Abstract
Literature reports indicate that the incidence of delayed gastric emptying (DGE) is higher after pylorus-preserving pancreaticoduodenectomy (PPPD) than after conventional pancreaticoduodenectomy (CPD), but DGE is traditionally diagnosed from patient-reported subjective sensations. Our clinical radiological experience suggests higher rates for physiological DGE post-CPD. We therefore sought to quantify rates of subjective DGE (sDGE, based on patient complaint) verses objective DGE (oDGE, based on scintography) post-CPD and post-PPPD. Contractile motility of post-PPPD residual stomach was also studied. For 21 PPPD and 33 CPD patients between October 1997 and June 2000, sDGE and oDGE data were collected preoperatively, on postoperative day 14, and 6 months postoperatively, with cholescintography for pylorus ring competency on postoperative day 14. The incidence of sDGE was higher for PPPD (42%) than for CPD (15%) at 14 days, with zero sDGE for both at 6 months. The incidence oDGE was higher for CPD (91%) than for PPPD (76%) at 14 days, with a 6-month incidence of 4.7% in PPPD but ~33% for CPD. Solid-phase emptying in PPPD showed that residual stomach retained partial gastric emptying function at 14 days but not at 6 months. Cholescintography showed abnormal pylorus closure function in 2 of 21 PPPD patients but was not related to DGE. Literature reports of higher DGE incidence post-PPPD are true only for subjective symptoms. Radiological measurement of oDGE shows that both CPD and PPPD manifest ~80% incidence of DGE in the early postoperative period. At 6 months, ~33% of CPD show persistent oDGE. We concluded that (1) the concept of DGE should distinguish between subjective and objective symptoms; (2) loss of distal stomach mechanoreceptors in CPD reduces patients sensation of oDGE, producing “silent” DGE;(3) both CPD and PPPD have high and approximately equal rates oDGE;(4) the previously unnoticed silent oDGE in CPD may contribute to the higher rates of ulceration and related morbidity in association with CPD
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A one-stage conventional pancreaticoduodenectomy (CPD) was reported by Whipple in 1941 [1]. A few years later Watson described a pylorus-preserving pancreaticoduodenectomy (PPPD) in which antrum, pylorus, and 1 inch of duodenum were preserved and the gastrointestinal tract was reestablished by duodenojejunostomy [2]. Conventional PD remained the preferred treatment for periampullary malignancy [1], but in 1978, to reduce marginal ulceration and improve postoperative nutritional status post-CPD, Traverso and Longmire revived PPPD [3]. However, PPPD presented a significant increase over CPD in delayed gastric emptying (DGE) [4], which increases parenteral nutrition complications and length of hospital stay. To date, most studies report early postoperative DGE incidence higher post-PPPD (25% to 40%) than post-CPD (6% to 23%) [5-8], although different opinions exist [9].
In clinical studies, DGE is traditionally diagnosed on the basis of patient-reported symptoms of abdominal discomfort, bloating or nausea [5, 6]. In these cases and also in postoperative patients with no subjective experience of discomfort, the presence or absence of physiological DGE is rarely confirmed. In our group’s as-yet-unpublished tests of postoperative procedures such as somatostatin prophylaxis to reduce pancreatic stump complications, conflict was noted between subjective patient reports and objective physiological testing by radiological gastric emptying scintigraphy. These differences so obvious that present investigation was undertaken to compare the incidence of DGE as determined by patient complaint and by radiological scintpgraphy measurement.
Both solid and liquid gastric emptying scintography were used to monitor actual mechanical emptying function preoperatively, on postoperative day 14, and 6 months postoperative. These data are compared with the subjective patient symptoms that constitute the traditional clinical diagnosis of DGE. We additionally used cholescintography to evaluate pylorus function by extent of enterogastric reflux, and determined the correlation of pyloric function with DGE.
Patients and Methods
Between October 1994 and June 2000, patients undergoing either PPPD or CPD at the National Cheng Kung University Hospital, Taiwan, routinely underwent preoperative solid and liquid phase gastric emptying scintography. Follow-up scintographic gastric emptying studies were done 14 days and 6 months postoperation. Subjective patient symptoms were also collected preoperatively, during the hospital stay, and at 6-month follow-up. Cholescintography for enterogastric reflux was performed to evaluate pyloric function 14 days postoperatively. After operation, all patients received antibiotics for 3 days and partial parenteral nutrition until recovery of food intake. The surgical suction drain was removed if there was no sign of pancreatic fistula or abscess. The pancreatic diversion duct was removed routinely 3 weeks postoperatively. If subjective DGE developed, then total parenteral nutrition was given via central line until the patient comfortably resumed a normal diet.
