Abstract
Although liver resection offers the only realistic chance of cure for patients with liver metastases from colorectal cancer, no consensus exists as to the procedure of choice for managing these tumors. Data from 193 patients who underwent hepatectomy for liver metastases from colorectal cancer and 26 of 193 patients who underwent repeat hepatectomy for recurrent metastases were collected. The suitability of resection was evaluated retrospectively based on known risk factors for recurrence and patterns of recurrence. On multivariate analysis, a positive surgical margin (SM+) was the only risk factor for recurrence after the initial resection (p < 0.01). SM+ (p < 0.01) and nonanatomic resection (p < 0·05) that was less than a sectionectomy (p < 0.05) were risk factors for recurrence after repeat hepatectomy. Multiple tumors (four or more) was the most common pattern of recurrence after initial hepatectomy, and recurrence close to the line of resection was most common after repeat hepatectomy. Based on tumor doubling times, recurrence after initial hepatectomy seemed to originate from the primary colorectal lesion, whereas recurrence after repeat hepatectomy was derived from a hepatic metastasis. Retrospective analysis suggests that hepatectomy with clear surgical margins is more important than anatomic resection for initial hepatectomy, and at least sectionectomy is necessary for repeat hepatectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Liver resection is the best available treatment for colorectal cancer metastases to the liver [1, 2, 3, 4, 5]. During the early days of hepatic surgery for colorectal metastases, only small, solitary unilobar lesions were resected. With the progress of surgical techniques and improved surgical skills, the indications for hepatectomy were extended to include large tumors, multiple unilateral metastases, and eventually multiple bilobar metastases when they could be removed entirely [6, 7]. Furthermore, several reports have now documented the benefit of repeat hepatectomy for recurrence in the liver remnant [8, 9]. Although general agreement has been reached that resection with a clear surgical margin is adequate for the initial resection, hepatectomy procedures still differ from facility to facility, and no uniform policy has been established. Standardization of procedures for repeat hepatectomy has not previously been attempted.
The current study was undertaken to develop guidelines for resecting primary and secondary liver metastases. We retrospectively analyzed risk factors for recurrence, patterns of recurrence, and estimates of tumor size at the time of colorectal resection based on tumor doubling times.
Materials and Methods
Patients
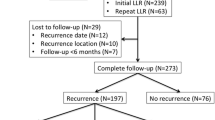
Between 1985 and 2000 a total of 193 patients underwent liver resection for colorectal cancer metastases. Among them, 26 patients underwent repeat liver resection for recurrent disease. Data from these patients were collected and reviewed. Operative feasibility and surgical outcome were compared between the initial and repeat hepatectomies. Risk factors for recurrence and patterns of recurrence after both the initial and repeat hepatectomies were compared.
Patterns of Recurrence
Patterns of recurrence were classified into three types, as described below. The pattern of multiple tumors was defined as four or more lesions. Near recurrence was defined as the development of fewer than four recurrent tumors near the original line of resection. Far recurrence was defined as the development of fewer than four recurrent tumors one segment or more distant from the original line of resection.
Calculation of Doubling Times of Tumors
The time course for determining the onset of recurrence was calculated using the doubling times (DTs) of the tumors. The tumor doubling time (T-DT) or carcinoembryonic antigen (CEA) doubling time (CEA-DT) was computed for each tumor. T-DT was calculated by the expression: log2/3 x [(T2 − T1)/(logD2 − logD1)], where T1 and T2 are the times, and D1 and D2 are the diameters at any two points in the clinical course before hepatectomy. CEA-DT was calculated by the expression: log2 x [(T2 − T1)/(logC2 − logC1)], where T is time, and C is the CEA level, as previously reported [10, 11, 12, 13, 14]. We also have shown that the T-DT of hepatic metastases is highly correlated with the CEA-DT in patients without extrahepatic metastases [13]. The T-DT does not correlate with the CEA-DT when extrahepatic metastases are present [14]. Therefore patients who had concomitant extrahepatic disease were excluded when only the CEA-DT was measurable. Consequently, data from 53 patients with liver recurrence after initial hepatectomy and from 9 patients with liver recurrence after repeat hepatectomy were collected to determine the onset of recurrence. When the tumor size was calculated to be less than 100 μm in diameter—the minimum implantable size [13, 15]—at the time of primary resection, we assumed that this recurrent tumor originated from the liver metastasis, not from the primary colorectal tumor.
