Abstract
Background
The epidemiology of trauma deaths in Europe is less than well investigated. Thus, our goal was to study the contemporary patterns of trauma deaths within a defined population with an exceptionally high trauma autopsy rate.
Methods
This was a retrospective evaluation of 260 consecutive trauma autopsies for which we collected demographic, pre-hospital and in-hospital data. Patients were analyzed for injury severity by standard scoring systems (Abbreviated Injury Scale [AIS], Revised Trauma Score [RTS], and Injury Severity Score [ISS]), and the Trauma and Injury Severity Scale [TRISS] methodology.
Results
The fatal trauma incidence was 10.0 per 100,000 inhabitants (17.4 per 100,000 age-adjusted ≥ 55 years). Blunt mechanism (87%), male gender (75%), and pre-hospital deaths (52%) predominated. Median ISS was 38 (range: 4–75). Younger patients (<55 years) who died in the hospital were more often hypotensive (SBP < 90 mmHg; p = 0.001), in respiratory distress (RR < 10/min, or > 29/min; p < 0.0001), and had deranged neurology on admission (Glasgow Coma Score [GCS] ≤ 8; p < 0.0001), compared to those ≥ 55 years. Causes of death were central nervous system (CNS) injuries (67%), exsanguination (25%), and multiorgan failure (8%). The temporal death distribution is model-dependent and can be visualized in unimodal, bimodal, or trimodal patterns. Age increased (r = 0.43) and ISS decreased (r = –0.52) with longer time from injury to death (p < 0.001). Mean age of the trauma patients who died increased by almost a decade during the study period (from mean 41.7 ± 24.2 years to mean 50.5 ± 25.4 years; p = 0.04). The pre-hospital:in-hospital death ratio shifted from 1.5 to 0.75 (p < 0.007).
Conclusions
While pre-hospital and early deaths still predominate, an increasing proportion succumb after arrival in hospital. Focus on injury prevention is imperative, particularly for brain injuries. Although hemorrhage and multiorgan failure deaths have decreased, they do still occur. Redirected attention and focus on the geriatric trauma population is mandated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Trauma remains a leading global cause of death. Knowledge of the epidemiological characteristics of trauma deaths is the backbone for trauma care planning, injury prevention, and systems improvements. In addition, this knowledge serves as an extremely useful baseline for determining health policy by directors of public health and for writing legislation at both the local and national levels. Seminal reports have set standards for textbook truisms and generated terms such as the “golden hour” and “trimodal distribution [1].” However, epidemiologic data from the past decades have yielded conflicting results, reflecting great geographic, socioeconomic, and regional differences [2–14]. In particular, when investigating the principle of trimodal death distribution, several authors have challenged the validity of this measure, both from the North American and the European perspectives [5, 7, 14]. Obviously, heterogeneity in the results from these studies makes general applicability difficult. In addition, autopsy rates as low as 10%, and rarely exceeding 60% in trauma [15], may further underscore the true injury spectrum in trauma deaths [16–20].
Reliable data on fatal trauma epidemiology is imperative for planning current and future trauma care in Europe. Although much can be learned from regional or national trauma registries, these data often preclude the availability and critical revision of autopsy data. Consequently, we sought to investigate the epidemiology of all trauma deaths over time in a representative North-European population, within a geographically defined area, and with a reliably high autopsy rate. Our primary aims were to document the demographic factors, the predominant injury mechanisms and severity, the causes of death, and the time distribution from injury to death after trauma.
Materials and methods
Study population
All trauma deaths occurring in the Stavanger area during a 9-year period were reviewed, beginning 1 January 1996 and ending 31 December 2004. Stavanger University Hospital (SUH) serves as the only primary trauma care facility for a mixed urban/rural population-based region of 290,000 inhabitants, and it covers trauma for a wider population approximating half a million. Annually, the SUH receives >300 patients whose Injury Severity Score (ISS) is ≥ 10 and/or whose injury mechanism require trauma team activation by preset criteria, as previously reported [21, 22]. About one quarter (80–85 patients/year) of all annual trauma admissions are severely injured patients with an ISS ≥ 16 (unpublished data from SUH Trauma Registry). The pre-hospital emergency medicine service (EMS) system is based on paramedic-manned ambulances, in addition to an anaesthesiologist-manned rapid-response car and helicopter system (HEMS), as previously described [23]. The hospital has a designated trauma team, which responds within 5 min of activation and is present in the trauma resuscitation room in the Emergency Department when the patient arrives [21]. The team leader is the senior surgical resident on-call. Consultant specialists (i.e., neurosurgery, vascular and thoracic, or gastroenterologic surgeons) are present by priority or are called in on a rapid-response basis (15 min) outside office hours.
