Abstract
Background
Implantation of exfoliated malignant cells has been suggested as a possible mechanism of tumor recurrence in colorectal anastomoses that might be prevented by cytocidal washout. The aim of our study was to assess whether malignant cells are likely to be collected by a circular stapler introduced transanally to perform an anastomosis and to observe local recurrences during follow-up, with special attention to the washout status of patients.
Methods
Between May 1999 and March 2004, 96 patients with carcinoma of the rectum and distal sigmoid colon undergoing anterior resection under the care of three surgeons (only one of whom routinely performed rectal washout) were prospectively studied. While 38 patients had rectal washout with 5% povidone-iodine before anastomosis, 58 patients did not. A circular stapler was used for anastomosis, and the stapler was immediately rinsed in 100 ml of saline. The fluid was then classified as “acellular,” “malignant cells identified,” or “benign cells identified” by pathologists.
Results
Malignant cells were collected from the circular stapler after use in 3 patients (8%) on whom rectal washout was performed and in 2 (3%) patients who did not have rectal washout performed (P = 0.631). Three patients (8%) in the washout group developed local recurrence, and 2 patients (3.4%) in the no-washout group had local recurrence (one was anastomotic recurrence) (P = 0.338). The median follow-up time was 23 (range: 9–70) months.
Conclusions
There were no differences in terms of the number of patients who had malignant cells collected from the circular stapler and local recurrence rates between the two groups. Although this is not a randomized study and size and mean follow-up time of the study were not sufficient, our results did not offer rational arguments in support of intraoperative rectal washout when a circular stapler is used after low anterior resection for carcinoma. Because of the limitations of our study, however, we are unable to arrive at a definite conclusion regarding rectal washout. There is a need for a randomized, controlled, large-scale, multicenter trial to establish the clinical relevance of intraoperative rectal washout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Implantation of exfoliated malignant cells has been suggested as a possible mechanism of tumor recurrences in colorectal anastomoses.1–3 There is clear experimental evidence that colorectal cancer cells are shed into the lumen of the bowel, they are viable, and they represent clones of cells capable of transplanting.4–6 The use of circular staplers introduced transanally to perform a low anterior anastomosis may be a danger in collecting exfoliated cells and possibly implanting them at the site of anastomosis. Several studies have shown that free malignant cells are collected on circular stapling devices during anterior resection.4,7 Although some reports8,9 have suggested that the use of staplers may result in a higher rate of locally recurrent tumors, no clear data documenting this occurrence have been reported when stapled and hand-sewn anastomoses are compared.10 If there is a risk of implantation of exfoliated malignant cells, such risk is more serious when performing transanal local excision. It is nearly impossible to avoid tumor cell implantation during conventional local excision or transanal endoscopic microsurgery for rectal cancer. The role of implantation of tumor cells is a controversial issue. Some authors11,12 strongly recommend introduction of a right-angled clamp first, followed by a cytocidal washout before cross-stapling, but the practice of a routine rectal washout during anterior resection is not universally accepted.
A number of studies have demonstrated that free malignant cells can be destroyed by effective rectal irrigation using cytotoxic agents, which have been shown to be effective in in vivo and in vitro studies.3,13,14 Two clinical reports7,14 demonstrated that during resection for rectal carcinoma, free malignant cells are collected on the circular stapler and that rectal irrigation effectively eliminates these cells. However, clinical relevance of intraoperative rectal washout—i.e., whether rectal washout causes a reduction in the incidence of local recurrence—was unclear. Recently in a retrospective study, the local recurrence rate was found to be identical between patients who underwent rectal washout using cetrimide and patients who did not have rectal washout before anastomosis.15 The aim of our study was to assess whether malignant cells are likely to be collected by a circular stapler introduced transanally to perform an anastomosis and to observe local recurrences during the follow-up period, with special attention to the washout status of patients.
PATIENTS AND METHODS
Between May 1999 and March 2004, in Colorectal Unit of Department of Surgery, Dokuz Eylul University Hospital, 96 patients with carcinoma of the rectum (n = 87) and distal sigmoid colon (n = 9) undergoing anterior resection under the care of three surgeons were prospectively studied. The study was approved by the Ethics Committee of Dokuz Eylul University, Faculty of Medicine.
