
Oldfield EH, Doppman JL, Nieman LK et al. (1991) Petrosal sinus sampling with and without corticotropoin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med 325:897–905
Bonelli FS, Huston J III, Carpenter PC et al. (2000) Adrenocorticotropic hormone-dependent Cushing’s syndrome: sensitivity and specificity of inferior petrosal sinus sampling. AJNR Am. J Neuroradiol 21:690–696
Obuobie K, Davies JS, Ogunko A et al. (2002) Venous thrombo-embolism following inferior petrosal sinus sampling in Cushing’s disease. J Endocrinol Invest 3:542–544
Lefournier V, Gatta B, Martinie M et al. (1999) One transient neurological complication (sixth nerve palsy) in 166 consecutive inferior petrosal sinus sampling for the etiological diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 84:3401–3402
Sturrock ND, Jeffcoate WJ (1997) A neurological complication of inferior petrosal sinus sampling during investigation for Cushing’s disease: a case report. J. Neurol. Neurosurg. Psychiatry 62:527–528
Miller DL, Doppman JL, Peterman SB et al. (1999) Neurological complications of petrosal sinus sampling. Radiology 185:143–147
Kaltsas GA, Giannulis MG, Newell-Price JD et al. (1999) A critical analysis of the value of simultaneous inferior petrosal sinus sampling in Cushing’s disease and the occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab 84:487–492
Doppman JL, Chang R, Oldfield EH et al. (1999) The hypoplastic inferior petrosal sinus: a potential source of false-negative results in petrosal sampling for Cushing’s disease. J Clin Endocrinol Metab 84:533–540
Lienhardt A, Grossman AB, Dacie JE et al. (2001) Relative contributions of inferior petrosal sinus sampling and pituitary imaging in the investigation of children and adolescents with ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab 86:5711–5714
Tabarin A, Vallil N, Chanson P et al. (1999) Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab 84:1193–1202
Granberg D, Sundin A, Janson ET et al. (2003) Octreoscan in patients with bronchial carcinoid tumours. Clin Endocrinol (Oxf) 59:793–799
Catargi B, Rigalleau V, Poussin A et al. (2003) Occult Cushing’s syndrome in type II diabetes. J Clin Endocrinol Metab 88:5808–5813
Markou A, Manning P, Kaya B et al. (2005) [18F]fluoro-2-deoxy-d-glucose ([18F]FDG) positron emission tomography imaging of thymic carcinoid tumor presenting with recurrent Cushing’s syndrome. Eur J Endocrinol 152:521–525
Utz AL, Swearingen B, Biller BMK (2005) Pituitary surgery and postoperative management in Cushing’s disease. Endocrinol Metab Clin North Am 34:459–478
Guilhaume B, Bertagna X, Thomsen M et al. (1988) Transsphenoidal pituitary surgery for the treatment of Cushing’s disease: results in 64 patients and long term follow-up studies. J Clin Endocrinol Metab 66:1056–1064
Arnott RD, Pestell RG, McKelvie PA et al. (1999) A critical evaluation of transsphenoidal pituitary surgery in the treatment of Cushing’s disease: prediction of outcome. Acta Endocrinol (Copenh) 123:423–430
Pieters GFFM, Hermus ARMM, Meijer E et al. (1989) Predictive factors for initial cure and relapse rate after pituitary surgery for Cushing’s disease. J Clin Endocriniol Metab 69:1122–1126
Graham KE, Samuels MH, Raff H et al. (1997) Intraoperative adrenocorticotropin levels during transsphenoidal surgery for Cushing’s disease do not predict cure. J Clin Endocrinol Metab 82:1776–1779
Semple CG, Thomson JA, Teasdale GM Transsphenoidal surgery for Cushing’s disease. Clin Endocrinol (Oxf) 21:621–629
Friedman RB, Oldfield EH, Nieman LK et al. (1989) Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg 71:520–527
Laws ER, Reigmeyer M, Thapar K et al. (2002) Cushing’s disease resulting from pituitary corticotrophic microadenoma. Treatment results from transsphenoidal microsurgery and gamma knife radiosurgery. Neuro-Chir 48:294–299
Leinung MC, Kane LA, Scheithauer BW et al. (1995) Long term follow-up of transsphenoidal surgery for the treatment of Cushing’s disease in childhood. J Clin Endocrinol Metab 80:2475–2479
Nasseri SS, Kasperbauer JL, Strome SE et al. (2001) Endoscopic transnasal pituitary surgery: report of 180 cases. Am J Rhinol 15:281–287
Chow JT, Thompson GB, Grant CS et al. (2007) Bilateral laparoscopic adrenalectomy for ACTH-dependent Cushing’s syndrome. Clin Endocrinol In Press, Available online from 29 October 2007
