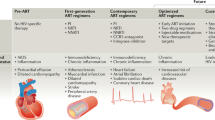
Abstract
Highly active antiretroviral therapy (HAART) has greatly reduced the risk of early death from opportunistic infections and extended the lifespan of people infected with the human immunodeficiency virus (HIV). Thus, many complications and organic damage in the HIV-infected population emerge. Cardiovascular disease as coronary artery disease has become a matter of particular concern. Its incidence is greatly increased in the HIV-infected population over that of people of the same age in the absence of general cardiovascular risk factors. Despite several clinical and laboratory studies in the association between HIV infection and cardiovascular disease, the pathogenic mechanisms of this significant clinical problem are largely unknown and are now under active investigation. Endothelial dysfunction is possibly the most plausible link between HIV infection and atherosclerosis. Increased expression of adhesion molecules such as intercellular adhesion molecule (ICAM)-1 and endothelial adhesion molecule (E-selectin) and inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL-6 has been reported in HIV-positive patients. The effect of HAART on endothelial function in HIV-positive patients is also demonstrated. In this review, we focus on the recent research update of HIV-associated vascular disease and vascular injury. We analyze and discuss the recent clinical and laboratory investigations on the effect of HIV, viral protein, and HAART therapy on endothelial injury and vascular disease; identify the areas of controversy and clinical relevance; and suggest some directions for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There were 39.4 million people who are living with human immunodeficiency virus (HIV) worldwide in 2004, of whom 4.9 million were newly infected. In the United States, over 1 million people are currently infected with HIV, and about 40,000 new cases are diagnosed each year. Acquired immune deficiency syndrome (AIDS) was responsible for the deaths of 16,000 in the US in 2004.1
Highly active antiretroviral therapy (HAART) has greatly reduced the risk of potential fatal opportunistic infections and thus has expanded the life span of these patients. Therefore, other possible causes of morbidity and mortality in the HIV-positive population have come to the forefront. In the past several years, several clinical studies have suggested that HIV-infected patients experience unexpectedly high rates of cardiovascular disease.2–5 Clearly, acute coronary syndromes, in particular, myocardial infarction, are among these potential causes of morbidity and mortality in the HIV-positive population.
The effects of many cardiovascular risk factors such as age, smoking, and hyperlipidemia on the incidence of coronary heart disease in the general population have been established. However, the pathogenic mechanisms of the increased incidence of HIV-associated vascular diseases are largely unknown. Thus, this important clinical problem is under active investigation. In this review, we focus on the recent advances in HIV-associated vascular disease and endothelial injury. Clinical investigations and possible mechanisms of atherosclerosis, pulmonary hypertension, and thrombosis in HIV-infected patients are discussed. Recent achievements in the molecular mechanism of HIV-associated endothelial injury including direct HIV infection, expression of cytokines and adhesion molecules apoptosis, and endothelial permeability are also discussed.
ATHEROSCLEROSIS
Clinical studies have suggested that HIV-infected patients experience unexpectedly high rates of atherosclerotic disease. Autopsy reports provided the first data suggesting an association between coronary artery disease and HIV infection. Atherosclerotic pathology occurred in the absence of traditional risk factors involving coronary, peripheral, and cerebral arteries in HIV-infected patients.6–11 In recent years, more and more clinical studies have indicated that people with HIV infection are epidemiologically at a significantly greater risk for coronary heart disease and myocardial infarction than uninfected people of the same age.2,3,12 A retrospective analysis in France reported that the incidence of coronary heart disease was between 5 and 5.5 per 1,000 person-years among HIV-infected people, which was at least a threefold increase over the incidence (1.52 per 1,000 person-years) in the general French male population.12 In another retrospective study of 28,513 HIV-infected people, HIV-infected men up to age 34 years and women up to age 44 years had a significantly higher incidence of cardiovascular disease than age-matched people without HIV infection (2.16–6.76 versus 1.53–2.47, P < 0.01).2 Furthermore, Klein et al. found that HIV-positive members of the Northern California Kaiser Permanente Medical Care Program, a large health maintenance organization, had a significantly higher rate of hospitalization for coronary heart disease than HIV-negative members (6.5 versus 3.8, P = 0.03) and that the rate of myocardial infarction was also greater (4.3 versus 2.9, P = 0.07).3
Many clinical ultrasound imaging studies have in fact shown an increased prevalence of subclinical atherosclerosis in patients receiving antiviral therapy.13–16 Several clinical studies further demonstrated the association of the increased prevalence of atherosclerosis with HAART.4,17–19 A study of 88,029 HIV patients in the French Hospital Database between 1996 and 199917 reported that the incidence of myocardial infarction is related to the duration of exposure to protease inhibitor (PI). Exposure to PIs was associated with a relative hazard rate for MI of 2.56 (95% confidence). The standardized morbidity rate of men exposed to PIs for 18 months, relative to the French general male population, was 0.8%, but it increased to 2.9% for men exposed to PIs for ≥ 30 months.17 In a prospective study by the HIV Outpatient Study (HOPS) for 5,672 HIV-positive patients (mean age = 42.6 years) from nine U.S. clinics, the incidence of MI was significantly increased in patients with PIs in a linear fashion.4 The Data Collection of Adverse Events Study (DAD) was a prospective observational study of 23,468 patients (median age = 39 years) from 11 cohorts in Europe, Australia, and the United States. The incidence of MI increased with increasing exposure to HAART, and the adjusted risk rate per year of exposure ranged from 0.32% for no HAART use to 2.93% for ≥ 6 years of HAART use.18 Because of the strong evidence from prospective, observational, and surrogate end point studies suggesting that HAART may be associated with an increased risk of cardiovascular events and that they may be related in part to dyslipidemia, the Infectious Disease Society of America (IDSA) and Adult AIDS Clinical Trials Group (AACTG) have updated their guidelines for the intensity of risk reduction therapy adjusted to the patient’s risk of developing cardiovascular disease in HIV-infected adults receiving antiretroviral therapy.19
HIV infection may also cause endothelial damage predisposing to atherosclerotic disease through its effect on triglyceride levels. The HIV infection itself is associated with dyslipidemia. Hypertriglyceridemia was well described in AIDS patients before the introduction of PI therapy and has been associated with elevations of circulating interferon-γ (IFN-γ).20
The antiviral therapy can cause hyperlipidemia, hyperglycemia, and central obesity.21 Cardiovascular risk is increased by these metabolic derangements, and premature atherosclerotic vascular disease may be the consequence. In addition, PIs such as ritonavir, indinavir, and amprenavir upregulate CD36, a scavenger receptor mediating cholesterol uptake in macrophages.22 Furthermore, PIs directly impair endothelium-dependent vasodilation, a marker of vascular damage preceding atherosclerotic changes.21
Inflammation induced by HIV contributes to the atherosclerotic formation. Bobryshev reported that immunohistochemical staining revealed significantly more HIV-1-infected dendritic cells in the atherosclerotic arteries than in the non-diseased segments, and he concluded that the accumulation of HIV-1 in dendritic cells in the arterial wall may influence the progression of atherosclerosis.23 Human immunodeficiency viral proteins may be able to affect smooth muscle cell (SMC) proliferation, which is a major event in vascular lesion formation; HIV gp120 was found to be a potent mitogen for rat SMCs in vitro.24 This effect seems to be mediated via gp120 sequences related to neuropeptide Y.24 Several mechanisms of HIV-induced endothelial injury or dysfunction described in the succeeding paragraphs may also contribute to the aggressive atherosclerosis formation in HIV infected patients.