To justify an epidemiological approach to a defined population of trauma deaths, the study population was limited to trauma deaths occurring within the Stavanger community area (with 290,000 inhabitants) and included victims holding residency within the defined region at the time of death (foreigners/tourists are often deferred for autopsy unless specifically requested). Thus, patients transferred from hospitals outside the area, as well as non-citizens (i.e., residents or visitors from other regions/countries) who died within the region during the study period were excluded. All trauma deaths with associated anatomical injury were included. Further, hangings, drownings, poisonings, and deaths from suffocation from non-traumatic causes were excluded, as were deaths after isolated femoral neck fractures, as previously reported [9, 10, 12]. Burns were also excluded, as these patients are transferred and cared for at the National Burn Unit at Haukeland University Hospital in Bergen, Norway. Population data for age- and sex-specific analysis were obtained from the Norwegian Bureau of Statistics for the fiscal year 2004 [24].
Autopsies
Autopsies were performed at the Stavanger University Hospital, Department of Pathology. Post-mortem examinations were conducted by protocol, depending on the forensic or medical designation and in general included a comprehensive description of all injuries and pathological findings and a determination of the weight of the brain, lungs, heart, liver, and kidneys—inclusive microscopic examination. Additional organs for histopathological study were included when necessary to determine the cause of death. Toxicology blood and urine screens were routinely performed in forensic autopsies. Postmortem radiological examinations were performed in select cases only.
Pre-hospital trauma deaths within the Stavanger County jurisdiction have a near 100% autopsy rate because of a general agreement between the Stavanger Police Department and the forensic pathologists at the hospital. The in-hospital autopsy rate for trauma deaths—based on data from a prospective intensive care unit registry and an in-hospital trauma registry for the years 2001–2004 (unpublished data)—exceeded 95% for all hospital-admitted fatal injuries in the selected population. Thus, with an overall annual autopsy rate > 95%, we believe the population serves as a reliable representation of a population-based Norwegian region.
Data collection and definitions
Charts of all medical and forensic autopsies performed in the study period (n = 3,932) were hand-searched by three of the investigators to retrieve all trauma-related deaths. Demographic data, injury pattern and severity, and physiological signs were obtained from pre-hospital trip charts, clinical charts, and forensic and medical autopsy records. Systolic blood pressure (SBP), respiratory rate (RR), and Glasgow Coma Scale (GCS) were recorded on arrival in the emergency department. To avoid missing values in physiological parameters, SBP, GCS, and RR were categorized on a five-point scale according to the Revised Trauma Score coded values [25], as reported by others [26]. Conservative scoring was achieved by not underscoring physiological signs if exact data were missing (i.e., if intubated, the patients’ GCS were scored from pre-intubation information, or as GCS = 8 [RTS code 2] if intubated and no other information on eye, verbal, or motor response was available).
Injury severity scoring was performed by a registrar (K.S.) trained and certified in the methods by AAAM using the Abbreviated Injury Score (AIS-90, 1998 update) [27]. Revised Trauma Score (RTS) [25], Injury Severity Score (ISS) [28], New Injury Severity Score (NISS) [29], and calculation of probability of survival (PS) for in-hospital deaths using the Trauma and Injury Severity Scale (TRISS) methodology [30].
Location of death was either pre-hospital or in-hospital, and time of death was defined as “found dead” (by police or other), “dead on scene” (when witnessed death) for pre-hospital deaths; or “acute” (within 48 h), “early” (within 3–7 days), and “late” (>7 days) for in-hospital deaths [1]. Further, temporal distribution was analyzed according to different time intervals, as previously defined [1] and reported by others [7, 9, 10]. The cause of death was defined as follows:
“Central nervous system” (CNS) if the cerebral, brainstem, or spinal injuries sustained were incompatible with life, regardless of other (severe or life-threatening) injuries.
“Exsanguination” if hemorrhage, as clinically visualized, or otherwise documented by anatomic disruption of large vessels or parenchymatous organs leading to the complete loss of blood volume, or hypovolemic cardiac arrest.
“Systemic inflammatory response syndrome/multiorgan failure syndrome” (SIRS/MOFS) as the clinical documentation, or findings on pathological examination, of renal, pulmonary, or hepatic failure (alone or in combination) as the immediate cause of death.
