Abstract
The etiology of pain in chronic pancreatitis may be ductal hypertension, increased parenchymal pressure, or neural damage. It is difficult to assess the severity of pain in this patient population, a problem made more challenging by the frequency of narcotic dependency. Therapeutic interventions developed to relieve the pain of chronic pancreatitis include denervation of the pancreas, decompression of the main duct of the pancreas, resection of part or all of the diseased pancreas, and reduction of pancreatic secretion. Operative intervention for patients with chronic pain is indicated when severe pain, complications of pain, or potential malignancy are present. The operations that consistently provide long-lasting pain relief all have in common resection of all or a portion of the head of the pancreas. Adverse effects on exocrine and endocrine function, nutrition, and quality of life are related to the amount of pancreas resected. The ideal procedure should be easy to perform, have a low morbidity and mortality rate, provide long-lasting pain relief, and not augment endocrine and exocrine insufficiency. No single operation fulfills this ideal. The local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy (LR-LPJ) proposed by Frey and the duodenum-preserving resection of the head of the pancreas (DPHR) proposed by Beger are discussed. The conceptualization, development, and technique of LR-LPJ are discussed, and comparisons of patient outcomes are made with the outcomes of other procedures for chronic pancreatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with chronic pancreatitis who require operative intervention include those with severe pain, those with the complications of chronic pancreatitis, and those in whom cancer cannot be ruled out.
Ideally, the operation for the patient experiencing pain from chronic pancreatitis should have a low mortality and morbidity rate, be easy to perform, provide long-lasting pain relief, and rectify the complications of chronic pancreatitis, and it should not augment exocrine and endocrine insufficiency.
There is no standard operation that is used both in the management of pain and the complications of chronic pancreatitis that fulfills the ideal, including local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy (LR-LPJ) (also known as the Frey procedure) or duodenum-preserving pancreatic head resection (also known as the Beger procedure. First, no single operation addresses all the structural abnormalities and complications associated with chronic pancreatitis. Second, selecting an operation for the patient when pain from chronic pancreatitis is the only symptom prompting a visit to the surgeon is challenging. Because the exact etiology of pain in patients with chronic pancreatitis is unknown, the surgeon must rely on empirically derived evidence about which operations have a respectable record of providing some pain relief.
There are many theories as to the cause of pain in chronic pancreatitis. Ductal hypertension and/or increased parenchymal pressure (compartment syndrome) [1] are known to be present in many patients with chronic pancreatitis, as is neural damage from inflammation and fibrosis. These causes, along with inflammation and infection, are given the most credence at this time [2, 3, 4, 5, 6, 7]. Moreover, it is not known whether there may be more than one etiology of pain, or whether the same patient may have more than one cause of pain. Recently, two reports have correlated the preoperative severity of pain with the pancreatic pathology found at operation. The presence of small cysts, acinar cell necrosis, and areas of acute inflammation in the pancreas were present in patients with the most severe pain [8, 9].The assessment of the severity of pain in individual patients is subjective, and comparison of severity between patients is impossible. Narcotic dependency is a problem common to patients with chronic pancreatitis. Narcotic addiction complicates our ability to determine if the patient has been relieved of pain postoperatively. Freedom from pain does not guarantee a reduction in narcotic use because patients can vary in their lengths of narcotic use according to their degree of drug dependency and their differences in motivation to overcome addiction. Considering the deficiencies in our knowledge of the cause of pain in chronic pancreatitis and our inability to accurately assess the motivation for and degree of narcotic dependence of our patients, it should not surprise anyone that even after total pancreatectomy, 20% of patients report that they still have pain [10, 11, 12].
Pain Relief
Historically a variety of therapeutic interventions have been initiated in hopes of relieving the pain of chronic pancreatitis. These include attempts to denervate the pancreas, to decompress the main duct of the pancreas, to resect all or part of the diseased pancreas, and to decrease pancreatic secretion to reduce pressure in the pancreatic duct system. Both main duct decompression and pancreatic resection have been shown to provide longer lasting, more complete pain relief than sensory denervation of the pancreas or reduction of pancreatic secretion.