THROMBOSIS
Many thromboembolic complications in HIV-infected patients have been described, among them, deep vein thrombosis (DVT), pulmonary embolism, thrombotic microangiopathy, and retinal venous thrombosis.25–30 Epidemiological studies with populations numbering from 60 to 42,935 showed that the occurrence of venous thromboembolic complications among HIV-infected people is twofold to tenfold higher than in healthy populations of comparable age.29,31–35 Furthermore, the development of venous thrombosis is found to be associated with the severity of HIV infection, with an incidence twice as high in AIDS patients as in simple HIV infection patients.36 Furthermore, a significantly higher incidence of thromboembolic events was observed in patients with low CD4 counts (< 200/mm3).28 Thromboembolic complications are also associated with HAART, with a dramatically increased incidence of venous thrombotic events from 0.19% before the introduction of PIs to 1.07% after the use of PIs.32
The pathogenesis of the increased risk of thrombosis in HIV-infected patients is not completely understood yet, but it is probably of a multifactorial nature. First of all, it might be related to a hypercoagulation state in HIV-infected patients.37 Many activated coagulation factors have been reported to be increased while anticoagulants are reduced or deficient in HIV-infected patients. For example, low levels of antithrombin (AT) were reported in HIV-infected patients with thrombosis.38 The prevalence of lupus anticoagulant in HIV infection has ranged as high as 70%.39 Anticardiolipin antibodies have been reported in 46%–90% of HIV-infected patients.40 Sugerman et al. have reported that acquired protein S deficiency is common in HIV-infected children (75%), significantly more prevalent in children with a CD4 count < 200/mm3.41 Decreased levels of protein C have also been detected in HIV-infected patients,42,43 and reduced protein S plasma levels and diminished activity were reported.44,45 Another physiological coagulation inhibitor, heparin cofactor II, has also been reported to be deficient in HIV infection. Other possible contributing factors are hypoalbuminemia-related fibrin polymerization defects, endothelial dysfunction, and abnormalities of the fibrinolytic system.39 Various markers of endothelial cell damage such as von Willebrand factor (vWF), soluble thrombomodulin (sTM), adhesion molecule E-selectin, tissue-type plasminogen activator (tPA), plasminogen activator inhibitor (PAI-1), fibronectin, angiotensin-converting enzyme (ACE), and endothelin have been shown to be increased in the course of HIV-1 infection.46–50 The secretion of tPA, (PAI-1), sTM, and vWF creates alterations in the coagulation cascade and could predispose to thrombosis. Furthermore, HIV gp120 could induce tissue factor expression in vascular SMCs, which may have potential effects on the arterial wall thrombogenicity.51
PULMONARY HYPERTENSION
Primary pulmonary hypertension, first described in hemophilic HIV-infected patients, occurs with a frequency of approximately 0.5% in patients with HIV infection, whereas the general yearly incidence is only approximately 1 per million people.52 Pulmonary hypertension in HIV infection showed plexogenic pulmonary arteriopathy in 95% of all cases,53 similar to the findings in immunocompetent patients. Antiretroviral therapy remains questionable in the treatment, even though the potential for slowing the progression of pulmonary hypertension or even causing its regression, has been observed.54,55
The pathogenesis of HIV-related pulmonary hypertension is not well understood. In vivo and in vitro studies show that HIV does not infect lung endothelial cells and HIV DNA, RNA, or p24 antigen could not be detected in the pulmonary vessels of patients with HIV-related pulmonary hypertension.56–58 It is possible, however, that HIV indirectly induces the development of pulmonary hypertension through increased production of inflammatory cytokines and chemokines by infected lymphocytes and alveolar macrophages.56–58 Endothelial dysfunction is central to the pathogenesis of pulmonary hypertension.59 Studies show that HIV and gp120 proteins can induce injury in lung endothelial cells.60 HIV may cause endothelial damage and mediator-related vasoconstriction through stimulation by the envelope gp120, including direct release and effects of endothelin-1 (vasoconstrictor), IL-6, and tumor necrosis factor (TNF)-α in the pulmonary arteries.55,61 Endothelin-1 levels are elevated in patients with pulmonary hypertension, and its plasma concentrations correlate with the severity of the disease.62 There is also increased expression of endothelin-1 in the vascular endothelium of patients with pulmonary hypertension, and this correlates with pulmonary vascular resistance.63,64 In vitro studies show that HIV gp120 stimulates the production of endothelin-1 in primary human lung endothelial cells,65,66 which further suggests that HIV and secreted viral proteins may play a role in the production of vasoconstrictors within the lung and contribute to the development of pulmonary hypertension.