To investigate differences (e.g., in age, gender, injury severity) during the study period, we arbitrarily defined the “early” study period as the 5-year period 1996–2000 and the “late” study period as the years 2001–2004 (the latter period coinciding with the establishment of a trauma registry).
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Comparison between continuous variables was performed with Student’s t-test, or by analysis of variance (ANOVA) followed by Bonferroni correction when appropriate. The χ2-test or Fisher’s exact test were used for categorical data. Correlations (i.e., between age, ISS, NISS, and time from injury to death) were assessed with Spearman’s rank correlation. Time from injury to death was analyzed by the Kaplan-Meier survival plot. All statistical tests were two-tailed, and the significance level was set at p < 0.05. Data are reported as mean ± SD or 95% confidence interval (95% CI), as indicated. Incidence rates were calculated from population statistics reported by Statistics Norway [24], using the two standard deviations of the mean.
Results
Demographic data
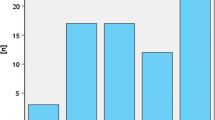
During a 9-year period, 260 trauma deaths occurred within the defined Scandinavian population under study. Demographic data are presented in Table 1. Mean age was 45.8 ± 25.1 years (median: 45 years; range: 2–100 years), and 160 (62%) patients were younger than 55 years. There were significantly more male deaths throughout all age groups (χ2-test, p = 0.027) (Fig. 1), although difference in gender distribution leveled off with increasing age (Spearman r = −0.174; p = 0.005). Injury mechanisms are presented in Figure 2A and 3B. Blunt injuries were the dominant mechanism in fatal trauma (Fig. 2B). A penetrating injury occurred in 34 (13%), caused by gunshot wounds (GSW; n = 17), shotgun wounds (SGW; n = 10), stab wounds (SW; n = 5), or blasts (n = 2). Most penetrating injuries were self-intentional/suicidal (n = 31; 94%), and only three were homicidal.
Injury mechanisms. A. Mechanism distribution according to place of death. B. Mechanism of trauma distributed in cake-plot. MVA motor vehicle accident; MCA motorcycle accident; Peds pedestrians; bikes bicycles; machinery any vehicle or heavy tools, i.e., as used in industry; violence any interpersonal or intrapersonal violent action
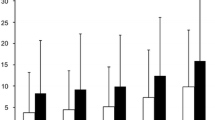
Age and injury severity according to cause of death. A. Age of death according to mode of death, showing fatal victims of exsanguination and central nervous system (CNS) injury being significantly younger than multiorgan failure (MOF) victims. B. Injury severity score is significantly lower in patients dying from MOF. Box-plot indicates medians, interquartile ranges, and total ranges (whiskers); *p < 0.001 on ANOVA (df = 2) and after Bonferroni adjustment
Injury severity
The median ISS was 38 (range: 4–75), and the median NISS was 57 (range: 6–75). The overall mean ISS score was significantly lower than the mean NISS (43.8 ± 23.2 versus 54.4 ± 19.9, respectively; p < 0.0001, t-test). Gender, age, ISS, and NISS are given in Table 2 and differed significantly among pre-hospital and in-hospital deaths, with pre-hospital deaths occurring more often in males, more often with a penetrating mechanism, at a younger age, with a higher ISS and NISS compared to the in-hospital deaths (Table 2). Comparing the same factors for in-hospital deaths remained statistically significant for acute, early, and late deaths (Table 2). In addition, the RTS and PS increased significantly from acute to early to late deaths when comparing in-hospital time to death distribution. In the pre-hospital group, 37% of the patients suffered very serious thoracic injuries (AIS of 5–6; i.e., ruptures of thoracic aorta or cardiac chambers), and 56% had very severe head and neck trauma (AIS of 5–6; i.e., brain avulsions, or high spinal cord transection), with combinations of multiple, potentially lethal injuries in many patients.
Vital signs
For in–hospital deaths, the total numbers with normotensive SBP (≥90 mmHg) versus hypotensive (<90 mmHg) were 65 and 58 patients, respectively. For normal respiration (RR 10–29/min) versus respiratory distress (RR > 29 or <10/min) the numbers were 55 and 68 patients, respectively; and for those with no or only minor head injury (GCS ≥ 9) versus moderate to severe head injury (GCS < 9), the numbers were 41 and 82 patients, respectively. Cross-tabulated for age, younger victims (<55 years) were significantly more often hypotensive (SBP < 90 mmHg; χ2-test, df = 1, p = 0.001), were in respiratory distress (RR < 10/min, or > 29/min; χ2-test, df = 1, p < 0.0001), and had a deranged cerebral status (GCS ≤ 8; χ2-test, df = 1, p < 0.0001) on arrival.