Operative procedures
We treated patients with colorectal carcinoma metastatic to the liver according to the following principles. (1) Hepatic resection was attempted irrespective of the number or distribution of liver metastases so long as all of tumors could be resected and residual liver function was adequate. (2) The presence of extrahepatic metastases other than resectable lung metastases was a weak contraindication to surgery, and these decisions were made on a case-by-case basis. Hepatectomy may or may not have conformed to principles of anatomic resection. A hepatectomy that ensured tumor-free margins was the guiding principle. When it was believed that resection could not be tolerated because the amount of residual liver was unlikely to meet metabolic needs, portal embolization or a two-stage hepatectomy was performed. Intraoperative ultrasonography (IOUS) was used during all hepatic resections to determine if any occult tumors that had not been detected preoperatively were present and to confirm the relation between the tumor and vasculobiliary structures.
Operative procedures were defined following the terminology proposed by Strasberg [16], where segmentectomy is resection of a Couinaud segment [17], and sectionectomy is resection of one of Healey’s segments [18]. Anatomic resections included segmentectomy, sectionectomy, hemihepatectomy, and trisectionectomy.
Calculation of Clinical Factors Affecting Hepatic Recurrence
Microscopic satellite lesions were defined as tumor cells invading the portal vein, hepatic vein, or intrahepatic bile duct irrespective of continuity or discontinuity with macroscopic liver tumors. Using Yasui’s classification, liver tumors were classified into simple nodular (SN) or confluent nodular (CN) types depending on the characteristics of the cut surface of the resected tumor [19].
Patient Follow-up
After discharge, patients were followed monthly at our outpatient clinic. Complete radiologic evaluation, including conventional computed tomography, ultrasonography, and chest roentgenography, was performed every 3 months. CEA levels were monitored in all patients throughout the follow-up.
Statistical Analysis
Data on these patients were collected retrospectively from the physician, hospital charts, and the patients themselves. Statistical comparisons of baseline data were performed using the χ2 test, Student’s t-test, or the Mann-Whitney U-test. The Cox proportional hazard model was used to assess the independent effect of various risk factors on patients’ disease-free survival. The disease-free survival rate was calculated by the Kaplan-Meier method. The significance of differences in the survival curves was determined by the log-rank test. A difference was considered significant at the level of p < 0.05.
Results
Mortality, Morbidity, Feasibility
One patient died within 60 days of the initial hepatectomy (0.52%, 1/193). Postoperative bleeding occurred in this patient, and she died of sepsis and multiple organ failure 10 days after anterior sectionectomy. There were no operative deaths among patients who underwent repeat hepatectomy. The morbidity rates were 26.3% for initial hepatectomy versus 30.0% for repeat hepatectomy (p = 0.729). The duration of hepatectomy, total blood transfusion volume, and hospital stay after hepatectomy (mean ± SD) were 487.1 ± 136.4 minutes (median 475 minutes; range 186–865 minutes), 479.1 ± 575.3 ml (median 280 ml; range 0–3040 ml), and 22.0 ± 11.2 days (median 19 days; range 9–66 days) for the initial hepatectomy compared to 546.1 ± 155.5 minutes (median 569 minutes; range 301–850 minutes), 829.3 ± 352.6 ml (median 480 ml; range 0–2800 ml), and 25.7 ± 14.8 days (median 22 days; range 8–53 days) for the repeat hepatectomy (p = 0.179, p = 0.184, and p = 0.384, respectively).