CYTOKINES AND ADHESION MOLECULES
Inflammation and endothelial dysfunction play important roles in atherosclerosis formation. Adhesion molecules enabling leukocytes to communicate and adhere to endothelial cells are essential for immunological and inflammatory responses.
HIV infection is associated with the inflammatory activation in the vascular wall. Tumor necrosis factor-α is an inflammatory cytokine that is expressed in large amounts by HIV-infected macrophages and can activate endothelial cells and enhance leukocyte adhesion.67,68 A clinical study conducted by de Larrañaga et al. found that HIV-infected patients had significantly higher plasma levels of TNF-α and IL-6 than uninfected controls. These levels correlated with viral load.69 Wolf et al. found that the levels of VCAM-1, intercellular adhesion molecule-1 (ICAM-1), and vWF were higher in untreated patients with HIV infection than in HIV-negative blood-donor controls, and the levels decreased significantly in all subjects in the HIV infection group after 5–13 months of antiviral treatment.70 Nordoy et al. also found significantly higher levels of TNF-α and neopterin, as well as soluble adhesion molecules sICAM-1 and sVCAM-1 in their HIV-infected patients than in controls.71
HIV activation of inflammatory pathways was suggested to be a specific effect of the transactivator of transcription protein (Tat). Buonaguro et al. showed that transduction of the Tat gene into a monocyte cell line led to TNF-β production, and the same effect was observed on T-lymphocyte and epithelial cell lines.72 The Tat protein also induces synthesis of NF-κB and IL-6 in both lymphoblastoid and epithelial cells.73 Furthermore, Westendorp et al. found that the Tat protein amplified TNF-α–induced activation of NF-κB by suppressing the synthesis of Mn-dependent superoxide dismutase, thus exposing the cells to oxidative stress. This effect was seen in both HeLa cells transfected with the Tat gene and soluble Tat protein-treated HeLa cells and T cells.74 The HIV Tat not only activates mononuclear cells to secrete cytokines but also activates endothelial cells to express inflammatory cytokines and adhesion molecules and contributes to angiogenesis and the development of Kaposi’s sarcoma (KS).75,76 Hofman et al. found that Tat appeared to bind to Flk-1/KDR, a VEGF-A tyrosine kinase receptor, as well as to mediate angiogenesis.77 Further studies discovered that Tat protein activated endothelial cells by increasing the expressions of E-selectin, IL-6, and IL-8.78,79 Interleukin-6 is considered to be an acute phase cytokine and can increase endothelial cell permeability, whereas IL-8 is chemotactic to leukocytes and assists in inflammatory cell recruitment. The HIV-1 Tat protein activated human umbilical vein endothelial cell E-selectin expression via an NF-kB–dependent mechanism.80 Tat protein also induced monocyte chemoattractant protein-1 (MCP-1) expression in brain astrocytes81 and human lung microvascular endothelial cells.82 Increased MCP-1 expression resulted in monocyte transmigration across the endothelial cells. Dhawan et al. showed that the HIV Tat protein also induced VCAM-I, ICAM-I, and endothelial cell adhesion molecule-I in cultured vascular endothelial cells.83 Furthermore, monocytes infected with HIV showed increased adherence to vascular endothelial cell layers. Infected monocytes also secreted gelatinase into the culture medium, markedly disrupting the endothelial cell layers with which they were co-cultured.84 Later research showed that these effects could be largely mimicked by extracellular treatment with Tat protein.85 Extracellular treatment with Tat protein also increased monocytes’ migratory behavior and their ability to penetrate reconstituted extracellular membranes.86
Besides the effect of Tat protein, HIV gp120 also has several effects relevant to endothelial cell functions. Furthermore, HIV gp120 has been shown to induce monocytes to express proinflammatory cytokines, thus activating endothelial cells., and HIV gp120 protein was able to increase the expressions of prostaglandin E2 and IL-1 in monocytes with a dose-dependent manner.87 Both IL-1 and prostanoids have profound effects on endothelial adhesive processes.88,89 Bragardo et al. studied two types of events mediating T cell–endothelial adhesion interaction: dynamic adhesion mediated by selectins and static adhesion mediated by integrins. Their study showed that gp120 enhanced dynamic adhesion, which involved CD31, CD38, and CD49d.90 In addition, HIV gp120 alone could also quickly cause monocytes to adhere to endothelial cells.91 Stins et al. demonstrated that soluble gp120 increased expression of ICAM-1, VCAM-1, and IL-6 in human brain microvascular endothelial cells from children and increased monocyte transmigration across the monolayer in vitro. They further discovered that the effect was mediated via CD4 in endothelial cells.92 In a HIV-1 g120 transgenic mouse model, ICAM-1, VCAM-1, and substance P were highly expressed in brain vessel endothelial cells, and there was a significant correlation between substance P and ICAM-1 expression.93 Furthermore, gp120 was able to enhance the TNF-α–mediated activation of NF-κB in Jurkat cells accompanied by increased formation of reactive intermediates such as H2O2.94 Recently, we have demonstrated that both soluble and membrane associated gp120 on virus-like particles could induce ICAM-1 expression on several types of cultured human endothelial cells and then increased monocyte adhesions to the endothelial monolayer.95
Expression of the HIV Nef protein in macrophages induces expression of two chemokines, macrophage inflammatory proteins-1α and -1β.96 Later work showed that treating monocytes with soluble Nef induced a variety of inflammatory factors—not only macrophage inflammatory proteins-1α and -1β, but also IL-1β, IL-6, and TNF-α. Furthermore, this release of inflammatory factors correlated with activation of NF-κB.97
APOPTOSIS
