Abstract
Introduction
Idiopathic granulomatous mastitis (IGM) is a rare benign inflammatory breast disease that presents with variable local manifestations. We describe here the different management protocols based on the clinical presentation of these patients.
Methods
A retrospective review of 20 histopathologic confirmed cases of IGM seen over a period of 10 years was performed.
Results
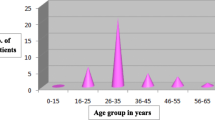
The median age was 34 years (age range: 21–45 years). All were married, parous with history of breast feeding. Ill-defined mass mimicking carcinoma was the commonest presentation (70%); however, with the presence of signs of inflammation like pain (55%), redness (40%), and peau d’orange (40%), an inflammatory process appeared more likely. Axillary lymph node enlargement was infrequently seen (40%). Radiologic findings (mammography and ultrasound) were nonspecific. Histopathology showed the characteristic lobular distribution of granulomatous inflammation in all cases. Surgically, 7 patients had abscess drainage with open biopsy, and 7 patients had lumpectomy. Six patients with diffuse breast involvement were diagnosed by core needle biopsy only. Microbial cultures showed no growth. Antibiotics were given empirically when signs of inflammation where present. Two patients needed further abscess drainage followed by persistent sinus excision 3–6 weeks later. The median follow-up was 24 months (range: 15–42 months). Seventeen patients (85%) were recurrence-free, and 3 patients (15%) were lost to follow-up.
Conclusions
Management of IGM cases needs to be tailored according to the clinical presentation. Precise radiologic and pathologic data interpretation by a multidisciplinary breast team will facilitate diagnosis and minimize unnecessary intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Idiopathic granulomatous mastitis (IGM) is a rare chronic inflammatory breast condition that was first described in 1972 [1]. Granulomatous changes occur around lobules and ducts in the breast in the absence of specific infection, trauma, foreign material, or evidence of sarcoidosis [2]. Although it is a benign condition, it can present with local manifestations that mimic carcinoma. Moreover, treatment is associated with recurrent attacks (a recurrence rate of 16%–50% has been reported) that might keep the patient under medical care for long periods [3–5]. Here we present our experience with 20 cases of IGM managed according to the clinical presentation.
Patients and methods
The database of the Breast Unit at King Fahd General Hospital, a tertiary center for medical and surgical care, Jeddah, Saudi Arabia was retrospectively reviewed from January 1996 to December 2005. During that period, 20 cases of histopathologically confirmed idiopathic granulomatous mastitis were seen. Patient characteristics, clinical presentation, radiologic work-up (mammography and/or breast ultrasound), microbiologic and histopathologic assessment, treatment modalities, and follow-up data were reviewed.
Diagnosis of IGM was confirmed histologically in all cases by core needle or open surgical biopsy. Special staining for tuberculosis and fungal infections, as well as microbial cultures (aerobic and anaerobic), were performed.
Results
During the study period, a total of 1,241 cases with different breast diseases were seen. Benign conditions (mastalgia, fibrocystic changes, fibro-adenomas, duct ectasia, simple cysts, and acute and chronic inflammatory conditions) represent 89% of the cases (1,106/1,241). Histopathologically confirmed IGM cases represent 1.8% of that 89% (20/1,106). Saudis represent 75% of those patients (15/20), and the other 5 were of various ethnic backgrounds (2 Sudanese, 2 Jordanian, and 1 Indonesian). The median age was 34 years (range: 21–45 years).
All patients were married and parous (parity range: 1–11; average, 5) with a history of breast-feeding. Two patients presented while lactating. Four used to breast feed from one breast, and they developed IGM on the contralateral side. One patient presented during pregnancy (first trimester). She was the only patient who had elevated body temperature and leukocytosis.
None of the patients had menstrual irregularities, hormonal disturbances, or used contraceptive medications. None of them smoke, and in no case was there a family history of breast diseases. Three patients were diabetic, with diabetes controlled with oral hypoglycemic agents; two where hypertensive on treatment; and one had had gestational diabetes in a previous pregnancy. One patient presented with concomitant polyarthritis that did not prove to have an autoimmune etiology. Four patients had had ipsilateral surgical drainage of breast abscesses at other medical centers 2–12 months earlier. In two of them the same breast quadrant was again involved.
Both breasts were equally involved in our series. All patients had unilateral disease. Breast quadrants were involved in the following order; more than one quadrant in 6 cases, upper outer quadrant in 5, retro-areolar area in 4, lower outer quadrant in 3; and inner quadrants in 2 (the least involved).
Patients presented with different local manifestations (Table 1). In patients with palpable breast masses, the average size of the mass was 8 cm (range: 2–20 cm). Duration of symptoms varied between 1 and 20 weeks (average: 8 weeks).
