Abstract
Background
The aim of this study was to analyze the prognostic factors associated with long-term outcome after liver resection for colorectal metastases. The retrospective analysis included 297 liver resections for colorectal metastases.
Methods
The variables considered included disease stage, differentiation grade, site and nodal metastasis of the primary tumor, number and diameter of the lesions, time from primary cancer to metastasis, preoperative carcinoembryonic antigen (CEA) level, adjuvant chemotherapy, type of resection, intraoperative ultrasonography and portal clamping use, blood loss, transfusions, complications, hospitalization, surgical margins status, and a clinical risk score (MSKCC-CRS).
Results
The univariate analysis revealed a significant difference (p < 0.05) in overall 5-year survival rates depending on the differentiation grade, preoperative CEA >5 and >200 ng/ml, diameter of the lesion >5 cm, time from primary tumor to metastases >12 months, MSKCC-CRS >2. The multivariate analysis showed three independent negative prognostic factors: G3 or G4 grade, CEA >5 ng/ml, and high MSKCC-CRS.
Conclusions
No single prognostic factor proved to be associated with a sufficiently disappointing outcome to exclude patients from liver resection. However, in the presence of some prognostic factors (G3–G4 differentiation, preoperative CEA >5 ng/ml, high MSKCC-CRS), enrollment of patients in trials exploring new adjuvant treatments is suggested to improve the outcome after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Liver resection is the gold standard approach for the treatment of liver metastases from colorectal cancer. A recent meta-analysis [1] revealed a survival rate for patients undergoing resection of metastases from colorectal cancer of 16% to 49% after 5 years and 17% to 33% after 10 years, with the operative mortality ranging from 0% to 9%. In contrast, chemotherapeutic treatments presented a median survival duration of 12 to 18 months [2], and patients not receiving any treatment had a median survival duration ranging from 6 to 12 months [3, 4].
Recent technologic advances and scientific developments have led to an expansion of the indications for liver resection to treat metastases from colorectal tumor. The development of surgical techniques, the introduction of intraoperative ultrasonography (IOUS) and modern parenchymal dissection tools, the evolution of perioperative management, the experience derived from hepatic resection for living donor liver transplants, and the development of dedicated surgical units [5] has led to surgery with the perioperative risk and biologic impact significantly reduced.
Indeed, the traditional limitation to surgery—intrahepatic extension of the tumor and subsequent unacceptable demolition of liver parenchyma—can today be at least partially dismissed owing to the possibility that the patient can undergo sequential resection (“two-stage hepatectomy” [6]). This is accomplished by inducing hypertrophy of the remaining liver parenchyma by means of preoperative percutaneous portal embolization or preoperatively reducing the extent of the disease by administering neoadjuvant chemotherapy. These techniques have been demonstrated to be effective in allowing surgery in up to 15% of patients whose tumor was originally considered unresectable [7]. Furthermore, concomitant extrahepatic disease and multiple bilobar lesions no longer represent absolute contraindications to liver resection, as significant survival has been described for these subgroups of patients [8–12]. Finally, resection of intrahepatic recurrent metastases after prior resection showed late results substantially similar to those for the first liver resection (34% after 5 years), thereby extending the indications for surgery to patients with recurrence [12] or re-recurrence [13] of liver metastases.
In addition, the last two decades have seen the introduction into clinical practice of new generations of chemotherapic drugs and of percutaneous-interstitial ablative methods and techniques that have proved to be effective in treating liver metastases from colorectal cancer, even if they do not achieve the results of hepatic resection. Treatment of liver metastases by radiofrequency ablation (RFA) allows a 4-year survival of 22%, even though it is inferior to that seen after liver resection (65%) [14].