HIV-1–induced CD4+ T cell apoptosis is a major mechanism of HIV pathogenesis.98 Furthermore, HIV-1 is able to induce apoptosis in other types of cells, including endothelial cells. The apoptosis of microvascular endothelial cells has been observed in the brain tissue of AIDS patients,99 and similar EC apoptosis was demonstrated in the brain tissue of macaque monkeys infected with simian immunodeficiency virus (SIV).100 In a HIV transgenic rat model infected with a HIV provirus with a functional deletion of gag and pol, endothelial cell apoptosis and microscopic hemorrhages were observed in the brain tissue.101
Several in vitro studies have reported that HIV-1 and gp120 can induce EC apoptosis. Soluble gp120 as well as virion- and cell membrane-associated gp120 are able to induce toxicity and apoptosis in human umbilical vein endothelial cells (HUVECs).102,103 The HIV gp120 protein is also toxic to human brain microvascular endothelial cells.104 and is able to alter the functional and molecular properties of the blood–brain barrier.105 Recently, Kanmogne et al. reported that HIV-1 gp120 protein directly induced apoptosis in primary human lung microvascular endothelial cells as determined by TUNEL assay, annexin-V staining, and DNA laddering. The gp120-induced lung endothelial cell injury was considered as a possible contributing factor in the development of HIV-associated pulmonary hypertension.66 A study to investigate the effect of antiviral drugs and HIV infection on endothelial damage indicated that HIV-1 and gp120, not antiviral drug (AZT), induced apoptosis of neonatal rat ventricular myocytes (NRVMs) and human coronary artery endothelial cells (HCAECs).106 Soluble gp120 can increase HUVEC apoptosis in a biphasic fashion suggesting that multiple cellular factors might be involved in the effect.103 The apoptotic effect was found to be mediated through two cell surface receptors (CCR5 and CXCR4) on HUVECs, and by protein kinase C activation.102 Further studies revealed that gp120 on cell membranes or on virion particles was an even more potent inducer of endothelial apoptosis than soluble gp120.103 Caspases played an important role in this process; the pretreatment of cells with a general caspase enzyme inhibitor decreased the extent of HUVEC apoptosis induced by gp120/160.107 In fact, caspase-3 was activated and the pro-apoptotic molecule Bax was increased by HUVECs following gp120/160 treatment.107 In addition, recombinant gp120 was observed to induce human lung microvascular endothelial cells (HLMEC) apoptosis.108
The HIV Tat protein has also been demonstrated to induce endothelial apoptosis. Kim et al. 109 reported that HIV-1 Tat induced apoptosis of human brain miscrovascular endothelial cells (HBMEC), as evidenced by changes in the cleavage of poly(A)DP-ribose polymerase, DNA laddering, and incorporation of fluorescein into the nicked chromosomal DNA (TUNEL assay). Park et al.108 reported that Tat induced apoptosis of primary microvascular endothelial cells of lung origin by a mechanism distinct from TNF secretion or the Fas pathway. Also, Tat treatment increased caspase 3 activity, but not caspase-9 activity. Tat-derived peptides were also able to induce endothelial apoptosis. Treatment with the Tat 21-40 or 23-34 peptides increased the percentage of apoptotic cells in HUVECs, and it blocked the anti-apoptotic effect of VEGF. The Tat 27–38 peptide produced a similar striking apoptotic effect, and the Tat 46–57 peptide alone induced a modest increase in annexin V staining, but it completely blocked VEGF-induced anti-apoptosis.110
ENDOTHELIAL PERMEABILITY
The normal endothelial cell layer constitutes a macromolecular barrier between the blood vessels and underlying tissues. Injury to the ECs and endothelial junction structures could increase endothelial permeability, which is one of the critical events in pathological conditions such as atherosclerosis, inflammation, and other vascular complications.
Approximately 20% of individuals with AIDS develop HIV encephalitis (HIVE), and 15% develop HIV-1–associated dementia (HAD) in the United States.111 Despite the initial drop as a result of HAART, the prevalence of both HIVE and HAD is again increasing as HIV-infected individuals are living longer.112–114 Human immunodeficiency virus enters the CNS early after the primary infection.115–117 Studies have suggested that this mechanism of viral entry into the brain is dependent on the transmigration of infected leukocytes across the blood–brain barrier (BBB) and into the CNS.81,118 The BBB is composed mainly of specialized ECs in contact with astrocytes, which are connected by tight junction proteins.119 Blood–brain barrier dysfunction is common in HIV-1–infected individuals and is implicated in the pathogenesis of HAD.120,121 Tight junction protein disruption has been detected in HIV encephalitic brain tissue.120,122 Dysfunction of brain endothelial cells caused by HIV-1 plays an important role in AIDS neuropathogenesis.123,124
In addition to the BBB dysfunction in HIV-associated diseases, other damage to endothelial cell functions related to membrane permeability has been reported. In HIV-associated ocular microangiopathic syndrome, both clinical and morphologic studies revealed endothelial permeability and vascular leakage in the ocular tissue from HIV-infected patients.125 Also, pathological observations in patients with HIV-associated pulmonary artery hypertension include lesions of the pulmonary endothelium and increased endothelial cell permeability.58,66,126
The mechanisms of HIV-associated endothelial permeability increase are under active investigation. In vitro studies have shown that HIV-1 gp120 elicited endothelial toxicity,104 leading to disruption and downregulation of TJPs in HBMECs.105 A recent study showed that HIV gp120 derived from the macrophage (CCR5)-tropic virus decreased BBB tightness, increased permeability in HBMECs, and enhanced monocyte migration across in vitro BBB models. In addition, HIV gp120 was observed to cause dysfunction of the BBB via protein kinase C (PKC) pathways and receptor-mediated [Ca2+] release.127