Surgical Technique
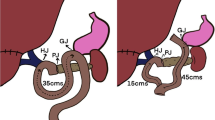
Patients underwent CPD or PPPD as described previously [10]. One volume surgeon performed all the operations using the same approach, technique, and anastomotic fashion to keep surgical variation to a minimum. In CPD, standard subtotal gastrectomy with sparing of the vagus nerve was done. In PPPD, duodenum was preserved longer than 2 cm if possible, and the right gastric artery was preserved if not tethering anastomosis. Regional lymph node dissection was performed for both PPPD and CPD. The sequence of gastrointestinal tract reconstruction was choledochojejunostomy, pancreaticojejunostomy, to gastrojejunostomy in CPD or to duodenojejunostomy in PPPD. End-to-side pancreaticojejunostomy in one layer suture was completed with a temporary diversion tube (trans-anastomotic stent) in the pancreatic duct using an appropriate pediatric suction tube to divert pancreatic juice, which can further damage surgically traumatized tissue. The diversion tube did not adhere directly to the abdomen wall and was left for 3 weeks, whereupon healing tissue was considered mature enough for restoration of normal (residual) pancreatic drainage. A closed-suction drain (Jackson Pratt) was placed in the operative dissection bed close to the pancreatic stump.
Gastric Emptying Scintography
Gastric emptying scintography was done according to our hospital’s standard technique. Test was performed after overnight fast. Patients were forbidden smoking and any pharmacenticals that would likely affect gastric motility. Single-phase gastric emptying was measured preoperatively, on postoperative day 14, and 6 months postoperatively. Radionucleolide formulae were: 0.5 mCi of Tc-99m sulfur colloid mixed with one scrambled egg (60 ± 3g, protein 7 g, fat 2 g, 75 kcal) for solid phase; 1 mCi of Tc-99m DTPA in 200 ml saline for liquid phase. Patients lay semi-upright (60°) under a single-headed gamma camera using the left anterior oblique (LAO) method [11]. The upper abdomen was scanned by every 20 seconds for 20 minutes for the liquid phase test and every 1 minute for 60 minutes for the solid phase test with a programmed computer. Generally, a gastric region of interest (ROI) was drawn on the computer for summed gastric imaging. After correction for decay and attenuation, a time-activity curve (TAG) was generated and a parameter of gastric emptying was calculated. The rate of gastric emptying was calculated by an exponential fitting curve (Fig. 1A). The time of maximal dosage of radionucleolide within the stomach (Tmax), the time for radionucleolide to appear within intestine (lag time), and the time of half clearance (T1/2, i.e., time half the food is emptied) were calculated. Normal control reference was obtained from 20 healthy volunteers: Tmax = 0.8 ± 1.1 minute, lag time = 3.1 ± 1.1 minutes, upper limit of normal range of T1/2 = 23 minutes for liquid phase gastric emptying test (LGE); Tmax = 3.3 ± 1.8 minutes, lag time = 14.2 ± 6.9 minutes, and T1/2 = 44.0 ± 7.2 minutes for solid phase gastric emptying test (SGE). In test patients, radiographic physiological DGE, referred to in the following as objective DGE (oDGE), was diagnosed if T1/2 > 23 minutes in LGE or if T 1/2 increased by more than two SD above the mean value in SGE.
Exponential fit of solid gastric emptying (SGE) curve. A. normal volunteer; B. PPPD patient at 14 days without sDGE, longer Tmax is observed; C. PPPD patient at 14 days with sDGE, longer Tmax and multiple fluctuations in emptying curve are observed; D. patient C at six months without sDGE, rapid emptying is observed; E. CPD patient at 6 months without sDGE, emptying curve is rapid and similar to curve D; F. CPD patient at 6 months with oDGE, late intestinal reflux results in longer T1/2. Term “Cts” indicates “counts.” Exponential fit = dashed line, solid food emptying curve = solid line.