Cause of death
The predominant cause of death was CNS injury in 67% (n = 175), followed by exsanguination in 25% (n = 65), and multiorgan failure in about 8% (n = 20). Age and ISS differed significantly according to cause of death, with older and less injured patients dying of MOF (Fig. 3A and 3B). To present the injury distribution in body region according to cause of death, we obtained the maximum AIS score (AISmax) for each region, and present the mean AISmax as an expression of body region injury severity (Table 3). Notably, CNS deaths had the highest AISmax in the head and neck region; exsanguinated victims sustained the highest AISmax in the thoracic and abdominal regions, whereas deaths from SIRS/MOFS were significantly less injured in all body regions. Also, although the total numbers of injuries with AIS ≥ 3 did not differ between the other groups, SIRS/MOFS patients also sustained significantly fewer numbers of moderate to severe injuries (Table 3).
Temporal distribution of time after injury to death
Death after injury occurred on a consecutive time scale from 0 h up to 7,320 h after injury (Fig. 4). Over 50% of victims died within the first hour after injury, and less than 10% of deaths occur beyond 96 h post-injury (Kaplan-Meier curve; Fig. 4). We recognized that the temporal distribution was an effect of the chosen time intervals and the stratification-or-not to the cause of death. When using the time intervals reported by others [5], no temporal distribution is depicted for all patients together, whereas a weak near-bimodal trend appears to be caused by CNS deaths (secondary brain injury) when stratified according to cause of death (data not shown). Using the intervals reported by Trunkey [1] (Fig. 5) depicts an overall bimodal distribution, but when stratified according to cause of death, a trimodal-like distribution (although not duplicating that by Trunkey) appears (see Figure 6).
Time distribution from injury to death stratified after cause of death. Depicted is the same time-scale used in Figure 6. Excluded are those found dead, or whose time of death could not be reliably estimated (n = 58). Arrows with percentages indicate the number of deaths within the first 4 h (55%), within the next 24 h (28%), and in the ensuing days and weeks (17%). Percentages are rounded. Small percentages are given for all patients in each time interval. A weak, but present trimodal temporal distribution can be seen
Of the 75 deaths that occurred <1 h from injury, 73 (95%) were in the pre-hospital group. Of the 37 deaths occurring in the next 1–4 h, 31 (84%) died in hospital. Near 55% died within the first 4 h of injury. There was a trend toward increasing age (Spearman r = 0.43, p < 0.001) and a decrease in ISS (Spearman r = −0.52, p < 0.001) and NISS (Spearman r = −0.46, p < 0.001) with longer time from injury to death. These correlations were even stronger for the 125 in-hospital deaths, with increasing age and PS (r = 0.29 and 0.60, respectively; p = 0.001, and p < 0.001, respectively) and decrease in ISS and NISS (r = −0.45, and −0.39, respectively; both p < 0.001) with longer time from injury to death.
Incidence of fatal trauma
The overall annual death rate remained about 10 deaths per 100,000 population throughout the study period, with the highest incidence (age-adjusted) in patients >54 years of age (Table 4). An annual mean of 14 in-hospital trauma deaths occurred, for an annual fatal trauma frequency estimation of 4%–5% of all trauma admissions (n = 3 00/year) with ISS ≥ 10 or preset trauma criteria [21, 22]; and about 17% of all severely injured (ISS ≥ 16) trauma patients admitted (n = 80–85/year). The overall median annual number of trauma deaths was 24 (range: 20–41).
Differences in the early versus late period
The mean age increased significantly from the early period of the study (1996–2000) to the late (2001–2004) period (41.7 ± 24 versus 50.5 ± 25 years; Student’s t-test, p = 0.04). Also, on cross tabulation, the number of in-hospital deaths increased from the early to the late period, with a shift in the pre-hospital:in-hospital death ratio from 1.5 (early period: 83 pre-hospital versus 56 in-hospital deaths) to 0.75 (late period: 52 pre-hospital versus 69 in-hospital deaths; χ2-test, p < 0.007). No statistically significant differences were found for ISS, NISS, or PS (for in-hospital deaths), nor in the number of admissions with GCS < 8 or SBP < 90 mmHg, nor in the cause (exsanguination, CNS, or MOF) or time (acute, early, or late) of death during the early and late study period. A total 7% reduction in number of fatal victims with high probability of survival (PS > 50%) was observed from the early to the late period (from 43% to 36%; p = 0.28).