Patient Outcome
Altogether, 4 of the 193 cases were not included in the evaluation (macroscopically noncurative hepatectomy in 2 and unknown outcome in 2). Of the remaining 189 patients, 128 (67.7%) developed recurrence (62 in the liver alone, 24 in the liver plus a distant site, 42 in a distant site alone) during a mean follow-up of 32 months (median 24 months; range 1–125 months). The 1-, 3-, and 5-year disease-free survival rates were 68.5%, 46.3%, and 42.6%, respectively. Of 62 patients (40.3%) who developed isolated hepatic recurrence, 25 underwent a second curative hepatectomy. One other patient in whom both liver recurrence and paraaortic lymph node metastases were present also underwent a second hepatectomy. Of these 26 patients, 18 (69.2%) had a second recurrence (9 in the liver alone, 2 in the liver plus a distant site, and 7 in a distant site alone) during a mean follow-up of 32.3 months (median 21 months; range 3–105 months). The 1-, 3-, and 5-year disease-free survival rates after repeat hepatectomy were 66.9%, 48.4%, and 48.4%, respectively (p = 0.683 vs. initial hepatectomy) (Fig. 1).
Risk Factors
A variety of factors affected hepatic recurrence after initial hepatectomy (Table 1). According to the univariate analysis, synchronous metastases (p = 0.027), bilobar metastases (p = 0.049), a large number of tumors (≥ 5) (p = 0.023), a positive tumor margin (p < 0.001), and the absence of postoperative intraarterial chemotherapy (p < 0.001) were risk factors for recurrence. Multivariate analysis using these five factors in the Cox proportional hazard method revealed that a tumor-free margin was the only independent factor for recurrence after initial hepatectomy (p < 0.001) (Table 2). Among the 26 patients who underwent repeat hepatectomy, univariate analysis showed that a positive surgical margin (p = 0.006) and nonanatomic resection (p = 0.025) of less than a sectionectomy (p = 0.016) were risk factors for recurrence after a repeat hepatectomy (Table 3).
Liver Recurrence Patterns
Patterns of recurrence were analyzed. After an initial hepatectomy, multiple lesions were present in 43 patients (50.0%): near lesions in 24 (27.9%) and far lesions in 19 (22.1%). Hepatic recurrence developed in 11 patients after repeat hepatectomy, with multiple lesions in 3 (27.3%), near lesions in 6 (54.5%), a far lesion in 1 (9.1%), and other (near plus for lesions) in 1 (p < 0.01) (Table 4).
Calculating the Onset of Recurrence
The time of hepatic metastasis could be calculated for 135 lesions in 53 of 86 patients with recurrence after the initial hepatectomy and for 23 lesions in 9 of 11 patients with a second recurrence after the second hepatectomy. DTs were used to calculate the size of the metastasis when the primary colorectal carcinoma was resected. The calculated diameter at that time was < 100 μm in 10.4% of recurrent tumors after the initial hepatectomy, whereas it was that size in 69.6% in patients with recurrent tumors after the second hepatectomy (p < 0.01) (Table 5).
Discussion
At present, 45% to 80% of patients who undergo hepatic resection of colorectal metastases to the liver develop recurrence [20, 21, 22, 23]. Isolated liver recurrence initially develops in approximately 30% of patients [22], one-third of whom are potential candidates for further resection [23, 24, 25, 26]. Moreover, one-third of the patients who suffer hepatic recurrence after a second hepatectomy underwent a third hepatectomy [27]. Although several reports have noted the benefit of repeat hepatectomy for liver recurrence [8, 9], the resectability rate is low. To improve both the disease-free interval and the overall survival rate, a surgical philosophy and technique must evolve.
It is reasonable to believe that most recurrences after hepatectomy are the result of occult tumors that are present at the time of resection [28]. It has been reported that microscopic satellite lesions are present in vessels and bile ducts around the macroscopic lesions [13]. These microscopic lesions are thought to originate from the primary colorectal tumor or from macroscopic metastases [13]. If the microscopic lesions represent intrahepatic spread similar to what occurs with hepatocellular carcinoma, anatomic resection with adequate surgical margins is essential. On the other hand, anatomic resection may not be necessary if the occult tumors originate from the primary colorectal tumor. Although distinguishing between these mechanisms is difficult, some means to do it must be developed to ensure that the most appropriate hepatectomy is performed in each case.