Cholescintography
Radionucleolide formula was 4 mCi of Tc-99m DISID A in 10 ml normal saline. After overnight fasting, the patients received infusion of radionucleolide and lay fully reclined under the scanning gamma camera, which captured an image every 5 minutes for the first 60 minutes after radionucleolide injection. The subject then drank 200 ml of milk to facilitate biliary flow. Imaging was continued for an additional 60 minutes while monitoring passage of radionucleolide (DISIDA) into jejunum or stomach. Enterogastric reflux was defined as radionucleolide detected in the stomach. The severity of enterogastric reflux was graded as 0 (absent), 1 (transient reflux into the antrum or body), 2 (persistence of isotope in the antrum or body), 3 (reflux into the gastric fundus), and 4 (reflux into the esophagus) [12]. Enterogastric reflux ≥ grade 2 is assumed to mean incompetent closure function of the pylorus. The postoperative status of bile in the jejunal limb is also used to detect slow transit of the jejunum after gastrointestinal reconstruction.
Delayed Gastric Emptying
Our hospital’s clinical definition of DGE is derived from previous studies [5, 6] whereby DGE is diagnosed if the nasogastric tube is in place at postopertive day 10 or more. This condition occurs basically as a result of patient-reported symptoms such as complaints of discomfort, pain, bloating, or nausea. In the following discussion, DGE diagnosed from subjective patient report is designated subjective DGE (sDGE). In previous studies [5, 6], the classification “early” is used for sDGE in the range of postoperative day 10 and “late” for sDGE symptoms in the range of 6 months postoperation. For easy comparison with the previous studies, we collect postoperative data on day 14 and again at 6 months. Cholescintography data are from postoperative day 14.
Statistical Analysis
Data are expressed as mean ± SD except Tmax and T1/2 values in the gastric emptying test. Tmax and T1/2 are presented as range (median). The Mann-Whitney U test was used for statistical analysis of Tmax and T1/2. Inter-group differences in postoperative morbidity and other categorical data were compared by Fisher’s exact test. A p value < 0.05 was regarded as statistically significant.
Results
Demographics and Postoperative Morbidity
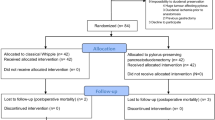
In the study period, 154 patients underwent either CPD or PPPD in our department. Of these, full data and patient consent was obtained for 63 patients who formed the initial study group. Nine patients were subsequently excluded from the study, one patient with palliative PD (positive margin of pancreatic remnant microscopically), 2 patients with septic shock at 6-month follow-up, and 6 patients with disease recurrence at 12-month follow-up. A total of 54 patients with complete examination were divided into two groups according to surgical procedure, group 1 for PPPD and group 2 for CPD (Table 1). Group 1 contained 21 patients with an age range of 38–84 years (median: 65 years) and a male/female ratio of 13:8. The follow-up period was 12–80 months (median: 40 months). Group 2 contained 33 patients with an age range 36–80 years (median:61 years) and a male/female ratio of 23:10. The follow-up period was 12–72 months (median: 35 months). Periampullary lesions accounted for 98.2% of patients, with one gallbladder carcinoma in group 1. In both groups carcinoma of the ampulla of Vater was the major disease: 52.4% of patients in group 1 and 42.4% in group 2. Pancreatic cancer accounted for only 9.5% and 9.1% of disease in groups 1 and 2, respectively.
The incidence of sDGE was significantly higher for PPPD than CPD: 9 of 21 PPPD patients (42%) versus 5 of 33 CPD patients (15%), p < 0.05. The measure “mean nasogastric tube days” was longer for PPPD patients, 1l.4 (4~36) days than for CPD patients, 6.7 (4~22) days. No significant intergroup difference was found in other postoperative morbidities (Table 1). There was no significant correlation between sDGE and postoperative morbidity. One PPPD patient with postoperative pancreatitis and two CPD patients with intraabdominal abscess did report 14-day sDGE, but they did not exhibit 6-month oDGE.
Stomach Emptying Function in PPPD and CPD
Preoperatively, no significant sDGE symptoms were noted in any patients. All patients underwent preoperative scintography, and approximately 20% exhibited some form of preoperative oDGE: in the PPPD group, 5 patients for LGE and 6 patients for SGE; in the CPD group, 3 patients for LGE and 7 patients for SGE. Because all of our patients were suffering initially from severe pancreatic or stomach disorder, this percentage of preoperative oDGE is not surprising. However, no correlation was found between incidence of preoperative oDGE sand postoperative sDGE, oDGE, or other morbidity.