Among the many operative procedures touted to provide pain relief in patients with chronic pancreatitis, the more successful ones provide long-lasting pain relief in about 75% to 80% of those treated [2, 10, 11, 12, 13, 14, 15, 16, 17, 18]. The operations that are consistently successful in providing long-lasting pain relief all have in common resection of all or a portion of the head of the pancreas. These include pancreaticoduodenectomy, duodenum-preserving resection of the head of the pancreas, local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy, 80% to 95% distal resection of the pancreas (Child operation), and total pancreatectomy with or without preservation of the duodenum. Unfortunately, the operations that require resection of significant portions of the gland have an adverse effect on endocrine (Table 1) and exocrine (Table 2)function, nutrition (Table 3), work status (Table 4), and quality of life that can roughly be correlated to the amount of pancreas resected—i.e., total pancreatectomy [7, 8, 16], 80% to 95% distal resection [13, 30], or pancreaticoduodenectomy [15, 16, 17].
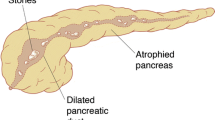
Main duct decompression in the form of the Partington-Rochelle longitudinal pancreaticojejunostomy [35] initially provides pain relief in 75% to 80% of patients [36, 37], but it fails to provide long-lasting pain relief in some patients followed for 5 years or longer [33, 38, 39, 40, 41]. The failures are believed to be the result either of the surgeon not opening the main duct all the way to the duodenum (Fig. 1), as described in the Partington-Rochelle longitudinal pancreaticojejunostomy, or failure of the operation itself to address disease associated with the other ducts in the head of the pancreas, i.e., the duct of Santorini, the duct to the uncinate, and the tributary ducts associated with all three ducts in the head of the pancreas (Wirsung, Santorini, and uncinate) (Fig. 2). When the main pancreatic duct is opened for only a few centimeters in the neck, body, or tail of the pancreas [33, 39], or when only the tail of the pancreas is decompressed (e.g., Duval procedure [26]), or the ampullary sphincter is divided [43], only 30% to 40% of patients obtain pain relief.
Sensory denervation of the pancreas in patients with chronic pancreatitis, except perhaps in some selected patients, seems to provide benefit for only a short time (12–18 months) [44, 45, 46, 47, 48]. Reduction of pancreatic secretion by enzyme [49] or somatostatin analog therapy [31] has not been proven to convey consistent long-term pain relief.
Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy (LR-LPJ) [2] consists of a resection of 4 to 12 g (average = 5.7 g) of diseased tissue (including contained nerve structures) in the head of the pancreas, and opening the main duct in the neck, body, and tail of the pancreas. All three ducts are decompressed or resected, along with their tributary ducts, by removing 4 to 12 g of tissue. The duct of Wirsung and the duct to the uncinate that traverse close to the posterior surface of the head are decompressed, and the duct of Santorini that traverses the head anteriorly and superiorly is excised (Fig. 3). Leaving the posterior capsule of the pancreatic head intact permits drainage of the head of the pancreas in continuity with the same Roux-en-Y limb used to drain the body and tail of the pancreas. In contrast, the posterior capsule is included in the resection of the head of the pancreas in the duodenum-preserving head resection (DPHR) of Beger. Thus, the (DPHR) requires two separate anastomoses to a Roux en-Y limb, one to the cut end of the pancreas and the other to the remnant of pancreatic tissue protecting the common bile duct. The LR-LPJ, in addition to decompressing the duct of Wirsung and the duct to the uncinate and excising the duct of Santorini, decompresses the main pancreatic duct in the neck, body, and tail of the pancreas. The LR-LPJ can be used in patients whose major duct in the body and tail of the gland is as small as 2–3 mm in diameter, because the anastomosis is to the capsule of the gland and not to the duct. The LR-LPJ operation is safe and relatively easy to perform compared with a pancreaticoduodenectomy or duodenum-preserving head resection of the pancreas. Although DPHR also addresses disease in the head of the pancreas, the DPHR unlike the LR-LPJ requires division of the pancreas at its neck. Division at the neck of the pancreas may be difficult in some patients if inflammatory changes or intrahepatic or extrahepatic portal hypertension is present causing increased vascularity and adherence of the pancreas to the underlying portal/superior mesenteric vein. Both operations can be useful in chronic pancreatitis in relieving pain; dealing with the complications of duct disruption (including pseudocysts, pancreatic ascites, pancreatic pleural effusions, and cardiac tamponade); common bile duct obstruction; and duodenal obstruction caused by a pseudocyst. Neither the LR-LPJ nor the DPHR is useful in patients with bleeding gastric varices from left-sided portal hypertension or pseudoaneurysms of the peripancreatic vasculature. Likewise, both operations are contraindicated in patients in whom the distinction between chronic pancreatitis and cancer of the pancreas cannot be made.
Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy successfully decompresses or resects the main duct of Santorini, duct to the uncinate, and tributary ducts in the head of the pancreas, and decompresses the main duct in the neck, body, and tail of the pancreas.
History of Development of the LR-LPJ
Gardner Child, former Chairman of the Department of Surgery at the University of Michigan, developed a large operative experience with an 80%–95% distal pancreatectomy to control pain in patients with chronic pancreatitis. After joining Dr. Child in 1964, I (C.F.F.) had the opportunity to perform the operation and do follow-up with the patients having this and all other operations for chronic pancreatitis at the University of Michigan [17]. Pain relief was achieved in 75% to 80% of patients observed during an average follow up of 6 to 9 years at the University of Michigan and other institutions using the operation [17]. Even though I was favorably impressed with the degree of long-lasting pain relief achieved, I was disappointed with the short- and long-term morbidity associated with the procedures. Patient discomfort and hospitalization were often prolonged, most frequently because of short-lived pancreatic fistulas and abscesses, which occurred in 40% of the patients. These complications resulted from collections of pancreatic juice arising from the cuff of pancreatic tissue preserved along the inner aspect of the duodenum; they often resulted in infection associated with the mandated use of open drainage with Penrose drains. The tissue adjacent to the duodenum was preserved because of the belief (since proved to be incorrect) that this tissue was necessary to preserve the blood supply to the duodenum [10]. Long-term sequelae of the 80%–95% distal pancreatectomy included the frequent development of exocrine and endocrine insufficiency in many patients. Insulin-dependent diabetes, for example, increased from a preoperative level of 9.1% to a postoperative level of 58%. Because of the high short- and long-term morbidity associated with the 80%–95% distal pancreatectomy, it has largely been abandoned, even though it provided long-term pain relief in 75% to 80% of patients.
With the availability of ultrasound, computerized tomography, and endoscopic retrograde cholangiopancreatography in the mid 1970s, it became possible to assess the structural pathology of the pancreas preoperatively, including its ductal system, and to identify patients who would be candidates for the Partington-Rochelle longitudinal pancreaticojejunostomy (LPJ). Concerned about the morbidity associated with 80%–95% distal resection of the pancreas and now able to identify patients with enlarged main ducts preoperatively, we became experienced in the performance of the Partington-Rochelle LPJ [35] in patients with pain from chronic pancreatitis (Fig. 2). It was widely believed at the time that it was not feasible to perform the operation unless the main duct was at least 6–7 mm in diameter. (Some surgeons believed the duct should have a minimum diameter of 1 cm [51]). We performed about 30 LPJs at the University of California, Davis, but subsequently discontinued the operation. Investigators began reporting variations in the duration of pain relief after LPJ [33, 35, 38, 39, 52]; initially, pain relief was reported to be present in 75 to 80% of the patients, but within 3 to 5 years pain relief had been maintained in only 50% or less of the patients [33, 38, 39, 41].