The concurrent development of two opposite trends in treating liver metastases from colorectal cancer—extension and repetition of surgical resection on the one hand and systemic chemotherapy and percutaneous ablation on the other—underline the need to determine which patients can benefit from surgical aggressiveness. Such categorization would identify patients with early postresection disease progression and poor prognosis so as to exclude them from liver resection and subject them to nonsurgical treatment. Selecting patients at high risk for recurrence after liver resection allows their enrollment in studies of adjuvant chemotherapeutic treatments that might improve their long-term prognosis.
The identification of prognostic factors for recurrence after surgery for colorectal liver metastases thus represents a primary aim for all professionals (surgeons, oncologists, interventional radiologists, pathologists) involved in the management of patients with liver metastases from a colorectal tumor and in the analysis and study of the progression of colorectal cancer.
Materials and methods
This analysis retrospectively examined 257 patients undergoing 297 liver resections for metastases from colorectal tumors performed at the San Raffaele Hospital Scientific Institute of Milan between 1982 and 2003 in the three general surgery units now affiliated with the Surgery Department.
Data concerning the examined patients were obtained from a review of medical records of the hospitalizations and ambulatory checks at the Surgery Unit or the Oncology Department, as well as a consultation of the archives of our institution (laboratory, instrumental, and pathologic data). Patients undergoing follow-up and/or postsurgical treatments in other institutions were called for a clinical evaluation, and follow-up data (when possible) and information on clinical outcome were updated by speaking with the relatives on the telephone. The obtained data were initially collected in three databases for the three units; afterward, however, having established unequivocal criteria for potentially heterogeneous parameters (e.g., stage of the primary tumor, type of resection, postoperative complications), a single institutional database was created. A single record was built for each liver resection. Thus, for patients undergoing a re-resection, a new record was included, considering that scientific literature agrees on stating that the outcome of the patient is similar after every resection [13, 15].
Classifications and definitions
The stage of the primary tumor was classified according to the UICC (TNM) and Dukes’ classifications. Liver resection was classified using the International Hepato-Pancreato-Biliary Association (IHPBA) Brisbane 2000 Terminology of Liver Anatomy & Resections [16].
The Memorial Sloan-Kettering Cancer Center Clinical Risk Score (MSKCC-CRS) with six categories (scoring 0–5), introduced by Fong et al. in 1999 [17], was used to define the risk classes for recurrence and progression of the tumor.
Each liver lesion that was present concomitantly with the primary colorectal cancer was considered a synchronous metastasis. Each liver metastasis diagnosed after colorectal tumor resection was considered a metachronous metastasis.
Anatomic liver resection indicated a resection performed with a preliminary vascular check and successive resection guided by ischemic demarcation lines. Any other resection was defined as a nonanatomic resection. Resection margins were considered positive whenever there was macroscopic or microscopic evidence of tumor on the resected margin (R1).
Indications for resection and preoperative evaluation
For all patients, the indication for liver resection was complete removal of the diseased portion of the liver, preserving a sufficient quantity of healthy residual liver parenchyma where no extrahepatic disease was detected by preoperative instrumental staging. The study included patients with extrahepatic disease on condition that it had been previously treated (e.g. local recurrence, lung metastases, locoregional lymph nodes, limited peritoneal localization) or removed simultaneously (intraoperative finding) with the liver resection.
The size of metastases, the presence of multiple metastases, the number of metastases, and bilateral localization did not represent single criteria for excluding the patient from liver surgery. In the case of synchronous metastases to the primary colorectal tumor, the choice of proceeding with liver resection at the same time or successively depended on the surgeon’s evaluation, which included several considerations (e.g., urgent or elective surgery, extension of liver resection, performance status of the patient, extension of colorectal surgery).
The resectability of liver lesions was evaluated by means of contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI). Preoperative staging included total body CT scanning and, more recently but not routinely, positron emission tomography (PET).