The rectum was defined as the portion of large bowel located between 0 and 15 cm from the anal verge.16,17 This was measured by rigid rectosigmoidoscopy. Tumor localization was subsequently subdivided into lower rectum (0 –5 cm from anal verge), midrectum (6–10 cm), upper rectum (11–15 cm), and distal sigmoid (16–20 cm).
The preoperative examination included general clinical examination, digital rectal examination, a complete blood test, chemistry profile, carcinoembryonic antigen (CEA) assessment, rigid proctosigmoidoscopy, colonoscopy, tumor biopsy, computed tomography (CT) of the abdomen and pelvis, and, chest radiography. Endorectal magnetic resonance imaging was used routinely to establish better preoperative staging from 2002 to date. CT of thorax was administered in patients who were candidate to preoperative chemoradiotherapy. Response to chemoradiotherapy was based on pre-treatment clinical stage versus the final pathologic stage.
Of the 96 patients, 17 (18 %) patients were found to have stage IV disease at presentation. The remaining 79 patients with stage I, II, and III disease underwent potentially curative surgery such as resection of all macroscopic tumor without positive margin and removal of the draining lymph nodes, with no evidence of distant metastases. Curative resection was defined as histologically complete resection of primary tumor with no metastasis to liver or peritoneum (R0).18,19 Patients who had microscopic residual tumor were classified as R1 and those who had macroscopic residual tumor were classified as R2.20
The surgical technique included a mesorectal excision as described previously.21 A total mesorectal excision (TME) was performed for tumors in the lower and midrectum, whereas the mesorectum was divided 5 cm distal to the tumor for upper rectal cancer. If the tumor was attached to adjacent parietals or viscera, en bloc excision was performed. The histopathological examination of the operative specimens was performed according to the principles of Quirke et al.22 The report included tumor staging according to the International Union Against Cancer/American Joint Committee on Cancer TNM staging system.23 Patients with stage II or III rectal carcinoma in the mid or lower rectum received preoperative chemoradiotherapy (1.8 Gy per day, 5 days per week to a total 25 fractions over a period of 5 weeks for a total of 4500 cGy + 5-fluorouracil 225 mg/m2/day infusion for 5 days/week over 5 weeks). Patients with stage II, III, and IV distal sigmoid colon and rectal cancer received 12 cycles of postoperative chemotherapy every 2 weeks (5-fluorouracil 400 mg/m2/day iv bolus + leucovorin 200 mg/m2/day iv over 2 hours then 5-fluorouracil 600 mg/m2/day 22 hours infusion for two days). Basic criteria for the administration of chemotherapy were adequate blood counts (WBC ≥ 4000/mm3 , Hb ≥ 11g/dl, PLT > 100.000/ mm3), and serum biochemistry (creatinine ≤ 1.5 mg/dl, aspartate aminotransferase [AST] and alanine aminotransferase [ALT] ≤ 2× upper limit of normal, bilirubin ≤ 2.0 mg/dl), as well as good performance status (ECOG [Eastern Cooperative Oncology Group] ≤ 2) in the absence of significant systemic disease.24 Local recurrences were defined as clinical, radiological, or histological evidence of recurrent tumor in the tumor bed, regional nodes, adjoining structures, anastomosis, pelvis, or perineum, and surgical scars regardless of whether new metastases are found elsewhere. Anastomotic recurrence was defined as isolated failures in the anastomosis.
All patients were routinely prepared with a low-residue diet and had mechanical bowel preparation using phosphosoda prep (Fleet, De Witt, USA) the 24 hours preceding the operation. On the morning of the procedure, all patients were offered preoperative phosphate enema (Fleet Ready-to-use Enema, De Witt, USA).
The three surgeons differed in their techniques for anterior resection, one routinely performing rectal washout and the others not. The patients were allocated to washout status as “washout group” and “no-washout group.” The patients undergoing rectal washout had standard mobilization of the colon and the rectum performed, with a cross-clamp applied just distal to the tumor. If placing a clamp distal to the tumor was not possible due to narrow pelvis, the bowel lumen was occluded by an encircling nylon tape distally to the tumor as previously described.25 A Foley catheter was introduced per rectum, and an average volume of 500 ml 5% povidone-iodine was used to irrigate the rectal stump. The rectum was then cross-stapled distal to the clamp or nylon tape and divided. If the tumor was so distal that it did not permit this technique, the lumen was washed out with no distal clamp, and the stapler was placed across the rectum. The patients having no rectal washout had also standard mobilization of the colon and the rectum. A cross-stapler was placed distal to the tumor and fired. A circular stapling device was used to fashion the anastomosis in all patients.