Probability of survival
Of the 123 in-hospital deaths, 47 (38%) had a PS > 0.5 (Table 5), and 37 of them were judged “frankly preventable” by the TRISS method (PS > 0.75). Of the possibly preventable deaths, 8 (80%) were the result of CNS injuries, and one each for exsanguination and MOF. Overall, MOF accounted for 19 deaths (40%) 18 of which were in the group with PS > 0.75. Further, CNS trauma accounted for 22 (47%) of the preventable deaths, and 14 of those were in the group with PS > 0.75. Exsanguination accounted for only 5 deaths (11%; <2% of all trauma deaths), all in the group with PS > 0.75. No formal peer review of all deaths was performed, as we intended to focus on groups rather than individual patients. However, closer review of the patients in the “preventable hemorrhage group” included the following: two patients with prolonged transport time resulting from difficult weather conditions developed hypothermia and acidosis (one with severe alcohol intoxication; one with near-complete torn thoracic aorta; the latter did not receive any beta-blocker before or during surgery), and both died in the operating room during surgery; one patient who underwent a damage-control procedure for severe abdominal/pelvic injuries but died in the ICU, likely as a result of continued profuse bleeding; one patient with bilateral thoracic injuries where autopsy revealed a misplaced endotracheal tube in the esophagus; and one older patient with coronary artery/ischemic heart disease who sustained moderate, combined thoracic and abdominal injuries and suffered cardiopulmonary arrest during (moderate) hemorrhage. Because only deaths (and no survivors) were included in this study, no attempts were made at comparing data, i.e., by Z- or M-statistics.
Discussion
Knowledge of the spectrum of trauma, in particular related to fatal outcome, is the backbone for trauma systems planning and injury prevention. Several recent studies have identified areas in which improvements have led to lower mortality [3, 31–33]. In modern trauma systems, fatalities still do occur but, compared to historical studies, with a somewhat changing “face and pace” [2, 5, 6, 9, 10, 12, 34, 35].
With this study we have confirmed that the “stereotypical” fatal trauma victim in a well-defined Scandinavian population is male, young, severely injured, and dying shortly after (blunt) injury. The incidence rate compares to other studies [7, 8], but it is lower than in regions with higher rates of penetrating trauma [5, 9, 36, 37] or in remote, rural areas with longer transport times [4, 13]. As reported in series of predominating blunt injury mechanism, head injury remains a frequent killer, as is the predominance of traffic-related accidents. The rate of penetrating injuries was low (13% total; lower for in-hospital deaths), mostly attributable to self-inflicted injuries in a recognized pattern [7, 8, 13].
Although much can be learned from regional or national registries, these data often preclude the availability, and critical revision, of autopsy data [16, 18]. In this study we found three areas that should receive greater focus in future studies.
First, the impact of fatal CNS trauma in our region remains significant, and further in-depth analysis to define potential protective and preventive areas appears key to reducing mortality. Secondary brain injuries contributed to a weak, but identifiable, second peak of trauma deaths. Identification of possible pre-hospital or in-hospital weaknesses in the treatment chain or suboptimal management strategies is necessary (i.e., triage and priority, intubation and ventilation strategies). However, the pattern is comparable to, and in line with other studies.
Second, although hemorrhage contributed to trauma deaths, the majority of these events occurred in patients who sustained critical anatomical injuries, and who had a poor chance of survival. In the “preventable” category we found that in most cases, the patient’s life could have been saved with optimal transport conditions and times or with an overall better health condition of the patient. There was also one case of delayed (unsuccessful) surgery and one in which a simple intubation error was identified. We stress that the latter two cases made up a minority of all patients dying from trauma in our region and, while errors are to be avoided, they still do occur in the most modern and mature level I centers [38]. We believe in open and transparent discussions of such cases to learn from mistakes [39], and in the latter study period we implemented regular trauma audits for this purpose.
Finally, MOF continues to be a late, fatal sequela in trauma—albeit at a much lower rate in recent years. The reported rate (8%) is in line with other current studies [5, 9, 40]. Of note, all MOF deaths were among older patients with significantly fewer or less severe injuries, compared to the other modes of death. Elderly trauma patients are more at risk after trauma [41, 42]. Although treatment and prevention of MOF in the general trauma population may have improved since early reports [1, 10, 43], the elderly trauma population appears to be more fragile and to have complex co-morbid patterns that make them prone to complications, including single organ or multiorgan failure. Increased awareness, followed by activation of the trauma team and early intensive monitoring, evaluation, and resuscitation of older trauma patients, has demonstrated better survival [44].