When a recurrence is derived from a metastatic lesion, one would expect the incidence of recurrence close to the line of resection to be high with the percentage of lesions < 100 μm in diameter at the time of colorectal resection also high. Recent reviews of the surgical procedures employed for initial resections concluded that the type of hepatectomy procedure (anatomic/major versus nonanatomic/limited) was not a prognostic factor [29, 30, 31, 32]. However, an inadequate surgical margin does have an adverse effect on outcome, and many surgeons advocate that a margin of at least 1 cm be used [33, 34, 35]. The present study also failed to identify the type of initial hepatectomy as a predictor of disease-free survival by univariate analysis (Table 1), although the multivariate analysis revealed that a tumor-free margin predicted disease-free survival. In contrast, although multivariate analysis was not performed because the type of hepatectomy and the presence or absence of a tumor-free margin were interdependent, both nonanatomic liver resection of less than a sectionectomy and inadequate tumor-free margin were risk factors for recurrence after repeat hepatectomy.
Multiple recurrence was the most common pattern after initial hepatectomy. In contrast, recurrence close to the line of resection was most common after repeat hepatectomy. These findings suggest that intrahepatic spread is more common with a second hepatic recurrence than with the first recurrence after the initial resection. Recurrence close to the line of resection seems to be most common after repeat hepatectomy because the extent of the surgical margin was limited by the effort to preserve adequate residual liver tissue. However, major hepatectomy (Couinaud’s three segments or more) was performed in only 2 of our 26 repeat hepatectomy cases at the initial resection. Therefore, if we performed major hepatectomy as the repeat hepatectomy in these patients, the estimated postoperative liver volume would be sufficient to avoid postoperative liver failure.
Furthermore, estimation of the time course indicated that the source of the recurrence after initial hepatectomy was the primary colorectal tumor but that the recurrences after repeat hepatectomy were derived from the original metastasis. These findings explain why a nonanatomic hepatectomy procedure was not a prognostic factor for the disease-free survival rate after the initial hepatectomy but was a predictor for recurrence after repeat hepatectomy.
Takahashi et al. [36] calculated that the minimum implantable size of a metastasis is 25 cells x 10 μm, the size of a single cancer cell [10]. In our previous study regarding microsatellite liver metastases [13, 14, 15], the minimum detectable size of microscopic lesions surrounding the macroscopic metastases was 100 μm. Therefore we assumed the minimum implantable size to be 100 μm the in present study.
In this series, the frequency of anatomic and anatomic + wedge resection for a repeat hepatectomy (57.6%, 15/26) (Table 3) was equivalent to that for initial hepatectomy (60.3%, 114/189) (Table 1) (p = 0.97). Consequently, operative feasibility was equivalent for initial and repeat hepatectomies. Although performing a second hepatic procedure is technically demanding, the complication rates and feasibility were comparable to those associated with primary hepatectomy. These results support a policy of performing radical hepatectomy for recurrence in the remnant liver. As we noted previously, only one-third of patients with an initial liver recurrence are potential candidates for repeat resection. Hence radical hepatectomy for an initial recurrence is indicated in only in a small number of patients. However, only one-third of the patients with recurrence after a second hepatectomy would undergo a third hepatectomy. Therefore to improve the outcome we believe that radical hepatectomy should be performed for the initial recurrence to reduce the possibility of a second recurrence after a second hepatectomy.
Liver recurrence after initial hepatectomy usually represents multiple metastatic foci from the primary disease rather than intrahepatic spread of existing metastases. Therefore most recurrences are not controllable, even by extensive hepatectomy. In contrast, it was presumed that recurrence after repeat hepatectomy predominantly represents intrahepatic spread. The results of this retrospective study suggest that resection with an adequate surgical margin, irrespective of hepatic anatomy, is all that is necessary for the initial hepatectomy. On the other hand, sectionectomy or a more extensive anatomic procedure should be performed when a second resection is indicated. The success reported in this series justifies a prospective randomized controlled study to define the role of hepatic resection for recurrence of hepatic metastases from colorectal carcinoma.