The donuts were detached and the stapler (anvil and instrument) was immediately rinsed in 100 ml of saline. The fluid was sent to the pathology laboratory as an unfixed fresh specimen. This fluid was poured into 100 ml plastic screw cap centrifuge tubes and centrifuged for 10 minutes at 2500 rpm. The supernatant was then poured off; the precipitate was poured into 10 ml tubes and once more centrifuged for 10 minutes at 2500 rpm. To deal with the low cellularity of the specimen, the cytocentrifuge preparations were prepared from the precipitate by using Shandon’s Cytospin 2 cytocentrifuge (Hermle Z 380, Hermle AG, Gosheim, Germany). The preparations were stained with hematoxylin and eosin and evaluated by two pathologists in a blinded fashion. The fluid was classified as “acellular,” “malignant cells identified,” and “benign cells identified.”
All patients were examined for local recurrence every 3 months by a surgeon who was masked to the patient’s rectal washout status. During each visit, the patients were offered clinical examination, CEA assessment, rigid rectosigmoidoscope, and abdominal ultrasonography every three months for the first 2 years; every 6 months for the next 3 years. Patients also had an annual chest x-ray, abdominopelvic CT, and colonoscopy. For levels of CEA > 10 ng/ml, a CT scan of the abdomen and pelvis was performed. For the purpose of this study, the last follow-up was done in July 2005. No patient was lost to follow-up. Statistical analyses were done with the chi-squared test, Student’s t-test, and Fisher’s exact test when considered appropriate.
RESULTS
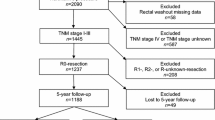
All treatment steps, local recurrences and the outcome of these patients are shown in Figure 1.
Treatment steps of 96 patients with rectosigmoid cancer. Preoperative course of chemoradiotherapy was completed in all (21/21) patients and there were no deaths in this treatment period. In the 17 (18%) patients with stage IV disease, distal margins were positive in two patients; distal as well as radial margins were positive in one patient. The perioperative mortality rate of series was 1.04% (1/96). Seventy-five patients who had stage II, III, and IV disease were found to be suitable for postoperative adjuvant therapy. Two patients refused the treatment. Remaining 73 patients received postoperative adjuvant therapy (chemotherapy in 36 patients and radiochemotherapy in 37 patients). Postoperative chemotherapy was completed in 72 (99%) patients. There was one death in this treatment period. Resectable liver metastases have been identified in 6 patients. In 3 of these patients, the metastases were identified at the time of initial surgery and proceeded to partial liver resections 8 weeks postoperatively. The remaining 3 patients were found on routine follow-up control, and also they underwent partial liver resection. All 6 patients remained disease free at the last follow up (July 2005). The median follow-up was 23 (range: 9–70) months.
The two groups were well matched for patient characteristics, in terms of age, sex, localization of the tumor, diameter of the tumor and stage of the tumor (Table 1), distal and radial clearance margins, differentiation and macroscopic appearance of the tumor, resection margins, and residual tumor status (Table 2). In the washout group, 5 patients had preoperative adjuvant therapy and 31 patients had postoperative adjuvant therapy, whereas in the no-washout group, 16 patients received preoperative adjuvant therapy, and 42 patients received postoperative adjuvant therapy. Each group was homogeneous with regard to preoperative and postoperative adjuvant therapy (P = 0.121). The donuts were complete and tumor free in all patients. The median follow-up time of all patients was 23 (range: 9–70) months. The mean follow-up time was similar in the two groups (33.17 ± 15.60 vs. 36.08 ± 19.48 months, P = 0.421). Although the number of patients who had neoadjuvant therapy was fewer in the washout group than in the no-washout group, the difference was not statistically significant (P = 0.131).