Preparation for liver resection included autologous blood donation when possible. For patients previously undergoing chemotherapy, a time interval of at least 2 weeks was observed before surgery to allow recovery from possible toxicity (hematologic, nephrologic, gastroenteric alterations). Whenever surgery required resecting more than 70% of the liver parenchyma, the patient underwent preoperative percutaneous portal embolization followed by liver resection 4 to 6 weeks later after CT evaluation of the hepatic volume.
Surgery
The surgical technique varied during the long period of data collection owing to the evolution and availability of surgical instruments (ultrasound dissector, Harmonic Scalpel, IOUS) and to establishing the three surgical groups. In any case, appropriate criteria for surgery were always respected. The dissection instruments and the option of parenchymal hemostasis as well as local treatments of the resected liver surface have been chosen by the surgeons throughout the years and therefore have not been considered in the present review.
Intraoperative ultrasonography has been available since 1993 for staging the disease and for evaluating hepatic vascular anatomy and the relation between the tumor and resection margins.
No specific guidelines or protocols for intraoperative management of patient’s volemia have been used. Postoperative monitoring was generally performed in the surgical department. Postoperative care in the intensive care unit was used only for patients at high surgical risk (e.g., extended liver resection, additional surgical procedures) or high anesthesiologic risk (American Society of Anesthesiologists class > 3).
Prognostic factors
To identify prognostic factors of long-term outcome we analyzed factors related to the patient (age, sex), factors related to the primary tumor (Dukes’ stage, grade of differentiation, state of colonic lymph nodes, colonic or rectal location of the primary tumor), factors related to the liver metastases [single or multiple metastases, number of lesions >3, size of the largest lesion >5 cm, synchronous or metachronous metastases, time interval between resection of primary tumor and appearance of liver metastases <12 months, monolobar or bilobar location of metastases, preoperative CEA values higher than the normal range (>5 ng/ml) and >40 times the normal range (>200 ng/ml), postresection adjuvant chemotherapy], factors related to the liver resection [type of liver resection (major, minor, anatomic, nonanatomic), use of IOUS, intraoperative blood loss >1000 ml, perioperative blood transfusions (heterologous, autologous), use and duration (>30 minutes) of portal clamping, postoperative complications and hospitalization, positive or negative resection margins]. The MSKCC-CRS risk score was calculated for each patient, and its prognostic value was analyzed.
Statistical analysis
Survival analysis was performed using the Kaplan-Meier method. The “log-rank” test was used for univariate analysis of survival curves. Multivariate analysis was performed using the Cox proportional risk regression model to identify those risk factors independently associated with survival that had been statistically significant in the univariate analysis. Differences were considered significant at p < 0.05.
Results
The results of case record analyses are shown in Table 1. The types of resection are shown in Table 2.
Long-term survival
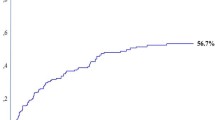
The long-term survival rate after liver resection was calculated using the Kaplan-Meier method, and the results are indicated in the respective survival curve in Figure 1). The median overall survival was 36 months, and the actuarial survival was 90.6% after 1 year, 51.0% after 3 years, 27.5% after 5 years, and 16.9% after 10 years.
Univariate analysis of prognostic factors influencing long-term survival
Demographic data
The sex and age of the patients did not show a statistically significant difference concerning overall survival.
Primary colorectal tumor-related factors
The localization and stage of the primary tumor did not show a statistically significant difference concerning survival after liver metastasis resection. On the other hand, a statistically significant difference (p = 0.0016) emerged regarding the survival of patients whose primary tumor was well differentiated [grade 1 (G1) and 2 (G2)] (median survival 41 months; 5-year survival 30.7%) with respect to patients with a less differentiated tumor (G3 and G4) (median survival 21 months; 5-year survival 14.4%).
Preoperative CEA values higher than the normal range (> 5 ng/ml) were found to worsen the long-term prognosis (median survival 30 vs. 70 months; 5-year survival 15.5% vs. 51.1%: p = 0.0016). Prognosis was even worse when preoperative CEA values were found to be >40 times the normal range (>200 ng/ml) (median survival 16 vs. 36 months; 5-year survival 17.4% vs. 27.9%: p = 0.0001).