Proximal stomach Function.
In 14-day LGE, both Tmax and T1/2 in PPPD with sDGE were significantly longer than in PPPD without sDGE; Tmax: 0–18.7 minutes (15.7) versus 0–1.3 minutes (1.0), T1/2: 73-(off scale) minutes (205.7) versus 6.7–182 minutes (46.9), respectively. No difference was observed between CPD patients with and without sDGE; Tmax: 0–7.3 minutes (0.3) versus 0–17.3 minutes (5.3), T1/2: 25–1041minutes (48) versus 20.3-(off scale) minutes (91). In 6-month LGE, T1/2 and Tmax had recovered to within normal range in 20 of the 21 PPPD patients. In contrast, Tmax and T1/2 had recovered to within normal range in only 21 of the 33 CPD patients (Table 2). The LGE emptying curves indicate that the slow return to normal resulted from intestinal reflux, presumably as a consequence of BII gastrojejunostomy.
Distal Stomach Function
In 14-day SGE, Tmax in most PPPD and CPD patients was longer than in normal volunteers. T1/2 in PPPD with sDGE was significantly longer than without sDGE; 79-(off scale) minutes (171.3) versus 9.5–68.3 minutes (33.1) (Table 3). Compared to PPPD without sDGE (Fig. 1B), multiple fluctuations in the emptying curve were observed in PPPD patients with sDGE (Fig. 1C). The values of T1/2 in most CPD patients were significantly longer than in normal volunteers, but there was no difference between CPD with and without sDGE.
In 6-month SGE, Tmax and T1/2 in 20 of the 21 PPPD patients had recovered to within normal range, but T1/2 was somewhat shorter than normal. A similar pattern was observed in 70% of the CPD group (Figs. 1D, 1E), but the remaining 30% of CPD patients showed oDGE at 6 months by SGE test. Excluding the 6-month oDGE CPD group, similar 6-month SGE emptying curves were observed in PPPD and CPD, patients indicating that the emptying function of the retained distal stomach was completely lost in PPPD patients. Longer T1/2 was observed in 30% oDGE CPD (Fig. 1F) and resulted from intestinal reflux.
Correlation Between sDGE and oDGE.
In our study group, 42% PPPD and 15% CPD had 14-day sDGE, with no 6-month sDGE. At postoperative day 14, oDGE was noted in 42% SGE and 76% LGE in PPPD, and in 88% SGE and 91% LGE in CPD. At 6 months scintographic data indicated oDGE in 4.7% SGE and 4.7% LGE in PPPD, and 30% SGE and 37% LGE in CPD (Fig. 2).
Pyloric Function in PPPD Patients
Cholescintography for enterogastric reflux was used to evaluate pylorus closure function. The distal stomach was preserved in PPPD and was completely resected in CPD, so the incidence of enterogastric reflux was higher in patients in the CPD group, 20 of 33 patients (60%) versus 4 of 21 patients (19.1%), p < 0.05. In the CPD group, 9 patients had significant enterogastric reflux (≥ grade 2): 8 patients with grade 2 and 1 patient with grade 3. In the PPPD group, 2 patients had significant enterogastric reflux: 1 patient with grade 2 and 1 patient with grade 3. The results indicate that the function of the pylorus ring was competent in 19 of the 21 PPPD patients.
Discussion
In general, the literature bases its human DGE data on patient-reported subjective symptoms such as discomfort, vomiting, or nausea, particularly because such symptoms constitute the conventional clinical determinants of whether to use parenteral nutrition or not. The issue of parenteral nutrition is relevant because (extended parenteral nutrition complicates care, increases costs, extends hospital stay, and increases infection risk. Use of scintography in our earlier clinical work and research (pending publication) resulted in questions regarding the conventional interpretation of the incidence of DGE post-CPD and post-PPPD and led to this study’s attempt to quantify the differences between subjective and objective DGE data.