Résumé
Bien que la résection offre la seule chance de cure pour les patients porteurs de métastases d’origine colorectale, il n’existe aucun consensus quant au procédé de choix pour la prise en charge de ces tumeurs. Les données provenant de 193 patients ayant eu une résection hépatique pour métastases d’origine colorectale et parmi ceux-ci, 26 patients qui ont eu une résection hépatique itérative pour récidive, ont été analysées. L’indication de résecabilité a été évaluée rétrospectivement, basée sur les facteurs de risque connus pour la récidive et pour leur mode de récidive. En analyse multivariée, une marge de résection chirurgicale positive (SM+) était le seul facteur de risque de récidive après résection initiale (p < 0.01). Une marge de résection (SM+) (p < 0.01) et une résection nonanatomique (p < 0.05) de moins d’une segmentectomie (p < 0.05) étaient des facteurs de risque de récidive après une résection hépatique itérative. Les tumeurs multiples (4) constituaient le mode le plus fréquent de récidive après résection initiale, alors que, la récidive près de la tranche de section était le mode de récidive le plus fréquent après résection itérative. Basé sur le temps de dédoublement tumoral, la récidive après résection hépatique initiale semble prendre son origine au niveau de la lésion colorectal primitive, alors que la récidive après résection itérative semble avoir comme origine la métastase hépatique. Une analyse rétrospective suggère que la résection hépatique avec une marge libre de tissu tumoral est plus importante qu’une résection anatomique lors de la résection initiale, alors qu’au moins une segmentectomie est nécessaire en cas de résection itérative.
Resumen
Aunque la resección hepática constituye la única posibilidad real de curación en pacientes con metástasis hepáticas de cáncer colorectal, aún no hay consenso sobre cual es el procedimiento de preferencia en el manejo de estas lesiones. Se recolectó la información de 193 pacientes sometidos a hepatectomía por metástasis hepáticas de cáncer colo-rectal y de 26 entre los 193 en quienes se practicaron hepatectomías repetidas por metástasis recurrentes. La adecuación de la resección fue evaluada retrospectivamente con base en factores de riesgo de recurrencia conocidos y en el patrón de recurrencia. En el análisis multivariado se encontró que un margen positivo en la resección (MR+) era el único factor de riesgo de recurrencia luego de la operación inicial (p < 0.01). El MR+ (p < 0.01) y una resección no anatómica (p < 0.05) aparecieron como factores de riesgo de recurrencia luego de hepatectomías repetidas. Tumores múltiples (4) apareció como el más importante patrón común de recurrencia luego de la hepatectomía inicial, en tanto que la recurrencia cerca de la línea de resección fue lo más común luego de hepatectomía repetida. Con base en los tiempos de doblaje del tumor, la recurrencia luego de la hepatectomía inicial pareció originarse a partir de la lesión colo-rectal primaria, pero la recurrencia luego de hepatectomía repetida se derivó de las metástasis hepáticas. El análisis retrospectivo sugiere que la hepatectomía con márgenes libres es más importante que la resección anatómica al practicar la operación inicial, pero por lo menos una seccionectomía es lo necesario en la hepatectomía repetida.
References
JG Fortner JS Silva RB Golbey et al. (1984) ArticleTitleMultivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer Ann. Surg. 199 306–316 Occurrence Handle1:STN:280:BiuC2M3pslA%3D Occurrence Handle6703792
MA Adson JA Heerden Particlevan MH Adson et al. (1984) ArticleTitleResection of hepatic metastases from colorectal cancer Arch. Surg. 119 647–651 Occurrence Handle1:STN:280:BiuB3s%2FmsVE%3D Occurrence Handle6732473
InstitutionalAuthorNameRegistry of Hepatic Metastases (1988) ArticleTitleResection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection Surgery 103 278–288
