Abstract
Pancreas-preserving total duodenectomy (PPTD) was first described by Chung et al. in 1994. Since then, several surgeons have used PPTD to treat diseases that involve the duodenum diffusely but not the head of the pancreas, mostly familial adenomatous polyposis (FAP). The PPTD method has been changed in each report and seems to have improved over time. We performed PPTD on three patients with different diseases-one with intestinal hemorrhage due to small intestinal amyloidosis; another with numerous duodenal gastrinomas in a patient with multiple endocrine neoplasia type 1 (MEN-1) and Zollinger-Ellison syndrome (ZES); and the third with numerous duodenal polyposis and FAP-using a new method that is simpler and safer than those previously reported. When resecting the whole duodenum, we performed mucosectomy of the major papillar portion and saved the structure of the major papilla. After an approximately 8 mm long sphincteropapillotomy, the opened major papilla was anastomosed to an incisional opening of the small intestine. The orifice of the main pancreatic duct (MPD) was stented by a catheter, and the MPD was kept intact under direct vision during the operative procedures. The head of the pancreas was fixed with the small intestine by interrupted 4-0 silk sutures. Reconstruction of the alimentary tract was performed after either the Billroth I or the Billroth II method. This is the first report of PPTD in which the entire MPD was preserved to simplify the biliopancreatic-ductal reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreas-preserving total duodenectomy (PPTD) has occasionally been reported as a surgical technique for familial adenomatous polyposis (FAP) [1, 2, 3, 4]. PPTD can be thought of as a counterpart to the duodenum-preserving pancreatectomy (DPP), which has usually been applied for benign or low malignant diseases of the head of the pancreas [5]. PPTD is useful for diffuse duodenal diseases that do not involve the head of the pancreas. The first patient on whom we performed PPTD was a patient with diffuse intestinal hemorrhage due to intestinal amyloidosis that involved the entire jejunum and duodenum. We used a new PPTD technique that preserves the structure of the major papilla and the entire main pancreatic duct. This technique was subsequently used in two other patients with a good postoperative course. We describe here our new technique precisely, as well as the indications for, and the merit of, this technique.
Patients and Methods
Patients
Three patients underwent PPTD with a new technique for the treatment of three different diffuse duodenal diseases: (1) duodenal bleeding due to hemorrhagic diffuse intestinal amyloidosis; (2) numerous duodenal gastrinomas in a patient with multiple endocrine neoplasic type/(MEN-1) and Zollingen-Ellison syndrome (ZES); and (3) multiple polyposis in a patient with FAP.
Surgical Technique
After mobilizing the whole duodenum from the retroperitoneum by Kocher’s maneuver, we perform a cholecystectomy and insert a catheter into the cut end of the cystic duct to pass it through the common bile duct into the duodenum. By palpating a catheter in the duodenum, we can easily identify the location of the major papilla. Then, the lower half of the duodenum is separated from the head of the pancreas at the level of the major papilla by dissecting the branches of the pancreaticoduodenal vessels using a Ligasure LAP (Valleylab, Boulder, Co, USA). The stomach is then transected at a line 5 cm to the oral side from the pylorus or on the line juxta-pylorus depending on the extension of the disease. Lifting up the cut stomach, the first part of the duodenum, or both, we carefully separate the upper half of the second part of the duodenum from the head of the pancreas with the Ligasure LAP. During these procedures, the duct of Santorini is identified and ligated twice, with 3-0 silk and 4-0 nylon. Several authors have reported that there was a dense adhesion between the upper half of the duodenum and the head of the pancreas [2, 6]. In such patients, however, we did not encounter any difficulty freeing this part of the duodenum (Fig. 1). The proximal jejunum is then cut, and the lower half of the duodenum is separated from the pancreas.
New pancreas-preserving total duodenectomy (PPTD) method. During surgery the head of the pancreas is preserved, and the duodenum is totally resected. When necessary, part of the stomach is also resected. The structures around the major ampulla are not cut at the line between the duodenum and the head of the pancreas, and only the mucosa over the ampulla is removed. Hence the submucosal structure of the major papilla is saved, as shown in Figure 2a. Thus, the whole tract of the main pancreatic duct (MPD) is preserved.
As the last step of the duodenal resection, we strip off the mucosal layer of the major papilla while palpating a catheter in the distal common bile duct. Thus, a part of the submucosal connective tissue and the muscular layer of the major papilla are saved. These procedures are shown (with the resection line superimposed) in figures that were drawn by Suda et al. and are used by courtesy of those authors (Fig. 2).
Reconstruction of the alimentary tract is performed after either the Billroth I or the Billroth II method. Anastomosis is started by the papillojejunostomy, which is the most important anastomosis in this operation (Fig. 3). Using a knife the major papilla is cut about 8 mm in length on the opposite wall of the orifice of the MPD. A catheter is inserted into the main pancreatic duct to prevent creating a stricture or kinking the MPD during the anastomosis procedures. Then the cut edge of the major papilla is anastomosed to a small incisional opening on the jejunum with about ten 5-0 interrupted absorbable sutures. During the anastomotic procedures the patency of the MPD is kept intact by palpating the stenting catheter in it (Fig. 3). The head of the pancreas, which had been attached to the duodenum, is covered with the small intestine using about 20 interrupted seromuscular sutures of 4-0 silk.