The overall mean age in our study was higher than in U.S. studies, probably because of a lower incidence of penetrating trauma (i.e., among adolescents) in our region. However, in the latter study period, we noted an overall increase in the age of trauma fatalities, a significant trend toward death in-hospital, but fewer decedents having a high likelihood of survival (PS > 0.5). The increase in the aging population is recognized in trauma centers [45], and the present study confirms this trend among Scandinavian trauma deaths. Recognition of the growing, elderly European population in general supports the need for increased focus on geriatric trauma care [46]. From our series, among patients with a PS > 0.5, the mean age was 68 years, and for those with PS > 0.75 the mean age was 72 years, findings that further mandate increased focus on the geriatric trauma population.
The temporal distribution of trauma deaths is poorly investigated in Europe, but a trimodal distribution has not been confirmed. In fact, the validity of a trimodal distribution has been called into question from numerous studies from both the United States and Europe [5, 7, 9, 14]. The frequently quoted paper by Trunkey [1] described the trimodal distribution of trauma deaths, which has been taught as textbook knowledge and perceived as a standard in most regions worldwide [1]. As depicted in the present study, the temporal distribution is an effect of the model used. The wide heterogeneity of time intervals used in various studies calls for standardized reporting of data after trauma [1, 5–7, 9–12, 14]. However, when using the Trunkey classification of immediate, early and late deaths, we found our data to be comparable with those previously reported [1, 5, 6, 12]. In particular, we noted a trend of deaths, clustering in a trimodal-like fashion when stratified according to cause of death, but with somewhat weaker peaks compared to studies from the 1980s. This is expected, as both preventive and therapeutic aspects of trauma, such as damage-control surgery and MOF management, have changed considerably. However, we believe that temporal distribution serves merely as an educational tool, because any time interval is prone to distort the actual negatively inverse relationship between the number of remaining survivors and increasing time from injury, as depicted in a Kaplan-Meier survival curve. In addition, major differences in trauma etiology, as well as factors related to transport and distance, may skew the time peaks, as noted in studies with (extremely) long transport times [4, 13]. In the present study we accrued patients who were subject to trauma within a geographic region with a maximum of 1–2 h transport distance to hospital (given optimal weather conditions).
Structural and trauma systems changes are taking place in many parts of Europe. For one, a formal trauma system is being evaluated for incorporation in Norway, and the other Scandinavian countries are likely to follow in due course (Denmark, Sweden). This study will serve as a baseline for evaluation of such efforts. Further, because of the shortcomings of U.S.-based analytical methods (e.g., the Major Trauma Outcome Study and the TRISS-method) [30, 47], there has been a call for alternative methods for trauma quality assessment in Scandinavia and other European countries [48]. Of notice, current progress in Scandinavian collaboration, together with a wider European network for trauma data collection and analysis, has the potential for significantly improving European trauma care. The data from the Stavanger area will serve as a good proxy for the general Scandinavian and European trauma population, and should thus be regarded a baseline for discussing strategies for improvement and prevention in trauma.
We believe one of the major strengths of this study to be its strictly population-based approach, with a geographically well-defined area, with a very unique and high autopsy rate (>95%), with all trauma scores performed by a certified scorer, and with trauma managed within a single-institution catchment area. Also, we included all trauma deaths occurring within the “trauma chain of survival,” ranging from out-of-hospital deaths to any in-hospital deaths or deaths after discharge [49].
Some points still must be mentioned. First, the retrospective design may potentially hamper the data quality. As such, the retrieval of data from autopsy records was, in general, very good, with very little missing information. Second, when clinical data were missing or poorly registered, such as respiratory rate and at times GCS, using the description of the patient’s vital signs allowed for classification according to RTS-coded values without loss of data for all in-hospital deaths [26].
In conclusion, this study supports the stereotypical view of the fatal trauma victim. Of note, however, the age-adjusted incidence of fatal trauma was highest among the elderly. In most victims the chances for survival are relatively poor, and death occurs immediately or very soon after injury. Major head and other CNS injuries are largely responsible for this pattern, in which prevention appears mandatory in order to decrease mortality. Fatal MOF occur primarily in older and less injured patients. Over time, the mean age increased significantly among the fatal victims, while injury mechanisms and severity remained largely unchanged. An increase in geriatric trauma patients can be expected in the future. Even though males dominate over females in all age groups, gender differences appear to level out with increasing age. In one quarter of deaths, intoxication by alcohol and cannabis was found. Penetrating trauma is rare in our region. However, when it does occur, it is directed at the head, often as an act of self-intentional harm, and almost all these patients die before they reach the hospital. The figures mandate further exploration of preventability issues, management improvements, and areas of clinical awareness that should apply not only to this trauma system, but also to similar systems in general.