We believe LPJ may fail to provide long-term pain relief in some patients for two reasons. First, the operation may not be performed properly. In patients on whom we have operated who had a failed LPJ performed at another institution, we often found that the main duct had not been opened in the head of the pancreas, leaving the head of the pancreas undrained and not decompressed. These failures were not the fault of the operation but of the surgeon for not performing the operation as Partington and Rochelle had recommended. Bradley and others found that when the main pancreatic duct is opened for short distances (2–4 cm), only 30% to 40% of patients obtain long-term pain relief [33, 39, 40]. Second, even when LPJ is performed correctly, it fails to address disease associated with the ducts of Santorini and the uncinate or with tributary ducts associated with the ducts of Wirsung, Santorini, or uncinate. Leaving disease in the head of the pancreas, “the pacemaker of the pancreas,” is recognized by many surgeons as an invitation for recurrent pain requiring further operative intervention [16, 20] The need for reoperation is a disaster for the patient and a failure for the surgeon. Some surgeons have added adjunctive procedures to the standard LPJ in hopes of improving the results of LPJ in pain relief. The need for these adjunctive procedures is a tacit admission of the shortcomings of the procedure. Intraoperative electrohydraulic lithotripsy was used by Rios and found to improve results over standard LPJ. (However, his was not a randomized control study, and procedures were compared during different time periods [53]). Chan et al. added absolute ethyl alcohol celiac plexus neurolysis to standard LPJ and also found improved results in a randomized controlled study [54].
Conceptualization of the LR-LPJ
Combining the head resection of the Child operation (80%–95% distal resection) [17] with the LPJ decompression of the main duct in the neck, body, and tail of the gland seemed like a solution to combine the best features and eliminate the worst features of both the Child and the LPJ operations (Fig. 3). The Roux-en-Y jejunal limb would cover and encompass the cored-out head of the pancreas and the main duct in the neck, body, and tail of the gland. The local resection of the head of the gland (like the Child operation and unlike the LPJ) removes diseased tissue associated with the ducts of Wirsung, Santorini, uncinate, and their tributaries. This resection theoretically eliminates pain, whether the pain is caused by ductal hypertension or nerve damage. By draining the secretions from the remnant of pancreatic tissue along the inner aspect of the duodenum into the Roux-en-Y limb, the problems of short-lived pancreatic fistulas and infected collections of pancreatic juice that plagued the Child operation were reduced. By using LPJ drainage of the neck, body, and tail of the pancreas, disease in the main duct in the form of obstructing calculi and strictures was addressed, and the mass of tissue (60%–65%) in the neck, body, and tail of the pancreas was preserved, thereby avoiding the surgically induced exocrine and endocrine insufficiency associated with the Child operation. This operation is a simpler operation to perform than pancreaticoduodenectomy or duodenum-preserving pancreatic head resection. The LR-LPJ does not require division of the neck of the pancreas, which may be technically difficult if inflammation and portal hypertension cause adherence of the portal-superior mesenteric vein to the pancreas. Furthermore, with the LR-LPJ, the pancreatic head can be drained in continuity with the Roux en-Y limb draining the body and tail of the pancreas. This avoids the two separate anastomoses required in the DPHR procedure, one to the divided pancreatic neck and the second to the remnant of tissue about the distal common bile duct.
Preoperative Assessment
An accurate assessment of the structural abnormalities of the pancreas, including its ductal system and vasculature, is essential to confirm the diagnosis of chronic pancreatitis and to plan operative strategy. At present, the helical computerized tomography (CT) scan with arterial and venous phase, endoscopic retrograde cholangiopancreatography (ERCP), and the serum markers CEA and CA19–9 are the screening tools that best evaluate the pancreas.