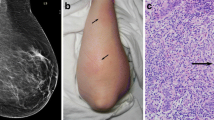
The clinical impression for each case dictated further diagnostic work-up and therapeutic approach (Table 2). Mammography and ultrasound were the standard imaging procedures performed. Mammography was performed in 8 patients only (patients presenting with mastitis or abscess were excluded). It showed focal asymmetric parenchymal densities in 3 (Fig. 1), architectural distortion in 2, ill-defined masses in 2 (Fig. 2), and diffuse increase in breast density in 1 patient. Benign microcalcifications were seen in one patient only. Ultrasound was performed in 13 patients. It was not performed for IGM cases presenting with breast abscess. It showed hypoechoic heterogeneous lesions with indistinct borders in 10, parenchymal heterogeneity with diffuse breast edema in 2, and irregular solid mass with cystic component and internal echoes in 1 patient.
Histopathologic assessment was performed on core needle biopsy (CNB) or lumpectomy specimens. Microscopically, all cases showed noncaseating granulomas involving the breast lobules with variable numbers of Langhans-type multinucleated giant cells, neutrophil polymorphs, lymphocytes, plasma cells, and eosinophils (Fig. 3). Micro-abscess formation and fat necrosis were frequently seen. Clear round spaces and foam cells were occasionally seen. Ductular damage with ulceration of ductular epithelium and the presence of polymorphs in the lumen was seen in all but two cases. There was no evidence of primary vasculitis, squamous metaplasia, keratin formation, or ductal dilatation. Refractile or birefrigent material was not identified. Special stains for micro-organisms (Gram), tuberculosis (Ziehl-Neelsen), and fungal infections (periodic acid–Schiff) were negative. In all cases, microbiologic cultures for both aerobes and anaerobes showed no bacterial growth.
Prophylactic broad-spectrum antibiotics (first-generation cephalosporin) were given to patients with signs of inflammation before open or core needle biopsy. No postoperative antibiotics were prescribed. Steroids were not used at any stage of treatment. One patient was placed on steroids as a treatment of polyarthritis, and she developed a sinus at the site of the CNB. One diabetic patient (Fig. 4) and one lactating patient needed repeat surgery 3 weeks later after formation of another abscess. Both of those patients developed a persistent sinus at the site of abscess drainage that had to be excised surgically. Our patients had regular clinical follow-up examination every 3–6 months in the outpatient department; no radiologic work-up was needed during that time. Some patients complained of minor discomfort at the operative site that was controlled with simple analgesics for short periods. The median period of follow-up was 24 months (range: 15–42 months). Seventeen patients (85%) were free of recurrence and 3 patients (15%) were lost to follow.
Discussion
Idiopathic granulomatous mastitis is a rare benign chronic inflammatory breast condition. The variability in clinical presentation and duration of symptoms reflects the heterogeneity of this entity. Although no ethnic predisposition has been documented before, prevalence of IGM in specific ethnic populations is observed; many reports come from Mediterranean countries (Turkey and Jordan) and Asia (Arabia, China and Malaysia) [3–11].
An autoimmune phenomenon has been suggested but was not proved [8, 12, 13]. There is no consistent relationship with breast-feeding, parity, and oral contraceptive hormone use [2, 8, 12, 14]. Our patients are young (median age: 34 years) and parous with a history of breast-feeding for variable periods but none used oral contraceptive hormones. Two of these patients presented during lactation, and they were treated with bromocriptin to reduced massive breast engorgement. It has been reported that prolonged breast-feeding might result in long-term distension of acini and ducts; this may facilitate rupture of these structures inducing granulomatous response [11]. This might be partially true; patients who breast-fed from one breast only developed IGM in the contralateral breast, which had sustained distension without relief. There is no clear reason why some nursing mothers prefer unilateral breast-feeding, but a significant number do so, and when breast pathology occurs later in life, it usually involves the contralateral side.
Local manifestations of IGM can mimic malignant lesions, especially when an irregular firm mass associated with nipple retraction or peau d’orange is found. Such a finding in a young patient should be interpreted with caution before making a specific diagnosis. Ipsilateral axillary lymph nodes involvement is an infrequent finding [4, 7, 10, 12].
Mammographic findings were non-specific, and sonography may better characterize such lesions, especially when they show as tubular hypoechoic foci associated with an irregular hypoechoic mass [5, 8–10].
Core needle biopsy confirmed the diagnosis in all cases that presented as a mass, but surgical excision was still performed because of the inconsistency between the radiologic and needle biopsy findings. The characteristic lobular distribution of granulomatous inflammation was seen in all cases. It is the hallmark of this lesion; hence the term “granulomatous lobular mastitis” has been recommended [2, 15]. This characteristic lobular distribution differentiates it from sarcoidosis, in which granulomas are diffusely present [13]. There was no ductal dilatation in our cases, which is a notable finding in duct ectasia/periductal mastitis (DE/PM), conditions that mimic IGM clinically and histologically [15].