Furthermore, HIV Tat may also have the capability to affect endothelial permeability. Tat protein significantly increases HUVEC monolayer permeability in a dose-dependent manner.128,129 The blocking experiments suggest that tyrosine kinase and MAP kinase, but not protein kinase G pathways, may mediate Tat-induced endothelial permeability.129 The Tat protein has also been shown to increase endothelial permeability in animal models.128,130 Tat synergized with basic growth factor (bFGF) induced vascular permeability and edema in guinea pigs and nude mice after systemic or local injection of Tat and bFGF.130 Kim et al. reported that HIV-1 Tat not only induced apoptosis of HBMEC but also significantly increased HBMEC permeability, resulting in the irreversible loss of BBB integrity.109 Avraham et al. reported that Tat treatment stimulated cytoskeletal organization and increased focal adhesion assembly leading to changes in the migration of HBMECs. Tat also induced BBB permeability, as observed in the endothelial cells isolated from HIV-1 Tat transgenic mice.131 Our recent study showed that HIV PI ritonavir increased endothelial permeability, decreases levels of tight junction proteins, and increased superoxide anion production in human dermal microvascular endothelial cells. These effects may underlie the mechanism of HIV PI-associated cardiovascular complications.132
Cellular molecules secreted from HIV-infected cells likely play an important role in BBB impairment and the development of HAD.133,134 Elevated levels of chemokine CC chemokine ligand 2 (CCL2), CCL5, and CXCL10 have been detected in the brain and cerebrospinal fluid (CSF) of patients with HIVE and HAD,135–138 suggesting that glial cells and endothelial cells, major sources of chemokines, may play a key role in the recruitment of uninfected and HIV-infected leukocytes into the CNS. Using a tissue culture model of the human BBB, Eugenin et al. demonstrated that HIV infection of human leukocytes resulted in their increased transmigration across the BBB in response to the chemokine CCL2, as well as in disruption of the BBB, as evidenced by enhanced permeability, reduction of tight junction proteins, and expression of matrix metalloproteinases (MMP)-2 and MMP-9. When HIV-infected cells were added to the model, they did not transmigrate in the absence of CCL2, nor did this condition alter BBB integrity.133 In the brain tissue from individuals with HIV encephalitis, there was an accumulation of cleaved and soluble forms of the extracellular region of the platelet/endothelial cell adhesion molecule (PECAM-1), i.e., sPECAM-1. In addition, HIV-infected individuals had elevated levels of sPECAM-1 in their sera. In vitro study demonstrated that HIV-infected leukocytes, when treated with CCL2, shed sPECAM-1, suggesting a mechanism of extracellular PECAM-1 cleavage and release dependent on HIV infection and CCL2.134
SUMMARY
With increased life expectancy in HIV patients, the prevalence of HIV-associated cardiovascular complications involving endothelial injuries has greatly increased in recent years, and it is a significant clinical concern. Dyslipidemia and other metabolic changes in patients with HIV and those using HAART may contribute to increased cardiovascular risk. Many clinical and laboratory studies have found that HIV infection causes profound functional alterations of the endothelium. The virus and its viral proteins such as gp120, Tat, and Nef are able to induce expression of several adhesion molecules and inflammatory cytokines such as ICAM-1, VCAM-1, E-selectin, TNF-α, and IL-6. Leukocyte adherence to the endothelium is enhanced as the expression of these cell adhesion molecules increases. Elevated circulating levels of vWF, a glycoprotein facilitating platelet adhesion, synthesized in endothelial cells and inflammatory cells, are elevated and correlate to circulating levels of inflammatory cytokines. A hypercoagulable state is induced depending on plasma HIV load. In addition, HIV and its viral proteins can also induce endothelial apoptosis and increase endothelial permeability. These effects could significantly contribute to vascular disease formation. However, the molecular mechanisms underlying HIV-associated vascular diseases and endothelial injury are not completely understood. Future studies on the associated mechanism of HIV infection and cardiovascular disease, as well as endothelial dysfunction, may contribute to the development of preventive and therapeutic approaches to the treatment of patients with HIV-associated cardiovascular disease.
References
AIDS epidemic update. Geneva Switzerland:World Health Organization, 2004:1–94
Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003;33:506–512
Klein D, Hurley LB, Quesenberry CP, Jr., et al. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr 2002;30:471–477
Holmberg SD, Moorman AC, Williamson JM, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet 2002;360:1747–1748
Bozzette SA, Ake CF, Tam HK, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med 2003;348:702–710
Joshi VV, Pawel B, Connor E, et al. Arteriopathy in children with acquired immune deficiency syndrome. Pediatr Pathol 1987;7:261–275
Paton P, Tabib A, Loire R, et al. Coronary artery lesions and human immunodeficiency virus infection. Res Virol 1993;144:225–231
Tabib A, Leroux C, Mornex JF, et al. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis 2000;11:41–46
Constans J, Marchand JM, Conri C, et al. Asymptomatic atherosclerosis in HIV-positive patients: a case-control ultrasound study. Ann Med 1995;27:683–685
Nair R, Robbs JV, Chetty R, et al. Occlusive arterial disease in HIV-infected patients: a preliminary report. Eur J Vasc Endovasc Surg 2000;20:353–357
Pinto AN. AIDS and cerebrovascular disease. Stroke 1996;27:538–543
Vittecoq D, Escaut L, Chironi G, et al. Coronary heart disease in HIV-infected patients in the highly active antiretroviral treatment era. AIDS 2003;17(Suppl 1):S70–S76
Depairon M, Chessex S, Sudre P, et al. Premature atherosclerosis in HIV-infected individuals—focus on protease inhibitor therapy. AIDS 2001;15:329–334
Maggi P, Serio G, Epifani G, Fiorentino G, et al. Premature lesions of the carotid vessels in HIV-1-infected patients treated with protease inhibitors. AIDS 2000;14:F123–128