However, quantifiable objective measurement of gastric activity and gastric emptying function is a difficult task. Many methods have been developed, including transcutaneous electrogastrography [13, 14] and indirect pharmacological tests [15], but direct gastric emptying scintography has become the standard clinical tool for investigating gastric motor behavior [16, 17]. Emptying rate is affected by many factors including patient physical status, meal volume, caloric level, concentration of nutrients, salinity, acidity, and viscosity. Increasing volume of solid food speeds gastric emptying, whereas a high-calorie meal entering the intestine stimulates hormone production, which inhibits further gastric emptying [12]. But when test meal caloric level is low, the solid gastric emptying test (SGE) is popular for evaluating distal stomach emptying function. The liquid gastric emptying test (LGE) uses clear liquids, which begin to empty immediately into the intestine with no lag phase as a function of volume [12], so it is popular for evaluating proximal stomach emptying. Because there is no distal stomach after CPD, LGE is used in this study for preoperative confirmation of normal stomach function and for post-PPPD evaluation of proximal emptying.
Incidence of early (postoperative day 14) sDGE in our study groups agrees with the literature, i.e., significantly higher sDGE was found post-PPPD than post-CPD; 42% of our PPPD patients and 15% of our CPD patients. These data match well the published 25%–40% sDGE in PPPD and the 6%–23% sDGE in CPD [5–8]. Also, as in the literature, 0% sDGE was found in PPPD and CPD patients at 6 months. However, in sharp contrast to the literature, our radiological data indicated very high incidence (over 80%) of early (day 14) oDGE in both PPPD and CPD patients, with the significantly higher incidence (~10%) being found in CPD. Radiologically, at 6 months, we found 4.7% oDGE in PPPD patients and 33% oDGE in CPD patients.
In LGE, proximal stomach emptying post-CPD and post-PPPD was abnormal early in the early postoperative period but recovered at 6 months. In SGE, we observed day 14 multi-fluctuations in the emptying curve with longer T1/2 in PPPD with-sDGE than without-sDGE, but no difference was observed between CPD with-/without sDGE. These 14-day emptying curve differences may be related to residual emptying function of the retained distal stomach. Six months later, however, similar emptying curves with shorter values of T1/2 were observed in all patients regardless of procedure and with or without early DGE, indicating complete loss of emptying function of the retained distal stomach in PPPD by 6 months. This finding is of significance because it appears to refute the pro-PPPD premise of continued functionality of the retained distal stomach.
Cholescintography on day 14 revealed a high incidence of enterogastric reflux in CPD, patients i.e., no pyloric function, but in all but 2 PPPD patients the pyloric closure function was competent. It would have been interesting to compare these results with the 6-month data, but we neglected to perform cholescintography at 6 months.
Previous work assumed normal proximal stomach function early post-PD [18]. The present study finds 14-day proximal dysfunction as indicated by increased LGE T1/2 in both PPPD and CPD, suggesting a weakening or inhibition of proximal stomach contraction. Proximal stomach motor function and its inhibition by feeding are normally hormonally regulated [19]. Most gastrointestinal hormones, including secretin, cholecystokinin, and motilin, are secreted from the portion of the duodenum that is removed during PPPD and CPD [20]. Also lost during these procedures are the pancreatic polypeptides (secreted primarily at non-ß islets of the head and uncinate process of the pancreas) implicated in feedback inhibition between pancreatic secretion and interdigestive cyclic events [21, 22]. In our procedure, juice from the remnant pancreas is diverted for 3 weeks postoperatively. It cannot be assumed that feedback inhibition between the vagus nerve and remnant pancreas is lost by duodenectomy and pancreatectomy or during the diverting period, but hormonal insufficiency may contribute to our LGE observation of early proximal stomach dysfunction post-PPPD and post-CPD.
Physiologically, the stomach consists of two functionally integrated but electromechanically distinct portions. Food entering the distal stomach stretches muscles and triggers peristalsis to grind and empty the food [23, 24]. Mechanoreceptor activation is necessary for proper peristalsis and emptying [25]. The mechanism responsible for DGE is unclear, but balloon activation of distal stomach mechanoreceptors of healthy humans can induce DGE-like symptoms such as gastric discomfort and nausea; overtactivation can induce gastric dysrhythmia [13]. Distal emptying requires coordination among antrum, pylorus, and duodenum [23, 24]. Pylorus opening is irrelevant for emptying of fluid and very fine particles, but it is required for fine and larger food particles. Pylorus opening is controlled by intrinsic downstream messages from the antrum, upstream inhibitory messages from the duodenum, and extrinsic stimulation from the vagus nerve [23–26]. Pyloric ring opening dysfunction can lead to inability to empty food, which causes distention of the distal stomach. However, cholescintography on day 14 revealed normal pyloric ring closure in over 90% of PPPD patients, yet over 40%, of PPPD patients experienced SGE-based oDGE, indicating a DGE mechanism that cannot be attributed to pylorus function.