Vella A, Thompson GB, Grant CS et al. (1998) Laparoscopic adrenalectomy for adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 83:348–352
Lairmore TC, Ball DW, Baylin SB et al. (1993) Management of pheochromocytomas in patients with multiple endocrine neoplasia type 2 syndrome. Ann Surg 217:595–601
Aniszewski JP, Sawka AM, Young WF Jr.(1999) Nelson-Salassa syndrome: a long-term follow-up study. 81st Annual Meeting of the Endocrine Society, San Diego, CA, June 15, p. 21
Assie G, Bahurel H, Bertherat J et al. (2004) The Nelson’s syndrome ... revisted. Pituitary 7:209–215
Gil-Cardenas A, Herrera MF, Diaz-Polanco A et al. (2007) Nelson’s syndrome after bilateral adrenalectomy for Cushing’s disease. Surgery 141:147–151
Plowman PN (1994) Pituitary adenoma radiotherapy—when, who, and how? Clin Endocrinol (Oxf) 51:265–271
Hentschel SJ, McCutcheon IE (2004) Stereotactic radiosurgery for Cushing disease. Neurosurg. Focus 16:1–7
Voges J, Kocher m, Runge M et al. (2006) Linear accelerator radiosurgery for pituitary macroadenomas: a 7-year follow-up study. Cancer 107:1355–1364
Hellman P, Linder F, Hennings J et al. (2006) Bilateral adrenalectomy for ectopic Cushing’s syndrome—discussions on technique and indication. World J Surg 30:909–916
Kollmorgen CF, Thompson GB, Grant CS et al. (1998) Laparoscopic versus open posterior adrenalectomy: comparison of acute-phase response and wound healing in the cushingoid porcine model. World J Surg 22:613–619
Walz MK (2004) Extent of adrenalectomy for adrenal neoplasm: cortical sparing (subtotal) versus total adrenalectomy. Surg Clin North Am 84:743–753
Inoue T, Ishiguro K, Suda T et al. (2006) Laparoscopic bilateral partial adrenalectomy for adrenocortical adenomas causing Cushing’s syndrome: report of a case. Surg Today 36:94–97
Shen WT, Sturgeon C, Duh Q-Y (2005) From incidentaloma to adrenocortical carcinoma: the surgical management of adrenal tumors. J Surg Oncol 89:186–192
Sturgeon C, Kebebew E (2004) Laparoscopic adrenalectomy for malignancy. Surg Clin North Am 84:755–774
Schulick RD, Brennan MF (1999) Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol 6:719–726
Ritchey M, Kinard R, Novicki DE (1987) Adrenal tumors: involvement of the inferior vena cava. J Urol 138:1134
Terzolo M, Angeli A, Fassnacht M et al. (2000) Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 356:2372–2380
Schteingart DE, Doherty GM, Gauger PG et al. (2005) Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr-Rel Cancer 12:667–680
Berruti A, Terzolo M, Pia A et al. (1998) Mitotane associated with etoposide, doxorubicin and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. Cancer 83:2194–2200
Figueiredo BC, Stratakis CA, Sandrini R et al. (1999) Comparative genomic hybridization analysis of adrenocortical tumors in childhood. J Clin Endocrinol Metab 84:1116–1121
Khan TS, Imam H, Juhlin C (2000) Streptozocin and o,p’-DDD in the treatment of adrenocortical cancer patients: long term survival in its adjuvant use. Ann Oncol 11:1281–1287
Schteingart DE, Homan D (2001) Management of adrenal cancer. In: Margioris AN, Chrousos G (eds) Adrenal Disorders. Humana Press, Totowa, p. 231–247
Wajchenberg B, Albergaria PM, Medonca B et al. (2000) Adrenocortical carcinoma: clinical and laboratory observations. Cancer 88:711–736
Williamsom SK, Lew D, Miller GJ et al. (2000) Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma. A Southwestern Oncology Study. Cancer 88:1159–1165
Riedl M, Maier C, Zetting G et al. (2006) Long term control of hypercortisolism with fluconazole: case report and in vitro studies. Eur J Endocrinol 154:519–524
Heaney AP (2004) PPAR-γ in Cushing’s disease. Pituitary 7:265–269
Greening JE, Brain CE, Perry LA et al. (2005) Efficient short-term control of hypercortisolaemia by low-dose etomidate in severe paediatric Cushing’s disease. Horm Res 64:140–143
Lindsay JR, Nieman LK (2005) The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocrinol Rev 26:775–799
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porterfield, J.R., Thompson, G.B., Young, W.F. et al. Surgery for Cushing’s Syndrome: An Historical Review and Recent Ten-year Experience . World J Surg 32, 659–677 (2008). https://doi.org/10.1007/s00268-007-9387-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9387-6