References
Trunkey DD (1983) Trauma. Sci Am 249:28–35
Acosta JA, Yang JC, Winchell RJ, et al. (1998) Lethal injuries and time to death in a level I trauma center. J Am Coll Surg 186:528–533
Cinat ME, Wilson SE, Lush S, et al. (2004) Significant correlation of trauma epidemiology with the economic conditions of a community. Arch Surg 139:1350–1355
Danne PD (2003) Trauma management in Australia and the tyranny of distance. World J Surg 27:385–389
Demetriades D, Kimbrell B, Salim A, et al. (2005) Trauma deaths in a mature urban trauma system: is “trimodal” distribution a valid concept? J Am Coll Surg 201:343–348
Demetriades D, Murray J, Sinz B, et al. (1998) Epidemiology of major trauma and trauma deaths in Los Angeles County. J Am Coll Surg 187(4):373–383
Di Bartolomeo S, Sanson G, Michelutto V, et al. (2004) Epidemiology of major injury in the population of Friuli Venezia Giulia-Italy. Injury 35:391–400
Hansen KS, Morild I, Engesaeter LB, et al. (2004) Epidemiology of severely and fatally injured patients in western part of Norway. Scand J Surg 93:198–203
Potenza BM, Hoyt DB, Coimbra R, et al. (2004) The epidemiology of serious and fatal injury in San Diego County over an 11-year period. J Trauma 56:68–75
Sauaia A, Moore FA, Moore EE, et al. (1995) Epidemiology of trauma deaths: a reassessment. J Trauma 38:185–193
Shackford SR, Mackersie RC, Davis JW, et al. (1989) Epidemiology and pathology of traumatic deaths occurring at a level I trauma center in a regionalized system: the importance of secondary brain injury. J Trauma 29:1392–1397
Shackford SR, Mackersie RC, Holbrook TL, et al. (1993) The epidemiology of traumatic death. A population-based analysis. Arch Surg 128:571–575
Wisborg T, Høylo T, Siem G (2003) Death after injury in rural Norway: high rate of mortality and pre-hospital death. Acta Anaesthesiol Scand 47:153–156
Wyatt J, Beard D, Gray A, et al. (1995) The time of death after trauma. BMJ 310:1502
Pollock DA, O’Neil JM, Parrish RG, et al. (1993) Temporal and geographic trends in the autopsy frequency of blunt and penetrating trauma deaths in the United States. JAMA 269:1525–1531
Sharma OP, Scala-Barnett DM, Oswanski MF, et al. (2006) Clinical and autopsy analysis of delayed diagnosis and missed injuries in trauma patients. Am Surg 72:174–179
Sharma BR, Gupta M, Harish D, et al. (2005) Missed diagnoses in trauma patients vis-a-vis significance of autopsy. Injury 36:976–983
Marx WH, Simon HM, Jumbelic M, et al. (2004) Severity of injury is underestimated in the absence of autopsy verification. J Trauma 57:46–49; discussion 49–50
Ong AW, Cohn SM, Cohn KA, et al. (2002) Unexpected findings in trauma patients dying in the intensive care unit: results of 153 consecutive autopsies. J Am Coll Surg 194:401–406
Hodgson NF, Stewart TC, Girotti MJ (2000) Autopsies and death certification in deaths due to blunt trauma: what are we missing? Can J Surg 43:130–136
Søreide K, Søiland H, Lossius HM, et al. (2007) Resuscitative emergency thoracotomy in a Scandinavian trauma hospital—is it justified? Injury 38:34–42
Lossius HM, Langhelle A, Pillgram-Larsen J, et al. (2000) Efficiency of activation of the trauma team in a Norwegian trauma referral centre. Eur J Surg 166:760–764
Langhelle A, Lossius HM, Silfvast T, et al. (2004) International EMS Systems: the Nordic countries. Resuscitation 61:9–21
Statistisk sentralbyrå (Statistics Norway). http://www.ssb.no/english/subjects/02/01/10/folkemengde_en/arkiv/tab-2004-03-12-15-en.html (28.05.2006)
Champion HR, Sacco WJ, Copes WS, et al. (1989) A revision of the Trauma Score. J Trauma 29:623–629
Moore L, Lavoie A, LeSage N, et al. (2005) Multiple imputation of the Glasgow Coma Score. J Trauma 59(3):698–704
AAAM (Association for the Advancement of Automotive Medicine) (2001) The Abbreviated Injury Scale (AIS) 1990 revision (Update 1998). Association for the Advancement of Automotive Medicine: Barrington, Illinois