Meng Q, Lima JA, Lai H, et al. Coronary artery calcification, atherogenic lipid changes, and increased erythrocyte volume in black injection drug users infected with human immunodeficiency virus-1 treated with protease inhibitors. Am Heart J 2002;144:642–648
Seminari E, Pan A, Voltini G, et al. Assessment of atherosclerosis using carotid ultrasonography in a cohort of HIV-positive patients treated with protease inhibitors. Atherosclerosis 2002;162:433–438
Mary-Krause M, Cotte L, Simon A, et al. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS 2003;17:2479–2486
Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003
Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003;37:613–627
Grunfeld C, Kotler DP, Hamadeh R, et al. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med 1989;86:27–31
Stein JH, Klein MA, Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation 2001;104:257–262
Dressman J, Kincer J, Matveev SV, et al. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest 2003;111:389–397
Bobryshev YV. Identification of HIV-1 in the aortic wall of AIDS patients. Atherosclerosis 2000;152:529–530
Kim J, Ruff M, Karwatowska-Prokopczuk E, et al. HIV envelope protein gp120 induces neuropeptide Y receptor-mediated proliferation of vascular smooth muscle cells: relevance to AIDS cardiovascular pathogenesis. Regul Pept 1998;75–76:201–205
Cohen JR, Lackner R, Wenig P, Pillari G. Deep venous thrombosis in patients with AIDS. NY State J Med 1990;90:159–161
Miller RF, Scully M, Cohen H, et al. Thrombotic thrombocytopaenic purpura in HIV-infected patients. Int J STD AIDS 2005;16:538–542
Ramanampamonjy RM, Ramarozatovo LS, Bonnet F, et al. [Portal vein thrombosis in HIV-infected patients: report of four cases]. Rev Med Intern 2005;26:545–548
Saif MW, Greenberg B. HIV and thrombosis: a review. AIDS Patient Care STDS 2001;15:15–24
Copur AS, Smith PR, Gomez V, et al. HIV infection is a risk factor for venous thromboembolism. AIDS Patient Care STDS 2002;16:205–209
Witz M, Lehmann J, Korzets Z. Acute brachial artery thrombosis as the initial manifestation of human immunodeficiency virus infection. Am J Hematol 2000;64:137–139
Hassell KL, Kressin DC, Neumann A, et al. Correlation of antiphospholipid antibodies and protein S deficiency with thrombosis in HIV-infected men. Blood Coagul Fibrinolysis 1994;5:455–462
George SL, Swindells S, Knudson R, et al. Unexplained thrombosis in HIV-infected patients receiving protease inhibitors: report of seven cases. Am J Med 1999;107:624–630
Sullivan PS, Dworkin MS, Jones JL, et al. Epidemiology of thrombosis in HIV-infected individuals. The Adult/Adolescent Spectrum of HIV Disease Project. AIDS 2000;14:321–324
Nordstrom M, Lindblad B, Bergqvist D, et al. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med 1992;232:155–160
Saif MW, Bona R, Greenberg B. AIDS and thrombosis: retrospective study of 131 HIV-infected patients. AIDS Patient Care STDS 2001;15:311–320
Laing RB, Brettle RP, Leen CL. Venous thrombosis in HIV infection. Int J STD AIDS 1996;7:82–85
Klein SK, Slim EJ, de Kruif MD, et al. Is chronic HIV infection associated with venous thrombotic disease? A systematic review. Neth J Med 2005;63:129–136
Toulon P, Lamine M, Ledjev I, et al. Heparin cofactor II deficiency in patients infected with the human immunodeficiency virus. Thromb Haemost 1993;70:730–735
Toulon P. [Hemostasis and human immunodeficiency virus (HIV) infection]. Ann Biol Clin (Paris) 1998;56:153–160
Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Med Clin North Am 1997;81:449–470
Sugerman RW, Church JA, Goldsmith JC, et al. Acquired protein S deficiency in children infected with human immunodeficiency virus. Pediatr Infect Dis J 1996;15:106–111
Feffer SE, Fox RL, Orsen MM, et al. Thrombotic tendencies and correlation with clinical status in patients infected with HIV. South Med J 1995;88:1126–1130
Erbe M, Rickerts V, Bauersachs RM, et al. Acquired protein C and protein S deficiency in HIV-infected patients. Clin Appl Thromb Hemost 2003;9:325–331
Stahl CP, Wideman CS, Spira TJ, et al. Protein S deficiency in men with long-term human immunodeficiency virus infection. Blood 1993;81:1801–1807
Bissuel F, Berruyer M, Causse X, et al. Acquired protein S deficiency: correlation with advanced disease in HIV-1-infected patients. J Acquir Immune Defic Syndr 1992;5:484–489
Schved JF, Gris JC, Arnaud A, et al. von Willebrand factor antigen, tissue-type plasminogen activator antigen, and risk of death in human immunodeficiency virus 1-related clinical disease: independent prognostic relevance of tissue-type plasminogen activator. J Lab Clin Med 1992;120:411–419
Lafeuillade A, Alessi MC, Poizot-Martin I, et al. Endothelial cell dysfunction in HIV infection. J Acquir Immune Defic Syndr 1992;5:127–131
Rolinski B, Geier SA, Sadri I, et al. Endothelin-1 immunoreactivity in plasma is elevated in HIV-1 infected patients with retinal microangiopathic syndrome. Clin Invest 1994;72:288–293
Seigneur M, Constans J, Blann A, et al. Soluble adhesion molecules and endothelial cell damage in HIV infected patients. Thromb Haemost 1997;77:646–649
Hadigan C, Meigs JB, Rabe J, et al. Increased PAI-1 and tPA antigen levels are reduced with metformin therapy in HIV-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab 2001;86:939–943
Schecter AD, Berman AB, Yi L, Mosoian A, et al. HIV envelope gp120 activates human arterial smooth muscle cells. Proc Natl Acad Sci USA 2001;98:10142–10147
Le Houssine P, Karmochkine M, Ledru F, et al. Primary pulmonary hypertension in human immunodeficiency virus infection. Study of 9 cases and review of the literature. Rev Med Intern 2001;22:1196–1203
Rerkpattanapipat P, Wongpraparut N, Jacobs LE, et al. Cardiac manifestations of acquired immunodeficiency syndrome. Arch Intern Med 2000;160:602–608
Speich R, Jenni R, Opravil M, Jaccard R. Regression of HIV-associated pulmonary arterial hypertension and long-term survival during antiretroviral therapy. Swiss Med Wkly 2001;131:663–665
Pellicelli AM, Palmieri F, D’Ambrosio C, et al. Role of human immunodeficiency virus in primary pulmonary hypertension—case reports. Angiology 1998;49:1005–1011