Nordlinger B, Jaeck D, Guiguet M. Traitement des métastases hépatiques des cancers colorectaux. In Monographies de l’Association Française de Chirurgie (AFC), Paris, Springer, 1992;129–146
J Scheele R Strangl A Altendorf-Hofmann et al. (1995) ArticleTitleResection of colorectal liver metastases World J. Surg. 19 59–71 Occurrence Handle1:STN:280:ByqB2Mbjs1E%3D Occurrence Handle7740812
M Minagawa M Makuuchi G Torzilli et al. (2002) ArticleTitleExtension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results Ann. Surg. 231 487–499 Occurrence Handle10.1097/00000658-200004000-00006
K Tanaka H Shimada S Togo et al. (2001) ArticleTitleIs hepatic resection for multiple liver metastases from colorectal carcinoma acceptable treatment? Hepatogastroenterology. 48 803–807
J Yamamoto T Kosuge K Shimada et al. (1999) ArticleTitleRepeat liver resection for recurrent colorectal liver metastases Am. J. Surg. 178 275–281 Occurrence Handle10.1016/S0002-9610(99)00176-2 Occurrence Handle1:STN:280:DC%2BD3c%2Flt1CltA%3D%3D Occurrence Handle10587183
S Suzuki T Sakaguchi Y Yokoi et al. (2001) ArticleTitleImpact of repeat hepatectomy on recurrent colorectal liver metastases Surgery 129 421–428 Occurrence Handle10.1067/msy.2001.112486 Occurrence Handle1:STN:280:DC%2BD3M3js12jsA%3D%3D Occurrence Handle11283532
VP Collins RK Loeffier H Tivey (1956) ArticleTitleObservations on growth rates of human tumors AJR. Am. J. Roentgenol. 76 988–1000 Occurrence Handle1:STN:280:CyiD3cfnslM%3D
HJ Staab FA Anderer A Hornung et al. (1982) ArticleTitleDoubling time of circulating CEA and its relation to survival of patients with recurrent colorectal cancer Br. J. Cancer 46 773 Occurrence Handle7171456
IJ Havelaar PH Sugerbaker M Vermes et al. (1984) ArticleTitleRate of growth of intraabdominal metastases from colorectal cancer Cancer 54 163–171 Occurrence Handle6722741
H Shimada M Nanko S Fujii et al. (1995) ArticleTitleTreatment strategies for hepatic metastasis from colorectal cancer J. Hepatobiliary. Pancreat. Surg. 2 116–121
S Fujii (1994) ArticleTitleHepatic metastases development from colorectal cancer in view of CEA doubling time Yokohama 45 627–635
M Nanko H Shimada H Yamaoka et al. (1998) ArticleTitleMicroscopic colorectal cancer lesions in the liver Surg. Today 28 707–713 Occurrence Handle10.1007/s005950050213 Occurrence Handle1:STN:280:DyaK1czmt1KjsA%3D%3D Occurrence Handle9697263
SM Strasberg (1997) ArticleTitleTerminology of liver anatomy and liver resections: coming to grips with hepatic Babel J. Am. Coll. Surg. 184 413–434 Occurrence Handle1:STN:280:ByiB2MrjsVc%3D Occurrence Handle9100690
C Couinaud (1954) ArticleTitleLobes et segments hepatiques: notes sur l’architecture anatomique et chirurgicale du foie Presse Med. 62 709–712 Occurrence Handle1:STN:280:CyuD1MjlvVI%3D Occurrence Handle13177441
JE Healey PC Schroy (1953) ArticleTitleAnatomy of the biliary ducts within the human liver: analysis of the prevailing pattern of branchings and the major variations of the biliary ducts Arch. Surg. 66 599–616 Occurrence Handle13039731
K Yasui T Hirai T Kato et al. (1997) ArticleTitleA new macroscopic classification predicts prognosis for patient with liver metastases from colorectal cancer Ann. Surg. 226 582–586 Occurrence Handle10.1097/00000658-199711000-00002 Occurrence Handle1:STN:280:DyaK1c%2FkvFamuw%3D%3D Occurrence Handle9389391
F Bozzetti R Doci P Bignami et al. (1987) ArticleTitlePatterns of failure following surgical resection of colorectal cancer for liver metastases: rationale for a multimodal approach Ann. Surg. 205 264–270 Occurrence Handle1:STN:280:BiiC2Mrltlc%3D Occurrence Handle3827362
P Hohenberger P Schlag V Schwarz et al. (1990) ArticleTitleTumor recurrence and options for further treatment after resection of liver metastases in patients with colorectal cancer J. Surg. Oncol. 44 245–251 Occurrence Handle1:STN:280:By%2BA2cfhvFA%3D Occurrence Handle2385101