The literature reports early post-PPPD abnormal function of the distal stomach, which recovers 6 months postoperatively [5, 6], but literature reports of recovery of distal stomach function are based on the absence of sDGE. The present study likewise finds no sDGE at 6 months, but it does find radiological emptying curve data indicating zero distal stomach emptying function in PPPD at 6 months. Thus, the early post-PPPD distal emptying function seems a “dying” function of the residual distal stomach. Parti et al. also reported 3 of 10 PPPD patients with abnormal SGE emptying curves 1–45 months after PPPD. This type of emptying curve is abnormally fast, indicates dysfunctional distal stomach emptying, and leads easily to our observation of complete loss of residual distal stomach emptying function at 6 months post-PPPD [27].
The commonly reported higher incidence of sDGE in PPPD relative to CPD has been among the primary rationales for continued preference of CPD over PPPD [6, 8]. Our study strongly contradicts the traditional data by showing a greater than 80% incidence of oDGE both post-PPPD and post-CPD on postoperative day 14. Further, we find a 33% incidence of persistent oDGE in CPD at 6 months, although this persistent oDGE is “silent”; i.e., the patient does not experience it subjectively. By conventional clinical practice, silent oDGE goes unnoticed and is treated as though it has no significant medical implications. However, this previously unsuspected silent oDGE may be a contributing factor to the well-known CPD complications of marginal ulceration and bleeding.
Although data are scant, the incidence of systemic sepsis seems higher in long-term follow-up of CPD and suggests that special attention be given the ~33% persistent oDGE in our CPD group. Silent oDGE might also correlate with incidence of respiratory infection from continued regurgitation of stomach contents. This condition is similar to the pulmonary aspiration in gastroesophageal reflux disease [28]. Therefore, it becomes necessary to consider the actual physiological effects of oDGE in general.
The mechanisms responsible for DGE are multi-factorial and have not been explained in this study. However, subjective and objective testing of DGE can give different results, which in turn can lead to different theoretical interpretations of DGE and optimal clinical management. We suggest that compromise or loss of mechanoreceptors produces low patient experience of oDGE, and that the total removal of distal stomach mechanoreceptors in CPD is the primary reason for the low DGE rates reported for CPD. In agreement with the general literature, the sDGE rate is low post-CPD and moderate post-PPPD, but we also observe higher oDGE rates (over 80%) in our CPD patients. The presence of this “silent” oDGE, whereby the physiological condition is present but the patient cannot sense it, is clearly an advantage post-CPD for minimizing hospital costs and time factors, but the question of whether silent oDGE is physiologically harmless remains unanswered. We suspect a higher rate of delayed morbidities, especially with the 33% silent oDGE in our CPD group at 6 months. Additionally, we have observed by LGE 14-day proximal stomach dysfunction both post-PPPD patients and post-CPD patients, as well as total loss of residual distal stomach emptying function in PPPD patients at 6 months. We have also shown that pyloric closure seems in general competent and so cannot be viewed as a primary contributor to the DGE mechanism. Our observation of a high rate of oDGE in CPD has been reported elsewhere. Sadowski et al. reported both high sDGE and oDGE in CPD [9], but they performed only antrectomy, while our procedure involved subtotal gastrectomy. As a result their CPD patients had greater numbers of the mechanoreceptors required for sDGE symptoms, making direct comparison of their data with ours difficult. It is hoped that further work will clarify these issues.