At the end of two cytological examinations, there was no contradictory classification. Three (8%) patients on whom rectal washout was performed had malignant cells collected from the circular stapler, whereas 2 (3%) patients who did not have rectal washout performed had malignant cells identified. There was no significant difference between the two groups regarding having malignant or benign cells in the centrifuged saline or having acellular cytology (P = 0.631) (Table 3). Of the five patients with malignant cytology, the original tumor was stage I in one patient, stage II in one, stage III in one, and stage IV in two. One patient had preoperative chemoradiotherapy. The rate of having malignant cells in the centrifuged saline in patients who had preoperative chemoradiotherapy and in patients who did not have preoperative chemoradiotherapy was similar (4.8% vs. 6%, P = 0.699).
In patients who had preoperative chemoradiotherapy (n = 21), 7 of them had no response and they had benign cells in the centrifuged saline; 2 patients showed complete response and they had benign cells in the centrifuged saline; 12 patients had partial response and 10 patients had benign cells in the centrifuged saline, 1 patient had malignant cells in the centrifuged saline, and 1 patient had acellular cytology.
Gross appearance of the tumor was either bulky-exophytic (3 patients) or infiltrative-ulcerated (2 patients) in patients who had malignant cells identified in the centrifuged saline.
Although distribution of tumor localizations were homogenous between groups (P = 0.062) (Table 1), there was an increase in low cancers in the no-washout group (10/58). The possible interaction between tumor localization and malignant cytology was tested by stratification. When tumor localizations were grouped as upper rectum tumors (distal sigmoid plus upper rectum) and lower rectum tumors (middle rectum plus lower rectum), there was no association between washout status and malignant cytology in any of the tumor localization strata (P = 0.068 for upper rectum group and, P = 0.52 for the lower rectum group). The local recurrence rate was 5.2% for all patients. Two patients with local recurrence were also noted to have widespread distant metastases at the time of local recurrence. Three (8%) patients in the washout group developed local recurrence. None of these recurrences were at the anastomosis. Two (3.4%) patients in the no-washout group had local recurrence, one of them an anastomotic recurrence (Table 1). There was no significant difference in the local recurrence rates between the two groups (P = 0.338).
The characteristics of four patients with recurrent disease are shown in Table 4. Of 3 patients with local recurrence on whom rectal washout was performed, two had benign cells collected from the circular stapler and one patient’s slides were acellular. Two patients with local recurrence on whom rectal washout was not performed had benign cells collected from the circular stapler. Of the f5 patients with local recurrence, the original tumor was stage I in one patient, stage II in one patient, stage III in two, and stage IV in one. Three patients with recurrent rectal cancer underwent salvage surgery and postoperative chemotherapy and chemoradiotherapy. One patient with stage IV disease underwent only radiotherapy. One patient’s local recurrence was diagnosed during her ninth cycle of postoperative chemotherapy. She developed intestinal obstruction and severe neutropenia and died before an attempt could be made to treat her local recurrence.
DISCUSSION
Exfoliated malignant cells implanting at distal sites within the bowel mucosa have been reported in the literature, and it has long been held that anastomotic recurrence is caused by this mucosal implantation.5,6,26–30 Local recurrence also may be attributable to inoculation of malignant cells when trocar punctures the sealed rectal stump in the cross staple technique. Moreover, it has been shown that viable colorectal cancer cells were able to cross an otherwise watertight anastomosis where they potentially might cause to locoregional recurrence.5,6 In theory, implantation of viable tumor cells may be responsible for some of the anastomotic recurrences as well as some of the other types of locoregional recurrence. Spillage of intraluminal tumor cells into the pelvis would be a logical mechanism, although one study reported that these cells were not viable30; others have demonstrated large numbers of viable intraluminal tumor cell4,31 that could produce tumors when injected into a mouse model.32 This provides an argument for the use of intraluminal washout during anterior resection.
Church et al., stated that thorough rectal irrigation probably eliminates exfoliated malignant cells, mainly by mechanical cleansing, rather than by any cytocidal effect of the irrigant; thus intraoperative rectal washout with normal saline before an anastomosis should be considered.12 With the use of saline alone, Jenner et al., have demonstrated that rectal washout mechanically removes exfoliated malignant cells from the distal rectum. In their study, none of 10 patients on whom rectal washout was performed had malignant cells collected from the circular stapler, but 8 of 10 patients who did not have rectal washout had malignant cells identified (P = 0.0007).14 Thus, these investigators strongly advise the routine use of rectal washout before anastomosis for all anterior resections for cancer.14 In our study, in spite of cytocidal rectal washout, 3 (8%) patients had malignant cells collected from the circular stapler, but none of them developed local recurrences in 6, 19, 24 months follow up time. Sayfan et al., also confirmed that free malignant cells are shed into the lumen of the rectum during anterior resection in patients with rectal cancer and distal sigmoid colon.13 They concluded that the volume of the lavage fluid should be larger than 500 ml to minimize the possibility of malignant cell entrapment in staple lines.13 In our study, cytological examination of circular stapler irrigation revealed that benign or malignant epithelial cells were present in 87% (33/38) of patients despite a rectal washout. Rectal washout with 500 ml volume of 5% povidone-iodine did not effectively achieve its goal of cellular eradication.