Baker SP, O’Neill B, Haddon W Jr, et al. (1974) The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 14:187–196
Osler T, Baker SP, Long W (1997) A modification of the injury severity score that both improves accuracy and simplifies scoring. J Trauma 43:922–925; discussion 925–926
Boyd CR, Tolson MA, Copes WS (1987) Evaluating trauma care: the TRISS method. Trauma Score and the Injury Severity Score. J Trauma 27:370–378
Mullins RJ, Diggs BS, Hedges JR, et al. (2006) Regional differences in outcomes for hospitalized injured patients. J Trauma 60:691–698; discussion 699–700
Liberman M, Mulder DS, Jurkovich GJ, et al. (2005) The association between trauma system and trauma center components and outcome in a mature regionalized trauma system. Surgery 137:647–658
Durham R, Pracht E, Orban B, et al. (2006) Evaluation of a mature trauma system. Ann Surg 243:775–783; discussion 783–775
Peng R, Chang C, Gilmore D, et al. (1998) Epidemiology of immediate and early trauma deaths at an urban level I trauma center. Am Surg 64:950–954
Nicholas JM, Rix EP, Easley KA, et al. (2003) Changing patterns in the management of penetrating abdominal trauma: the more things change, the more they stay the same. J Trauma 55:1095–1108; discussion 1108–1010
Meel BL (2004) Incidence and patterns of violent and/or traumatic deaths between 1993 and 1999 in the Transkei region of South Africa. J Trauma 57:125–129
Solagberu BA, Adekanye AO, Ofoegbu CP, et al. (2003) Epidemiology of trauma deaths. West Afr J Med 22:177–181
Gruen RL, Jurkovich GJ, McIntyre LK, et al. (2006) Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg 244:371–380
Stewart RM, Corneille MG, Johnston J (2006) Transparent and open discussion of errors does not increase malpractice risk in trauma patients. Ann Surg 243:645–649; discussion 649–651
Demetriades D, Murray J, Charalambides K, et al. (2004) Trauma fatalities: time and location of hospital deaths. J Am Coll Surg 198:20–26
Grossman MD, Miller D, Scaff DW, et al. (2002) When is an elder old? Effect of preexisting conditions on mortality in geriatric trauma. J Trauma 52:242–246
Bergeron E, Lavoie A, Clas D, et al. (2003) Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma 54:478–485
Border JR (1995) Death from severe trauma: open fractures to multiple organ dysfunction syndrome. J Trauma 39:12–22
Demetriades D, Karaiskakis M, Velmahos G, et al. (2002) Effect on outcome of early intensive management of geriatric trauma patients. Br J Surg 89:1319–1322
Schwab CW, Kauder DR (1992) Trauma in the geriatric patient. Arch Surg 127: 701–706
Kuhne CA, Ruchholtz S, Kaiser GM, et al. (2005) Mortality in severely injured elderly trauma patients—when does age become a risk factor? World J Surg 29:1476–1482
Champion HR, Copes WS, Sacco WJ, et al. (1990) The Major Trauma Outcome Study: establishing national norms for trauma care. J Trauma 30:1356–1365
Skaga NO, Eken T, Steen PA (2006) Assessing quality of care in a trauma referral center: benchmarking performance by TRISS-based statistics or by analysis of stratified ISS data? J Trauma 60: 538–547
Hannan EL, Farrell LS, Mottley L (2001) Motor vehicle crashes in New York State: importance of accounting for emergency department deaths when assessing differences in in-hospital mortality by level of care. J Trauma 50:1117–1124
Acknowledgments
The authors thank Kjell Tjosevik, RN, for help with extracting data from the Trauma Registry at the Stavanger University Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Søreide, K., Krüger, A.J., Vårdal, A.L. et al. Epidemiology and Contemporary Patterns of Trauma Deaths: Changing Place, Similar Pace, Older Face. World J Surg 31, 2092–2103 (2007). https://doi.org/10.1007/s00268-007-9226-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9226-9