Liver metastasis-related factors
No statistically significant difference was found for survival of patients with multiple metastases and with more than three metastases with respect to patients with a single metastasis. Furthermore, a diameter of the largest lesion > 5 cm was a negative prognostic factor (median survival 29 vs. 42 months; 5-year survival 18.8% vs. 30.0%: p = 0.0074), as was the distribution of liver lesions on both hepatic lobes (median survival 30 vs. 41 months; 5-year survival 15.5% vs. 31.9%: p = 0.039).
The occurrence of liver lesions after a disease-free interval from colorectal tumor lasting less than 12 months was found to be a negative prognostic factor concerning survival after liver resection (median survival 32 vs. 45 months; 5-year survival 23.0% vs. 36.1%: p = 0.042).
Surgery-related factors
Extension and type of resection, use and duration of portal clamping, intraoperative blood loss, and use of IOUS were not of significant importance as prognostic factors for long-term survival. Nor did neoplastic infiltration of resection margins or a resection margin free from disease of <1 cm prove to be negative prognostic factors for survival. Postoperative complications and postoperative care in the hospital for more than 2 weeks also did not appear to have a negative influence on long-term survival.
Patients requiring heterologous blood transfusions had a survival similar to that of patients not receiving blood transfusions (median survival 35 vs. 33 months, 5-year survival 26.0% vs. 24.5%: p = 0.665). However, when considering autologous blood transfusions, a statistically significant difference emerged concerning the survival curves of the three groups. There was longer survival of patients who were given autologous blood transfusions (median survival 51 months; 5-year survival 44.5: p = 0.045).
Factors related to postresection adjuvant treatment
Adjuvant treatment with systemic or locoregional intraarterial chemotherapy did not significantly influence survival after liver resection.
Risk classes for recurrence
Considering risk classes defined according to the MSKCC-CRS [17], a statistically significant difference emerged on the overall survival of the six risk classes considered singly (p = 0.043). The six classes were also classified into two groups—low risk (score 0–2) and high risk (score 3–5)—confirming the negative prognostic value of high-risk classes according to the MSKCC-CRS (median survival 30 vs. 41 months; 5-year survival 16.3% vs. 36.4%: p = 0.017).
Multivariate analysis of prognostic factors influencing long-term survival
Multivariate analysis of risk factors for long-term survival considered the following parameters: moderate or poor differentiation grade (G3–G4), preoperative CEA values >5 ng/ml and >200 ng/ml, disease-free interval after resection of colorectal tumor <12 months, diameter of the largest lesion >5 cm, bilobar distribution of hepatic lesions, need for perioperative heterologous or autologous blood transfusions, risk class according to the MSKCC-CRS considering the six classes or the two groups (low risk versus high risk). Of the above-mentioned variables, differentiation grade G3–G4 (Fig. 2), preoperative CEA values higher than the normal range (>5 ng/ml) (Fig. 3), and the high-risk class according to the MSKCC-CRS (considering the six classes singly) (Fig. 4) proved to be independent negative prognostic factors.