Finally, the stomach is anastomosed to the jejunum in a fashion similar to either the Billroth I or Billoth II method. With the Billroth I method, the stomach is anastomosed to the jejunum about 10 cm oral from the papillojejunostomy. With the Billroth II method, the stomach is anastomosed to the retrocolically shifted jejunum about 30 cm anal from a papillojejunostomy (Fig. 4).
Alimentary tract is reconstructed by the Billroth I method or the Billroth II method. With the Billroth I method, the stomach is anastomosed to the jejunum about 10 cm oral from the papillojejunostomy (a). With the Billroth II method, the stomach is anastomosed to the retrocolically shifted jejunum about 30 cm anal from the papillojejunostomy (b).
Results
Case 1
A 65-year-old man was admitted with an acute abdomen on October 20, 2002. Emergency laparotomy revealed extensive necrosis of the jejunum including the duodenum. The jejunum was totally resected to stop jejunal bleeding, but the duodenum was left unresected and a side-to-side duodenoileostomy was performed. Pathology examination of the resected jejunum revealed diffuse hemorrhagic enteritis due to severe intestinal amyloidosis. Postoperatively, duodenal bleeding continued because of intestinal amyloidosis. Therefore PPTD was performed with the technique described above on November 22, 2002. Laparotomy was done through an upper midline incision, and the ileoduodenal anastomosis was identified. The ileum was then transected 5 cm from the anastomosis to mobilize the whole duodenum from the retroperitoneum. After total separation of the duodenum from the head of the pancreas, the whole duodenum was resected with part of the gastric antrum and an anastomotic part of the ileum. Reconstruction was performed as described above. The opened ampulla of Vater was anastomosed to an incisional opening of the ileum, and the stomach was anastomosed to the ileum after the Billroth II method. The patient’s postoperative course was uneventful, and he has been well without any gastrointestinal bleeding for 18 months postoperatively.
Case 2
A 52-year-old woman with MEN-1 was admitted to our department complaining of hypergastrinemia with duodenal ulcers. It is well known that in patients with MEN-1 and ZES gastrinomas are located in the duodenum. As they are often multiple, recurrence of ZES has not been rare [7, 8]. In this patient, preoperative duodenoscopy revealed more than five mucosal duodenal tumors and multiple ulcer scar-like lesions in the duodenum. Therefore we thought that PPTD was indicated. PPTD was performed using our method on November 6, 2003. The duodenum was totally resected with part of the gastric antrum, and a gastroenteric reconstruction was performed after the Billroth II method. Pathology examination of the resected specimen revealed numerous mucosal gastrinomas in the duodenum. Postoperatively, her serum gastrin levels normalized, evidence that all the gastrinomas were resected. She has been well without any sign of recurrence of gastrinomas for 7 months postoperatively.
Case 3
A 40-year-old man with FAP underwent the same PPTD method for duodenal polyposis on November 11, 2003. This time, to save the stomach and the pylorus, the oral cut line of the alimentary tract was juxtasubpyloric. After resecting the whole duodenum, gastroenteric reconstruction was performed after the Billroth I method (i.e., an end-to-end pylorojejunostomy) because we thought that this reconstruction would make it easier for him to undergo endoscopic surveillance of the intestinal polyps postoperatively. He has been well for 8 months since the operation.
Discussion
We described here a new operative technique for PPTD that has been successfully performed on three patients for the treatment of three duodenal diseases: duodenal bleeding due to hemorrhagic intestinal amyloidosis; numerous duodenal gastrinomas in a patient with MEN-1 and ZES; and multiple polyposis in a patient with FAP. This is the first report that a patient with MEN-1 and ZES underwent a curative resection of multiple duodenal gastrinomas with PPTD.
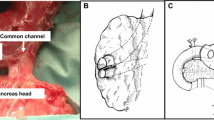
PPPD is simpler and less invasive that Whipple’s operation because it avoids unnecessary resection of the head of the pancreas [2]. In previous reports on PPTD, the distal choledochus was cut on the line between the duodenum and the pancreas. Accordingly, the MPD has been cut because it usually opens close to the orifice of the major papilla (Fig. 2) [9]. Suda et al. [9] examined the anatomy of the choledochopancreatic junction in 130 Japanese adults and classified it into three patterns according to Tokuyama [7]. They found that in only four patients (3.1%) did the MPD have an orifice separate from that of the common bile duct (CBD) (type 1). In 19 patients (14.6%), the MPD and the CBD run into the duodenum together without forming a common channel (type 2). In all of the other 107 patients (82.3%) a common channel existed (type 3), and the choledochopancreatic junction was located in the mucosa or submucosa (type 3a) (Fig. 5a). Only in patients with biliary atresia or a choledochal cyst was the choledochopancreatic junction located below the muscular layer of the duodenum (type 3b) (Fig. 5b). Thus, in most patients without an abnormal biliopancreatic anastomosis, the MPD opens into the mucosal or submucosal layer of the duodenum.