Kanmogne GD, Kennedy RC, Grammas P. Is HIV involved in the pathogenesis of non-infectious pulmonary complications in infected patients? Curr HIV Res 2003;1:385–393
Klings ES, Farber HW. The pathogenesis of HIV-associated pulmonary hypertension. Adv Cardiol 2003;40:71–82
Pellicelli AM, D’Ambrosio C, Vizza CD, et al. HIV-related pulmonary hypertension. From pathogenesis to clinical aspects. Acta Cardiol 2004;59:323–330
Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004;109:159–165
Kanmogne GD, Kennedy RC, Grammas P. Analysis of human lung endothelial cells for susceptibility to HIV type 1 infection, coreceptor expression, and cytotoxicity of gp120 protein. AIDS Res Hum Retroviruses 2001;17:45–53
Pellicelli AM, Barbaro G, Palmieri F, et al. Primary pulmonary hypertension in HIV patients: a systematic review. Angiology 2001;52:31–41
Cody RJ, Haas GJ, Binkley PF, et al. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation 1992;85:504–509
Mesa RA, Edell ES, Dunn WF, et al. Human immunodeficiency virus infection and pulmonary hypertension: two new cases and a review of 86 reported cases. Mayo Clin Proc 1998;73:37–45
Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993;328:1732–1739
Ehrenreich H, Rieckmann P, Sinowatz F, et al. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J Immunol 1993;150:4601–4609
Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun 2005;333:1107–1115
Krishnaswamy G, Smith JK, Mukkamala R, et al. Multifunctional cytokine expression by human coronary endothelium and regulation by monokines and glucocorticoids. Microvasc Res 1998;55:189–200
Herbein G, Keshav S, Collin M, et al. HIV-1 induces tumour necrosis factor and IL-1 gene expression in primary human macrophages independent of productive infection. Clin Exp Immunol 1994;95:442–449
de Larrañaga GF, Petroni A, Deluchi G, et al. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinolysis 2003;14:15–18
Wolf K, Tsakiris DA, Weber R, et al. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis 2002;185:456–462
Nordoy I, Aukrust P, Muller F, et al. Abnormal levels of circulating adhesion molecules in HIV-1 infection with characteristic alterations in opportunistic infections. Clin Immunol Immunopathol 1996;81:16–21
Buonaguro L, Barillari G, Chang HK, et al. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol 1992;66:7159–7167
Scala G, Ruocco MR, Ambrosino C, et al. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J Exp Med 1994;179:961–971
Westendorp MO, Shatrov VA, Schulze-Osthoff K, et al. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. Embo J 1995;14:546–554
Liu NQ, Lossinsky AS, Popik W, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol 2002;76:6689–6700
Mitola S, Soldi R, Zanon I, et al. Identification of specific molecular structures of human immunodeficiency virus type 1 Tat relevant for its biological effects on vascular endothelial cells. J Virol 2000;74:344–353
Albini A, Soldi R, Giunciuglio D, et al. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med 1996;2:1371–1375
Hofman FM, Wright AD, Dohadwala MM, et al. Exogenous tat protein activates human endothelial cells. Blood 1993;82:2774–2780
Hofman FM, Chen P, Incardona F, et al. HIV-1 tat protein induces the production of interleukin-8 by human brain-derived endothelial cells. J Neuroimmunol 1999;94:28–39
Cota-Gomez A, Flores NC, Cruz C, et al. The human immunodeficiency virus-1 Tat protein activates human umbilical vein endothelial cell E-selectin expression via an NF-kappa B-dependent mechanism. J Biol Chem 2002;277:14390–14399
Weiss JM, Nath A, Major EO, et al. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood–brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol 1999;163:2953–2959
Park IW, Wang JF, Groopman JE. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood 2001;97:352–358
Dhawan S, Puri RK, Kumar A, et al. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood 1997;90:1535–1544
Dhawan S, Weeks BS, Soderland C, et al. HIV-1 infection alters monocyte interactions with human microvascular endothelial cells. J Immunol 1995;154:422–432
Lafrenie RM, Wahl LM, Epstein JS, et al. HIV-1-Tat modulates the function of monocytes and alters their interactions with microvessel endothelial cells. A mechanism of HIV pathogenesis. J Immunol 1996;156:1638–1645
Lafrenie RM, Wahl LM, Epstein JS, et al. HIV-1-Tat protein promotes chemotaxis and invasive behavior by monocytes. J Immunol 1996;157:974–977
Wahl LM, Corcoran ML, Pyle SW, et al. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc Natl Acad Sci USA 1989;86:621–625
Frenette PS, Wagner DD. Adhesion molecules—Part II: Blood vessels and blood cells. N Engl J Med 1996;335:43–45
Frenette PS, Wagner DD. Adhesion molecules—Part 1. N Engl J Med 1996;334:1526–1529
Bragardo M, Buonfiglio D, Feito MJ, et al. Modulation of lymphocyte interaction with endothelium and homing by HIV-1 gp120. J Immunol 1997;159:1619–1627
Stefano GB, Salzet M, Bilfinger TV. Long-term exposure of human blood vessels to HIV gp120, morphine, and anandamide increases endothelial adhesion of monocytes: uncoupling of nitric oxide release. J Cardiovasc Pharmacol 1998;31:862–868
Stins MF, Shen Y, Huang SH, et al. Gp120 activates children’s brain endothelial cells via CD4. J Neurovirol 2001;7:125–134
Toneatto S, Finco O, van der Putten H, et al. Evidence of blood–brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS 1999;13:2343–2348
Shatrov VA, Ratter F, Gruber A, et al. HIV type 1 glycoprotein 120 amplifies tumor necrosis factor-induced NF-kappa B activation in Jurkat cells. AIDS Res Hum Retroviruses 1996;12:1209–1216
Ren Z, Yao Q, Chen C. HIV-1 envelope glycoprotein 120 increases intercellular adhesion molecule-1 expression by human endothelial cells. Lab Invest 2002;82:245–255
Swingler S, Mann A, Jacque J, et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med 1999;5:997–103
Olivetta E, Percario Z, Fiorucci G, et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-kappa B activation. J Immunol 2003;170:1716–1727