Discussion
The present study retrospectively analysed 297 liver resections for colorectal cancer metastases with the aim of identifying prognostic factors for long-term outcome. The present population is one of the widest data collections described by Italian authors in the English-language literature [18–21], presenting the experience of an institution that represents more than a single center. However, it has to be said that even if the present series includes the experience of three groups it is more homogeneous than retrospective studies and multicentric surveys. This is because it is characterized by elements common to the three groups (diagnostics, anesthesiology management, pathology, medical oncology), which have been determined to have real homogeneity in obtaining many of the analyzed data. Moreover, no statistically significant differences emerged in the distribution of the analyzed factors (data not shown), indicating that there have been no substantial differences in the three groups regarding the following: selection of patients undergoing liver resection (age, single or multiple lesions, unilobar or bilobar lesions, number or size of lesions), in the choice of the surgical approach (liver resection concomitant with or successive to colorectal surgery, use and duration of portal clamping), in the surgical “performance” (percentage of positive resection margins and width of the margins, perioperative morbidity/mortality, perioperative transfusions, postoperative hospitalization), or in the postresection oncologic protocols (adjuvant chemotherapy) able to determine significant differences in long-term outcome. In fact, the three groups of patients had similar 5-year survivals and disease-free intervals (data not shown). This study includes liver resections performed over a period of almost 22 years. The study period was split into two intervals, each lasting 11 years (1982–1992, 1993–2003). No significant difference was found between the two time periods with regard to patient survival. The favorable outcome of the first-decade patients—despite the absence of the positive impact of modern chemotherapy regimens and the development of surgical techniques and preoperative staging characterizing the management of patients during the last years—is probably related to the more restricted indications for surgery (as regards the number and diameter of the lesions or inclusion of elderly patients with concomitant cardiopulmonary diseases—data not shown).
The overall 1-, 3-, 5-, and 10-year survivals were 90.6%, 51.0%, 27.5%, and 16.9%, respectively, similar to data obtained from the scientific literature [17, 22], once again confirming the generally accepted opinion that liver resection is the most effective treatment for liver metastases from colorectal tumor. The overall morbidity was 17%, and mortality was 0.7%. Our data are similar to the suggested standards for major liver surgery [1, 5, 23] and confirm the safety of liver resection for metastases from colorectal cancer.
As for factors related to the primary tumor, Dukes’ staging, colonic lymph node neoplastic infiltration, and colonic or rectal location of the cancer did not have significant prognostic value. As has been noted before, the prognostic value of these factors is still debated, with some authors declaring that it has value and others denying it [17, 18, 22, 24–28]. It is possible that adjuvant chemotherapy given after colorectal resection—which in our series was performed in various centers with different indications, types, and schedules of drug administration—could have masked the prognostic value of some factors related to the primary tumor, especially concerning infiltration of visceral serosa and colonic lymph nodes. The present study, based on univariate and multivariate analyses, indicates a prognostic value for the CEA level, which correlates with a significant difference in the 5-year survival for values of >200 ng/ml (5-year survival for CEA <200 ng/ml vs. CEA >200 ng/ml: 27.9% vs. 17.4: p = 0.0001), as indicated by several authors [17, 28, 29], and for values just above the normal range (5-year survival for CEA <5 ng/ml vs. CEA >5 ng/ml 51.1% vs. 15.5%: p = 0.0016), as stated by others [30]. The negative prognostic value of a high preoperative CEA level was also described by Fong et al. [17] and was used to elaborate the MSKCC-CRS. Our experience suggests that even a moderate increase in CEA values must be considered a relevant negative prognostic factor for long-term survival. Our results show also that the differentiation grade of the tumor correlates with the long-term outcome of patients (5-year survival for G1–G2 vs. G3–G4 30.7% vs. 14.4%: p = 0.0016) and is an independent prognostic factor at the multivariate analysis, as is the preoperative CEA level. This result appears reasonable considering that poor differentiation of a tumor cell is generally associated with greater aggressiveness of the cancer.
In our case series, high preoperative CEA and high grading (G3–G4) identified the patients with the poorest prognosis after liver resection for metastases from colorectal cancer. Patients with high preoperative CEA as well as patients with unfavorable grading show a median survival (30 and 21 months, respectively) higher than that obtainable with chemotherapy regimens, which is usually <19 months, based on the best results described in the literature [31, 32]. Therefore, elevated CEA and an unfavorable grade cannot be used to identify groups of patients to be excluded a priori from liver resection. In fact, Bismuth, in the discussion of Fong et al.’s article [17], stated, “my policy is to try to resect when it is technically possible in terms of anatomy and function of the remaining liver…. We must try to give the patient the chance, even small, of a cure”.