Suda et al. examined the anatomy of the choledochopancreatic junction in 130 Japanese adults and classified it into three patterns according to Tokuyama [9]
. They found that in only four patients (3.1%) did the MPD have an orifice separate from that of the common bile duct (CBD) (type 1). In 19 patients (14.6%), the MPD and the CBD ran into the duodenum together without forming a common channel (type 2). In all of the other 107 patients (82.3%) a common channel existed (type 3), and the choledochopancreatic junction was located in the mucosa or submucosa (type 3a) (a). Only in patients with biliary atresia or a choledochal cyst was the choledochopancreatic junction located below the muscular layer of the duodenum (type 3b) (b). Thus in most patients without an abnormal biliopancreatic anastomosis, the MPD opens into the mucosal or submucosal layer of the duodenum.
To preserve the orifice of the MPD, we resected the duodenal wall around the major papilla, as shown in Fig. 2a. This was easily achieved by palpating a catheter inserted into the distal CBD. Some of the submucosal connective tissue around the choledochopancreatic junction was saved, as was part of the sphincter of the major papilla (Fig. 2b). The biliopancreatic duct was reconstructed after a sharp papillotomy about 8 mm in length on the opposite wall of the MPD opening. A catheter was inserted into the MPD to prevent kinking or stenosis during the anastomotic procedure, and the cut edge of the major papilla was anastomosed to the small opening in the ileum using 5-0 interrupted absorbable sutures. This is a simple, safe method that avoids anastomosing a nondilated MPD to the jejunum.
Regarding invasion of pancreatic tissue into the upper half of the second part of the duodenum [6], we examined 10 cadavers and found invasion of pancreatic tissue in 7 of them. These results were compatible with the report of Kimura et al. [6]. In these 10 cadavers the length of the common channel was 2.9 ± 1.9 mm, which was also compatible with the results of Suda et al. [9].
It is well known that in patients with MEN-1 and ZES duodenal gastrinomas cause ZES [7, 8, 10]. Based on our observations and others, these duodenal gastrinomas are often multiple and recur even after extirpation of multiple duodenal gastrinomas [7, 8]. In case 2, the postoperative pathology study revealed numerous mucosal gastrinomas in the duodenum, although fewer gastrinomas had been diagnosed preoperatively. She has been well without any sign of recurrence after PPTD. We think that PPTD might become a standard method for the curative resection of multiple duodenal gastrinomas in patients with MEN-1 and ZES.
In conclusion, PPTD with our new method seems to be a safe, useful technique for patients with various diffuse duodenal diseases.
References
RS Chung JM Church R Stolk Particlevan (1995) ArticleTitlePancreas-sparing duodenectomy: indications, surgical technique, and results Surgery 117 254–259
GG Tsiotos MG Sarr (1998) ArticleTitlePancreas-preserving total duodenectomy Dig. Surg. 15 398–403 Occurrence Handle10.1159/000018652
JM Sarmiento GB Thompson DM Nagorney et al. (2002) ArticleTitlePancreas-sparing duodenectomy for duodenal polyposis Arch. Surg. 137 557–563 Occurrence Handle10.1001/archsurg.137.5.557
MF Kalady BM Clary DS Tyler et al. (2002) ArticleTitlePancreas-preserving duodenectomy in the management of duodenal familial adenomatous polyposis J. Gastrointest. Surg. 6 82–87 Occurrence Handle10.1016/S1091-255X(01)00005-1
HG Begen C Witte W Krautzberger et al. (1980) ArticleTitleErfahrung mit einer das Duodenum eahaltenden Pancreaskopfresektion bei chronischer Pankreatitis Chirurg 51 303
W Kimura H Nagai (1995) ArticleTitleStudy of surgical anatomy for duodenum-preserving resection of the head of the pancreas Ann. Surg. 221 359–363
M Imamura K Takahashi K Isobe (1992) ArticleTitleClinicopathological characteristics of duodenal microgastrinomas World J. Surg. 16 703–709
M Pipeleers-Marichal G Somers G Willems et al. (1990) ArticleTitleGastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome N. Engl. J.Med. 16 703–710
K Suda K Miyamoto K Hashimoto (1980) ArticleTitleThe choledocho-pancreatico-ductal junction in infantile obstructive jaundice diseases Acta Pathol. 30 187–194
NW Thompson Vinik Al FE Eckhauser (1989) ArticleTitleMicrogastrinomas of the duodenum Ann. Surg. 209 396–402
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imamura, M., Komoto, I., Doi, R. et al. New Pancreas-preserving Total Duodenectomy Technique. World J. Surg. 29, 203–207 (2005). https://doi.org/10.1007/s00268-004-7585-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-004-7585-z