References
Whipple AO. Pancreaticoduodenectomy for islet carcinoma: a five-year follow up. Ann. Surg. 1945;121:847–852
Watson K. Carcinoma of the ampulla of Vater. Successful radical resection. Br. J. Surg. 1944;31:368–373
Traverse LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg. Gynecol. Obstet. 1978;146:959–962
Warshow AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreatectomy. Surg. Genecol. Obstet. 1985;160:1–4
Hunt DR, McLean R. Pyloras-preserving pancreatectomy: functional results. Br. J. Surg. 1989;76:173–176
Grace PA, Pitt HA, Longmire WP. Pyloras-preserving pancreaticoduodenectomy: an overview. Br. J. Surg. 1990;77:968–974
Braasch J, Rossi R, Deziel D, et al. Pyloric- and gastric- preserving pancreatic resection. Ann. Surg. 1986;204:411–418
Lin PW, Lin YJ. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br. J. Surg. 1999;86:603–607
Sodowski C, Uhl W, Baer HU, et al. Delayed gastric emptying after classic and pylorus-preserving Whipple procedure: a prospect study. Dig. Surg. 1997;14:159–164
Lin PW, Lee JC, Lee PC, et al. A simple, secure, and universal pancreaticojejunostomy following pancreaticoduodenectomy. HPB Surg. 1997;10:305–310
Urbain J-L C, Vekemans MM, Malmud LS. Esophageal transit, gastroesophageal reflux, and gastric emptying. In Sandler MP, et al., editors, Diagnostic Nuclear Medicine, 4th edition, Philadelphia, WW Lippincott, 2003;chap 24: 487–502
Thomas WEG, Cooper MJ, Mortensen NJ, et al. The clinical assessment of duodenogastric reflux by scintography and its relation to histological changes in gastric mucosa. Scand. J. Gastroenterol. 1984;(Suppl 92) 19:195–199
Ladabaum URI, Koshy SS, Woods ML, et al. Differential symptoms and electrogastrographic effect of distal and proximal human gastric distension. Am. J. Physiol. 1998;275:G418–G424
Chen JDZ, Lin Z, Pan J, et al. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig. Dis. Sci. 1996;41:1538–1545
Heading RC, Mimmo J, Prescott LF, et al. The dependence of paracetamol absorption on the rate of gastric emptying. Br. J. Pharmacol. 1973;47:415–421
Camilleri M, Brown ML, Malagelada J-R. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology 1986;91:94–99
Urbain J-L C, Charkes ND. Recent advances in gastric emptying scintography. Semin. Nucl. Med. 1995;25:318–325
Kobayashi I, Miyachi M, Kanai M, et al. Different gastric emptying of solid and liquid meals after pylorus-preserving pancreaticoduodenectomy. Br. J. Surg. 1998;85:927–930
Thomas PA, Kelly KA. Hormonal control of intergestive motor cycles of canine proximal stomach. Gastroenterology 1979;E192–E197.
Tanaka M, Sarr MG. Role of the duodenum in the control of canine gastrointestinal motility. Gastroenterology 1988;94: 622–629
Zimmerman DW, Sarr MG, Smith CD, et al. Cyclic interdigestive pancreatic exocrine secretion: is it mediated by neural or hormonal mechanism? Gastroenterology 1992;102:1378–1384
Malfertheiner P, Sarr MG, DiMango EP. Role of pancreas in the control of interdigestive gastrointestinal motility. Gastroenterology 1989;96:200–205
Hasler WL. The physiology of gastric motility and gastric emptying. In Yamada T, editor. Textbook of Gastroenterology, 2nd ed., Philadelphia, JB Lippincott, 1995; chap 8, 181–206
Kelly K. Gastric emptying of liquids, solids: role of proximal and distal stomach. Am. J. Physiol. 1980;239:G71–G76
Dochray GJ. Neurochemical basis of reflex relaxation in gastric corpus. Dig. Dis. Sci. 1994;39:82S–85S
Lindeotrön LM, Ekalad E. Origins and projections of nerve fiber in rat pyloric sphincter. Auton. Neurosci. Basic Clin. 2002;97:73–82
Patti MG, Pellegrini CA, Way LW. Gastric emptying and small bowel transit of solid food after pylorus-preserving pancreaticoduodenectomy. Arch. Surg. 1987;122:528–532
Patti MG, Debas HT, Pellegrini CA. Esophageal manometry and 24-hour pH monitoring in the diagnosis of pulmonary aspiration secondary to gastroesophageal reflux. Am. J. Surg. 1992;163:401–406
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shan, YS., Tsai, ML., Chiu, NT. et al. Reconsideration of Delayed Gastric Emptying in Pancreaticoduodenectomy. World J. Surg. 29, 873–879 (2005). https://doi.org/10.1007/s00268-005-7473-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-005-7473-1