As regards metastasis-related factors, bilobar location, major diameter of the larger lesion >5 cm, time interval between resection of the primary tumor, and appearance of metastases <12 months were significantly correlated with long-term outcome according to the univariate analysis, even if none of these factors emerged as an independent prognostic factor during the multivariate analysis. No prognostic value emerged regarding single or multiple metastases, number of lesions >3, synchronous or metachronous metastases, or postresection adjuvant chemotherapy. These results are frequently described in the literature, where the prognostic meaning of these factors has been variously confirmed or denied, regardless of whether considered singly or in combination. Fong et al. [17] identified the interval between colorectal resection and appearance of liver metastases at <12 months and the diameter of the largest lesion as >5 cm. These parameters have been included in the MSKCC-CRS. The multivariate analysis of the 1001 resections performed by Fong et al. also included in the prognostic factors the number of metastases, which did not emerge as relevant in our series.
It is interesting to note that the most important prognostic factor of extreme significance in terms of overall survival and disease-free survival in all the studies in which it had been considered is involvement of lymph nodes of the hepatic hilum. This parameter was not analyzed in our study because lymph node sampling or peduncle lymphadenectomy have not been routinely performed during liver surgery at our institution. This finding is in contrast with the supposed ability of systematic lymphadenectomy of the liver hilum to ameliorate the prognosis of patients undergoing liver resection for metastases from colorectal tumor [33, 34]. In fact, the present case series includes 300 liver resections without peduncle lymphadenectomy, showing a survival similar to that reported by other authors for patients undergoing liver resection and hilum lymphadenectomy [33, 34]. Indeed, the absence of lymph node sampling did not allow identification of groups of patients with a poor prognosis in which specific adjuvant treatments could have further ameliorated the overall survival.
Surgery-related factors—type of liver resection (major, minor, anatomic, nonanatomic), use of IOUS, intraoperative blood loss >1000 ml, perioperative transfusions (heterologous, autologous), use and duration (>30 minutes) of portal clamping, postoperative complications and hospitalization, positive or negative resection margins, distance of the disease from the resection margin >1 cm or <1 cm—did not show any prognostic value at multivariate analysis, with the exception of perioperative transfusions, whose value has been noted by other authors [17, 35–37]. On the other hand, the negative prognostic value of transfusions did not emerge at multivariate analysis, indicating a secondary role in long-term survival with respect to factors more strictly correlated with the tumor (CEA, grade).
In fact, the prognostic value of surgery-related factors is still debated; but certainly the choice of strategy for the resection does not seem to influence the patient’s outcome so long as the tumor is completely resected, even with suboptimal (<1 cm) or infiltrated margins. In the present study, infiltration of the resection margins (R1) was found in 19% of the patients, for whom the 5-year survival was reported to be 30.6%, without significant differences compared to those who had resections with a disease-free margin. The absence of a prognostic value of the resection margins status confirms the recent criticism on the absolute need for a disease-free margin at least 1 cm wide [24, 38–40]. As already mentioned, rather than excluding patients from surgery, even a minimal free resection margin or a margin with macroscopic or microscopic evidence of cancer can be accepted. Indeed, complete resection of the disease is crucial. In fact, residual disease beyond the resection margin is associated with a survival similar to that of patients not undergoing liver resection [41]. In the present series, the value of IOUS was not evident, probably due to the small number of patients who would benefit from this technique.