In the study of Dehni et al., despite the fact that all patients had rectal washout with 5% povidone-iodine both at the start of procedure and after rectal mobilization, 3 (3/255) patients developed local recurrence at the anastomosis.33 It has been shown that blood makes povidone-iodine and chlorhexidine/cetrimide much less efficient at killing colorectal cells.34 It is not clear whether cytocidal agents are effective in preventing anastomotic recurrences.
The clinical importance of cytocidal rectal washout—effect of cytocidal rectal washout on the incidence of local recurrence—was recently tested in a retrospective clinical study.15 Ninety patients with rectal cancer underwent rectal washout with cetrimide before anastomosis. Fifty-one patients with rectal cancer did not have rectal washout before anastomosis. There was no significant difference in the local recurrence rates between two groups (4.4% vs. 5.9%, P = 0.0653). Although, the study population was small, the author concluded that the true benefit of cytocidal washout was small.15 The American Society of Colon and Rectal Surgeons published practice parameters for the management of rectal cancer in 2005.35 They concluded that there was insufficient evidence to recommend intraoperative rectal washout.35 In our study local recurrence rates were similar in the rectal washout group and the no-washout group. None of the patients who developed local recurrence had malignant cells collected from circular stapler, but the only patient who developed anastomotic recurrence was in the no-washout group.
When interpreting this result one should take into account the present study’s limitations. The first is that the size of our study was not sufficient to detect the difference of local recurrence rates with a good statistical power. The second, although no patient was lost during the follow-up period, the mean follow-up time of our study was less than 5 years, and that was not sufficient time to allow all potential recurrences to become apparent. Third, although the study was prospective in terms of data collection, the study design was not randomized due to having surgeons with obvious preferences on rectal washout administration. Thus, allocation of patients was based on the surgeon’s preference.
In conclusion, there were no differences in terms of the number of patients who had malignant cells collected from the circular stapler and local recurrence rates between two groups. Our results did not offer rational arguments in support of rectal washout when a circular stapler is used following low anterior resection for carcinoma. However, because of the limitations of our study, we are unable to draw a definitive conclusion regarding rectal washout. There is need for a randomized, controlled, large-scale, multicenter trial to establish the clinical relevance of intraoperative rectal washout. In addition, the optimum volume of lavage fluid and the value of using cytocidal agents instead of normal saline are still unclear issues.
References
McGregor JR, Galloway DJ, McCulloch P, et al. Anastomotic suture materials and implantation metastasis: an experimental study. Br J Surg 1989;76:331–334
Hubens G, Lafullarde T, Van-Marck E, et al. Implantation of colon cancer cells on intact and damaged colon mucosa and serosa: an experimental study in the rat. Acta Chir Belg 1994;94:258–262
Tsunoda A, Shibusawa M, Kawamura M, et al. Recurrent colonic cancer developing at the site of a stapled stump: report a case. Surg Today 1997;27:457–459
Umpleby HC, Fermor B, Symes MO, et al. Viability of exfoliated colorectal carcinoma cells. Br J Surg 1984;71:659–663
O’Dwyer PJ, Martin EW. Viable intraluminal tumor cells and local/regional tumor growth in experimental cancer. Ann R Coll Surg Engl 1989;71:54–56
Leather AJM, Yiu CY, Baker LA, et al. Passage of shed intraluminal colorectal cancer cells across a sealed anastomosis. Br J Surg 1991;78:756
Gertsch P, Baer HU, Kraft R, et al. Malignant cells are collected on circular staplers. Dis Colon Rectum 1992;35:238–241