Surgical drainage of abscesses in IGM differs from that in acute pyogenic abscesses. In IGM the process is more diffuse and the amount of pus is minimal and always present in multiple small loculi that communicate through tiny channels. Removing these loculi is mandatory to avoid non-healing and sinus formation. Wide excision of the whole inflammatory mass is not indicated and might even be impossible, as the cosmetic outcome would be unacceptable, especially when the disease involves more than one quadrant. Contrary to what has been reported, neither wide complete excision [7] nor conservative treatment [3] should be taken as a standard treatment for all cases of IGM. Treatment should be tailored for each case according to the clinical presentation (Table 2).
Steroids were not used in treating our patients. Because an autoimmune phenomenon was not proven and steroids are not without side effects, we believe that they can be reserved for refractory cases with diffuse breast involvement.
The course of the disease is characterized by slow resolution, which is often punctuated by abscess or discharging sinus formation, particularly after large CNB [6]. In our series, 3 patients required sinus excision 3–6 weeks after the initial intervention. One of them was receiving steroids as a treatment of polyarthritis, one was diabetic, and one was lactating. Whether the presence of co-morbid conditions interfered with healing and promoted sinus formation or whether it is the natural course of IGM by itself remains to be understood, and it deserves further study on larger number of cases. The median period of follow-up was 24 months (range: 15–42 months), during which there was no recurrence in 85% of the patients. Recurrence rates up to 50% have been reported; however, how long these patients need to be followed is not decided yet [3–5]. Because the time frame for recurrence is unknown, and because our knowledge of the fate of IGM is limited, we prefer to have our patients return for regular follow-up every 3–6 months, with clinical examination during the first 2 years and annually thereafter. Additional symptoms and clinical findings should dictate further diagnostic work-up.
Conclusions
The pathogenesis of IGM is not fully understood. Lack of evident predisposing factors and the variable forms of clinical presentation make its diagnosis a challenge. Parity, history of breast feeding, and ethnicity are associated factors that need to be confirmed by studying larger number of cases. We believe that managing IGM patients by a multidisciplinary breast team able to provide precise interpretation of breast imaging and histologic findings will help in treatment planning and avoiding unnecessary surgery.
References
Kessler E, Wolloch Y (1972) Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 58:642–646
Tavassoli FA (1999) Pathology of the Breast, 2nd Edition, New York, McGraw-Hill, p 793
Lai EC, Chan WC, Ma TK, et al. (2005) The role of conservative treatment in idiopathic granulomatous mastitis. Breast J 11:454–456
Bani- Hani KE, Yaghan RJ, Matalka II, et al. (2004) Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J 10:318–322
Azlina AF, Ariza Z, Arni T, et al. (2003) Chronic granulomatous mastitis: diagnostic and therapeutic considerations. World J Surg 27:515–518
Tse GM, Poon CS, Law BK, et al. (2003) Fine needle aspiration cytology of granulomatous mastitis. J Clin Pathol 56:519–521
Asoglu O, Ozmen V, Karanlik H, et al. (2005) Feasibility of surgical management in patients with granulomatous mastitis. Breast J 11:108–114
Han BK, Choe YH, Park JM, et al. (1999) Granulomatous mastitis: mammographic and sonographic appearances. Am J Radiol 173:317–320
Memis A, Bilgen I, Ustun E, et al. (2002) Granulomatous mastitis: imaging findings with histopathologic correlation. Clin Radiol 57:1001–1006
Kocaoglu M, Somuncu I, Ors F, et al. (2004) Imaging findings in idiopathic granulomatous mastitis. A review with emphasis on magnetic resonance imaging. J Comput Assist Tomogr 28:635–641
Kaur AC, Dal H, Muezzinoglu B, et al. (1999) Idiopathic granulomatous mastitis. Report of a case diagnosed with fine needle aspiration cytology. Acta Cytol 43:481–484
Fletcher A, Magrath IM, Riddell RH, et al. (1982) Granulomatous mastitis: a report of seven cases. J Clin Pathol 35:941–945
Jorgensen MB, Nielsen DM (1992) Diagnosis and treatment of granulomatous mastitis. Am J Med 93:97–101
Gombos EC, Esserman LE, Weisberg S, et al. (2004) Granulomatous mastitis. J Women’s Imaging 6:136–139
Going JJ, Anderson TJ, Wilkinson S, et al. (1987) Granulomatous lobular mastitis. J Clin Pathol 40:535–540
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baslaim, M.M., Khayat, H.A. & Al-Amoudi, S.A. Idiopathic Granulomatous Mastitis: A Heterogeneous Disease with Variable Clinical Presentation. World J Surg 31, 1677–1681 (2007). https://doi.org/10.1007/s00268-007-9116-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9116-1