In the present study, the MSKCC-CRS [17] was calculated, and the long-term outcomes of the six classes (0–5) were compared, worsening as Fong’s score increased. The 5-year survivals of classes 0–3 were, respectively, 43.7%, 46.2%, 28.6%, and 21.8%; and no patients with a score of 4 or 5 survived 5 years after resection. The MSKCC-CRS revealed a significant prognostic value at univariate analysis (p = 0.0043) and at multivariate analysis (p = 0.008); moreover, a comparison between the classes with a good prognosis (CRS 0–2, 59.5% of the patients) and the classes with a poor prognosis (CRS 3–5, 40.4% of the patients) confirmed the prognostic value of the score: the 5-year survival of low-score classes (0–2) was definitely higher than the survival of high-score classes (3–5): 36.4% and 16.3%, respectively. Therefore, the MSKCC-CRS, already validated in studies [42, 43] subsequent to Fong’s original description [17], proved to be a valid instrument to identify the risk for recurrence and progression of postresection liver disease in our study.
Conclusions
The experience of our institution confirmed the safety and efficacy of liver resection for metastases from colorectal cancer. The present series did not identify single prognostic factors that were absolute contraindications to liver resection; nor did it predict a long-term outcome that was so discouraging it was, a priori, a reason to exclude patients from surgery.
Some of the potential prognostic factors that have been analyzed have identified classes of patients with poor outcomes (CEA, tumor grade); and the predictive score chosen (MSKCC-CRS) was effective in identifying low-risk and high-risk patients. The latter should be enrolled in protocols that are evaluating new diagnostic procedures and, above all, new neoadjuvant treatments with the aim of staging the disease more accurately during the preoperative phase and protecting patients more effectively from the risk of disease recurrence after liver resection.
References
Memom MA, Beckingham IJ (2001) Surgical resection of colorectal liver metastases. Colorectal Dis 3:361–373
Chang AE, Schneider PD, Sugarbaker PH, et al. (1987) A prospective randomized trial of regional vs. systemic continuous infusion 5-FU chemotherapy in the treatment of colorectal metastases. Ann Surg 206:685–693
Jaffe BM, Donegan WL, Watson F (1968) Factor influencing survival in patients with untreated hepatic metastases. Ann Gynecol Obstet 127:1–11
Bengmark S, Hafstrom L (1969) The natural history of primary and secondary malignant tumors of the liver: the prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 23:198–202
Glasgow R, Showtack J, Katz P, et al. (1999) The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg 134:30–35
Adam R, Laurent A, Azoulay D, et al. (2000) Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 232:777–785
Adam R, Huguet D, Azoulay D, et al. (2003) Hepatic resection after down-staging of unresectable hepatic colorectal metastases. Surg Oncol Clin N Am 12:211–220
Elias D, Ouellet JF, Bellon N, et al. (2003) Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg 90:567–574
Inoue M, Ohta M, Iuchi K, et al. (2004) Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 78:238–244
Bolton J, Fuhrman M (2000) Survival after resection of multiple bilobar hepatic metastases from colorectal cancer. Ann Surg 7:743–751
Imamura H, Sano K, Harihara Y, et al. (2003) Complete remission of disease for 5 years following initial and repeat resection of the liver for the removal of 22 metastases of colorectal origin. J Hepatobiliary Pancreat Surg 10:321–324
Nagakura S, Shirai Y, Wakai T, et al. (2003) An 11-year survivor who underwent ten sequential resections of colonic adenocarcinoma metastases. Hepatogastroenterology 50:1032–1033
Petrowsky H, Gonen M, Jarnagin W, et al. (2002) Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg 235:863–871
Abdalla EK, Vauthey JN, Ellis LM, et al. (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239:818–825
Adam R, Pascal G, Azoulay D, et al. (2003) Liver resection for colorectal metastases: the third hepatectomy. Ann Surg 238:871–883
Terminology Committee of the International Hepato-Pancreato-Biliary Association (2000) IHPBA Brisbane 2000 Terminology of Liver Anatomy & Resections. HPB 2:333–339