Anderberg B, Enbald P, Sjodahl R, et al. Recurrent rectal carcinoma after anterior resection and rectal stapling. Br J Surg 1984;71:98–100
Hurst PA, Prout WG, Kelly JM, et al. Local recurrence after low anterior resection using staple gun. Br J Surg 1982;69:275–276
Leff EI, Shaver JO, Hoexter B, et al. Anastomotic recurrence after low anterior resection. Stapled vs. hand-sewn. Dis Colon Rectum 1985;28:164–167
Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583–596
Church JM, Gibbs P, Chao M W, et al. Optimizing the outcome for patients with rectal cancer. Dis Colon Rectum 2003;46:389–402
Sayfan J, Averbuch F, Koltun L, et al. Effect of rectal stump washout on the presence of free malignant cells in the rectum during anterior resection for rectal cancer. Dis Colon Rectum 2000;43:1710–1712
Jenner DC, de Boer WB, Clarke G, et al. Rectal washout eliminates exfoliated malignant cells. Dis Colon Rectum 1998;41:1432–1434
Agaba EA. Does rectal washout during anterior resection prevent local tumor recurrence? Dis Colon Rectum 2004;47:291–296
Sjödahl R. Do we need adjuvant treatment for rectal cancer? Ann Med 1997;29:91–93
Zaheer S, Pemberton JM, Farouk R, et al. Surgical treatment of adenocarcinoma of the rectum. Ann Surg 1998;227:808–811
Wood CB, Dawson PM, Habib NA. The sialomucin content of colonic resection margins. Dis Colon Rectum 1985;28:260–261
Pietra N, Sarli L, Costi R, et al. Role of follow up in the management of local recurrences of colorectal cancer. Dis Colon Rectum 1998;41:1127–1133
UICC, TNM Supplement 1993. A Commentary on Uniform Use. Berlin: Springer-Verlag
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–1482
Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical excision. Histopathological study of lateral tumor spread and surgical excision. Lancet 1986;2:996–999
Greene FL et al. (eds). AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag, 2002
Begg CB, Carbone PP. Clinical trials and drug toxicity in the elderly. The experience of the Eastern Cooperative Oncology Group. Cancer 1983;52:1986–1992
Nicholls RJ, Moskowitz RL. A clampless method of rectal division during anterior resection. Surg Gynaecol Obstet 1988;166:357
Norgren J, Svensson JO. Anal implantation metastasis from carcinoma of the sigmoid colon and rectum—a risk when performing anterior resection with the EEA stapler. Br J Surg 1985;72:602
Goligher JC. Surgery of the Anus, Rectum, and Colon. 5th. Ed. London: Bailliere-Tindall, 1984;454–456
Killingback M, Wilson E, Hughes ESR. Anal metastasis from carcinoma of the rectum and colon. Aust N Z J Surg 1965;34:178–187
Rollinson PD, Dundas SA. Adenocarcinoma of sigmoid colon seeding into pre-existing fistula in ano. Br J Surg 1984; 71:664–665
Rosenberg IL. The aetiology of colonic suture line recurrence. Ann R Coll Surg Engl 1979; 61:251–257
Skipper D, Cooper AJ, Marston JE, et al. Exfoliated cells and in vitro growth in colorectal cancer. Br J Surg 1987;74:1049–1052
Fermor B, Umpleby HC, Lever J, et al. The proliferation and metastatic potential of exfoliated colorectal carcinoma cells. L Natl Cancer Inst 1984;74:1161–1168
Dehni N, Caplin S, Frileux P, et al. Cancer recurrence along the pouch longitudinal suture line after colonic J pouch-anal anastomosis. Br J Surg 2002;89:206–207
Docherty JG, McGregor JR, Purdie CA, et al. Efficacy of tumorocidal agents in vitro and in vivo. Br J Surg 1995;82:1050–1052
Tjandra JJ, Kilkenny J 3rd , Buie WD, et al. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum 2005;48:411–423
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented at the American Society of Colon and Rectal Surgeons Annual Meeting, June 21–26, 2003, New Orleans, LA (in the Resident/Fellow Presentations as the presentation of International Scholarship Winner).
Rights and permissions
About this article
Cite this article
Terzi, C., Ünek, T., Sağol, Ö. et al. Is Rectal Washout Necessary in Anterior Resection for Rectal Cancer? A Prospective Clinical Study. World J. Surg. 30, 233–241 (2006). https://doi.org/10.1007/s00268-005-0300-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-005-0300-x