Herbeuval JP, Shearer GM. Are blockers of gp120/CD4 interaction effective inhibitors of HIV-1 immunopathogenesis? AIDS Rev 2006;8:3–8
Shi B, De Girolami U, He J, et al. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest 1996;98:1979–1990
Adamson DC, Dawson TM, Zink MC, et al. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med 1996;2:417–428
Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA 2001;98:9271–9276
Huang MB, Bond VC. Involvement of protein kinase C in HIV-1 gp120-induced apoptosis in primary endothelium. J Acquir Immune Defic Syndr 2000;25:375–389
Huang MB, Khan M, Garcia-Barrio M, et al. Apoptotic effects in primary human umbilical vein endothelial cell cultures caused by exposure to virion-associated and cell membrane-associated HIV-1 gp120. J Acquir Immune Defic Syndr 2001;27:213–221
Kanmogne GD, Kennedy RC, Grammas P. HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: possible pathway for HIV entry into the brain and HIV-associated dementia. J Neuropathol Exp Neurol 2002;61:992–1000
Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol 2005;64:498–505
Fiala M, Murphy T, MacDougall J, et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc Toxicol 2004;4:327–337
Ullrich CK, Groopman JE, Ganju RK. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood 2000;96:1438–1442
Park IW, Ullrich CK, Schoenberger E, et al. HIV-1 Tat induces microvascular endothelial apoptosis through caspase activation. J Immunol 2001;167:2766–2771
Kim TA, Avraham HK, Koh YH, et al. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J Immunol 2003;170:2629–2637
Jia H, Lohr M, Jezequel S, et al. Cysteine-rich and basic domain HIV-1 Tat peptides inhibit angiogenesis and induce endothelial cell apoptosis. Biochem Biophys Res Commun 2001;283:469–479
Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol 2003;9:222–227
Dore GJ, Correll PK, Li Y, et al. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 1999;13:1249–1253
McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol 2004;157:3–10
Kaul M, Zheng J, Okamoto S, et al. infection and AIDS: consequences for the central nervous system. Cell Death Differ 2005;12(Suppl 1):878–892
Anderson E, Zink W, Xiong H, et al. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr 2002;31(Suppl 2):S43–54
Rappaport J, Joseph J, Croul S, et al. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J Leukoc Biol 1999;65:458–465
Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001;410:988–994
Price RW, Brew B. Infection of the central nervous system by human immunodeficiency virus. Role of the immune system in pathogenesis. Ann NY Acad Sci 1988;540:162–175
Rubin LL, Staddon JM. The cell biology of the blood–brain barrier. Annu Rev Neurosci 1999;22:11–28
Dallasta LM, Pisarov LA, Esplen JE, et al. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol 1999;155:1915–1927
Avison MJ, Nath A, Greene-Avison R, et al. Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmunol 2004;157:140–146
Petito CK, Cash KS. Blood–brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol 1992;32:658–666
Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukocyte Biol 2003;74:691–701
Toborek M, Lee YW, Flora G, et al. Mechanisms of the blood–brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol 2005;25:181–199
Gariano RF, Rickman LS, Freeman WR. Ocular examination and diagnosis in patients with the acquired immunodeficiency syndrome. West J Med 1993;158:254–262
Pellicelli AM, Palmieri F, Cicalini S, et al. Pathogenesis of HIV-related pulmonary hypertension. Ann NY Acad Sci 2001;946:82–94
Kanmogne GD, Schall K, Leibhart J, et al. HIV-1 gp120 compromises blood–brain barrier integrity and enhances monocyte migration across blood–brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab 2007;27:123–134
Arese M, Ferrandi C, Primo L, et al. HIV-1 Tat protein stimulates in vivo vascular permeability and lymphomononuclear cell recruitment. J Immunol 2001;166:1380–1388
Oshima T, Flores SC, Vaitaitis G, et al. HIV-1 Tat increases endothelial solute permeability through tyrosine kinase and mitogen-activated protein kinase-dependent pathways. AIDS 2000;14:475–482
Toschi E, Barillari G, Sgadari C, et al. Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol Biol Cell 2001;12:2934–2946
Avraham HK, Jiang S, Lee TH, et al. HIV-1 Tat-mediated effects on focal adhesion assembly and permeability in brain microvascular endothelial cells. J Immunol 2004;173:6228–6233
Chen C, Lu XH, Yan S, et al. HIV protease inhibitor ritonavir increases endothelial monolayer permeability. Biochem Biophys Res Commun 2005;335:874–882
Eugenin EA, Osiecki K, Lopez L, et al. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 2006;26:1098–1106
Eugenin EA, Gamss R, Buckner C, et al. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J Leukocyte Biol 2006;79:444–452
Conant K, Garzino-Demo A, Nath A, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA 1998;95:3117–3121
Kelder W, McArthur JC, Nance-Sproson T, et al. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus–associated dementia. Ann Neurol 1998;44:831–835
Kolb SA, Sporer B, Lahrtz F, et al. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol 1999;93:172–181
McManus CM, Weidenheim K, Woodman SE, et al. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol 2000;156:1441–1453
Acknowledgments
This work was partially supported by research grants from the National Institutes of Health (NIH) (P.H.L.: HL076345; Q.Y.: DE15543 and AT003094; and C.C.: HL65916, HL72716, EB-002436 and HL083471).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented at the Molecular Surgeon Symposium on Vascular Injury, Repair and Remodeling at the Baylor College of Medicine, Houston, Texas, May 15 and 16, 2006. The symposium was supported by a grant from the National Institutes of Health National Institute of Health (to C. Chen: R13 HL0836500)
Rights and permissions
About this article
Cite this article
Mu, H., Chai, H., Lin, P.H. et al. Current Update on HIV-associated Vascular Disease and Endothelial Dysfunction. World J. Surg. 31, 632–643 (2007). https://doi.org/10.1007/s00268-006-0730-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-006-0730-0