KS Hughes R Simon S Songhorabodi et al. (1986) ArticleTitleResection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence Surgery 100 278–284 Occurrence Handle1:STN:280:BimB1M%2FgtVc%3D Occurrence Handle3526605
B Nordlinger JG Vaillant M Guiguet et al. (1994) ArticleTitleSurvival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases; Association Française de Chirurgie J. Clin. Oncol. 12 1491–1496 Occurrence Handle1:STN:280:ByuB1Mzgsl0%3D Occurrence Handle8021741
JG Fortner (1988) ArticleTitleRecurrence of colorectal cancer after hepatic resection Am. J. Surg. 155 378–382 Occurrence Handle1:STN:280:BieC2cfitFA%3D Occurrence Handle3344897
F Bozzetti P Bignami F Montalto et al. (1992) ArticleTitleRepeat hepatic resection for recurrent metastases from colorectal cancer Br. J. Surg. 79 146–148 Occurrence Handle1:STN:280:By2B3cfmvVQ%3D Occurrence Handle1555063
Y Fong LH Blumgart A Cohen et al. (1994) ArticleTitleRepeat hepatic resections for metastatic colorectal cancer Ann. Surg. 220 657–662 Occurrence Handle1:STN:280:ByqD2s%2FjvFQ%3D Occurrence Handle7979614
J Yamamoto T Kosuge K Shimada et al. (1999) ArticleTitleRepeat liver resection for recurrent colorectal liver metastases Am. J. Surg. 178 275–281 Occurrence Handle10.1016/S0002-9610(99)00176-2 Occurrence Handle1:STN:280:DC%2BD3c%2Flt1CltA%3D%3D Occurrence Handle10587183
KD Griiffith PH Sugarbaker AE Chang (1990) ArticleTitleRepeat hepatic resection for colorectal metastases Surgery 81 101–104
RL Jamison JH Donohue DM Nagorney et al. (1997) ArticleTitleHepatic resection for metastatic colorectal cancer results in cure for some patients Arch. Surg. 132 505–511 Occurrence Handle1:STN:280:ByiA3crhtFw%3D Occurrence Handle9161393
B Ohlsson U Stenram KG Tranberg (1998) ArticleTitleResection of colorectal metastases: 25-year experience World J. Surg. 22 268–277 Occurrence Handle10.1007/s002689900381 Occurrence Handle1:STN:280:DyaK1c7lsFGhsQ%3D%3D Occurrence Handle9494419
J Yamamoto K Shimada T Kosuge et al. (1999) ArticleTitleFactors influencing survival of patients undergoing hepatectomy for colorectal metastases Br. J. Surg. 86 332–337 Occurrence Handle10.1046/j.1365-2168.1999.01030.x Occurrence Handle1:STN:280:DyaK1M3hsF2mtQ%3D%3D Occurrence Handle10201774
N Kokudo K Tada M Seki et al. (2001) ArticleTitleAnatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma Am. J. Surg. 181 153–159 Occurrence Handle10.1016/S0002-9610(00)00560-2 Occurrence Handle1:STN:280:DC%2BD3MzmtlGgsA%3D%3D Occurrence Handle11425058
J Scheele R Stangl A Altendorf-Hofmann et al. (1991) ArticleTitleIndicators of prognosis after hepatic resection for colorectal secondaries Surgery 110 13–29 Occurrence Handle1:STN:280:By6A38jivFU%3D Occurrence Handle1866690
M Rees G Plant S Bygrave (1997) ArticleTitleLate results justify resection for multiple hepatic metastases from colorectal cancer Br. J. Surg. 84 1136–1140 Occurrence Handle10.1046/j.1365-2168.1997.02749.x Occurrence Handle1:STN:280:ByiH38vlsV0%3D Occurrence Handle9278662
K Shirabe K Takenaka T Gion et al. (1997) ArticleTitleAnalysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin Br. J. Surg. 84 1077–1080 Occurrence Handle10.1046/j.1365-2168.1997.02743.x Occurrence Handle1:STN:280:ByiH38vltlU%3D Occurrence Handle9278644
Y Takahashi M Mai T Akimoto (1983) ArticleTitleStudy on natural history of gastric cancer with liver metastasis Jpn. J. Gastroenterol. Surg. 16 2067–2073
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tanaka, K., Shimada, H., Ohta, M. et al. Procedures of Choice for Resection of Primary and Recurrent Liver Metastases from Colorectal Cancer. World J. Surg. 28, 482–487 (2004). https://doi.org/10.1007/s00268-004-7214-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-004-7214-x