Fong Y, Fortner J, Sun RL, et al. (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318; discussion 318–321
Ercolani G, Grazi GL, Ravaioli M, et al. (2002) Liver resection for multiple colorectal metastases: influence of parenchymal involvement and total tumor volume, vs number or location, on long-term survival. Arch Surg 137:1187–1192
Doci R, Gennari L, Bignami P, et al. (1995) Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg 82:377–381
Ferrero A, Polastri R, Muratore A, et al. (2004) Extensive resections for colorectal liver metastases. J Hepatobiliary Pancreat Surg 11:92–96
Nuzzo G, Giuliante F, Giovannini I, et al. (1997) Resection of hepatic metastases from colorectal cancer. Hepatogastroenterology 44:751–759
Nordlinger B, Guiguet M, Vaillant JC, et al. (1996) Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve selection, based on 1568 patients. Cancer 77:1254–1262
De Santibanes E, Lassalle FB, McCormack L, et al. (2002) Simultaneous colorectal and hepatic resections for colorectal cancer: postoperative and longterm outcomes. J Am Coll Surg 195:196–202
Yamada H, Kondo S, Okushiba S, et al. (2001) Analysis of predictive factors for recurrence after hepatectomy for colorectal liver metastases. World J Surg 25:1129–1133
Seifert JK, Bottger TC, Weigel TF, et al. (2000) Prognostic factors following liver resection for hepatic metastases from colorectal cancer. Hepatogastroenterology 47:239–246
Laurent C, Sa Cunha A, Coudere P, et al. (2003) Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br J Surg 90:1131–1136
Yamada H, Katoh H, Kondo S, et al. (2002) Mesenteric lymph nodes status influencing survival and recurrence pattern after hepatectomy for colorectal liver metastases. Hepatogastroenterology 49:1265–1268
Choti MA, Sitzmann JV, Tiburi MF, et al. (2002) Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 235:759–766
Yamamoto J, Sugihara K, Kosuge T, et al. (1995) Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg 221:74–78
Imamura H, Matsuyama Y, Shimada R, et al. (2001) A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol 96:3178–3184
Douillard JY, Cunningham D, Roth AD, et al. (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first line treatment for metastatic colorectal cancer: a multicentric randomised trial. Lancet 355:1041–1047
De Gramont A, Figer M, Seymour M, et al. (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Ercolani G, Grazi GL, Ravaioli M, et al. (2004) The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg 239:202–209
Laurent C, Sa Cunha A, Rullier E, et al. (2004) Impact of microscopic hepatic lymph node involvement on survival after resection of colorectal liver metastasis. J Am Coll Surg 198:884–891
Imamura H, Seyama Y, Kokudo N, et al. (2004) Single and multiple resections of multiple hepatic metastases of colorectal origin. Surgery 135:508–517
Rosen CB, Nagorney DM, Taswell HF, et al. (1992) Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 216:493–505
Kooby DA, Stockman J, Ben-Porat L, et al. (2003) Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 237:860–869
Elias D, Cavalcanti A, Sabourin JC, et al. (1998) Results of 136 curative hepatectomies with a safety margin of less than 10 mm for colorectal cancer. J Surg Oncol 69:88–93
Pawlik TM, Scoggins CR, Zorzi D, et al. (2005) Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241:715–722
Bodingbauer M, Tamandl D, Schmid K, et al. (2007) Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg 94:1133–1138
Bakalakos E, Kim JA, Young DC, et al. (1998) Determinants of survival following hepatic resection for metastatic colorectal cancer. World J Surg 22:399–405
Mala T, Bohler G, Mathisen O, et al. (2002) Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome? World J Surg 26:1348–1353
Jarnagin WR, Conlon K, Bodniewicz J, et al. (2001) A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer 91:1121–1128
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arru, M., Aldrighetti, L., Castoldi, R. et al. Analysis of Prognostic Factors Influencing Long-term Survival After Hepatic Resection for Metastatic Colorectal Cancer. World J Surg 32, 93–103 (2008). https://doi.org/10.1007/s00268-007-9285-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9285-y