Patients thought to be candidates for pain relief or alleviation of the complications of chronic pancreatitis should undergo an assessment of suitability for operation by a surgeon, gastroenterologist, and psychiatrist. Baseline studies include exocrine, endocrine, and nutritional function; work status; quality of life; pain severity; and narcotic usage. These studies yield essential data to be used for comparison with postoperative results to assess the expected success or failure of the operation. We believe pain severity is best assessed by the patient’s use of a visual analog scale in which zero indicates no pain and 10 the worst pain imaginable. Preferably the pain scale results should be kept as a daily diary. Narcotic use should be recorded as none, minimal (hydrocodone bitartrate equivalent used 1 to 3 times a month), moderate (hydrocodone bitartrate equivalent used daily or weekly), or major (meperidine hydrochloride used daily, weekly, or monthly).
Structural Pathology in the Pancreas
The nature of the structural pathology in the pancreas and its ductal system influences the selection of the operation most suitable to correct the patient’s problem(s).
-
1.
Intrapancreatic biliary strictures from chronic pancreatitis associated with proximal dilation of the common bile duct may lead to cholangitis (9.4%) or biliary cirrhosis (5.2%) and should be bypassed or eliminated.
-
2.
Intraductal calculi, fibrosis, and retention cysts in the ducts of Wirsung, Santorini, and uncinate may create a large, bulky, pancreatic head “inflammatory mass” that is not adequately decompressed by LPJ. Fig. 4 shows calculi/calcification in head of the pancreas.
-
3.
Main ducts in the body and tail of the pancreas as small as 3 mm in diameter may be decompressed if the surgeon sews to the capsule of the pancreas rather than to the mucosa of the duct.
-
4.
Multiple sites of strictures in the main duct in the neck, body, and tail of the pancreas require opening the main duct throughout its length to eliminate obstructed segments of the main duct. In areas of discontinuity of the main duct in the body and tail of the pancreas a V-shaped incision following the course of the main duct may be used to create a main duct channel. [55]
-
5.
One or more pseudocysts, even when widely separated, can usually be drained by the same Roux-en-y limb of jejunum.
Operative Indications
Operative indications include severe, unremitting, or frequently recurring epigastric or back pain; a pseudocyst or a bile duct obstruction, a complication of pancreatitis; or the inability of the physician to distinguish cancer from chronic pancreatitis. Preservation of exocrine and endocrine function by duct decompression, a concept advanced by Nealon and Thompson and associates, may also be an important indication for surgery in chronic pancreatitis, even in the absence of pain [24, 95]. However, further clarification is needed before this latter indication can be accepted as a valid indication for surgery. Malka et al. reported that operative decompression did not delay the onset of exocrine and endocrine insufficiency in their patients [56].
Pain
For patients with chronic pancreatitis and pain, operative management is the only acceptable option when therapies with fat restriction, enzymes, and non-narcotic analgesics have failed to relieve the pain. Providing the patient with ever increasing doses of narcotics in the hopes of avoiding operative intervention is misguided and creates greater challenges for the patient and subsequent care givers. It also diminishes the chances of ever achieving adequate pain relief and a better quality of life.
Selection of Operation.
There has been discussion among surgeons of what minimum duct diameter is required to open and decompress the main duct and surrounding parenchyma. With the LR-LPJ and DPHR procedures, this discussion applies only to the main duct in the neck, body, and tail of the pancreas, as the tissue overlying the ducts is either unroofed or excised in the head of the pancreas. The technical feasibility of managing small-diameter ducts has evolved over the years. We have found that sewing to the capsule of the pancreas rather than to the duct mucosa allows us to decompress ducts as small as 3 mm in diameter. Izbicki recently described a longitudinal V-shaped excision of pancreatic tissue along the course of the main duct that includes the main duct. A substitute duct channel is thus created in patients with very small (less than 3-mm ducts) or in patients whose ducts have been partially obliterated. This allows the Roux en-Y limb to be sewn to the capsule of the pancreas. This new procedure may reduce the need for distal pancreatectomy in patients with small ducts and disease limited only to the tail of the pancreas [55].
Complications of Chronic Pancreatitis
Fibrosis and inflammation of the pancreatic parenchyma, as well as ductal disruption and stricturing, can cause many of the complications of chronic pancreatitis. Fibrosis and inflammation may cause obstruction and/or stricture of the biliary and pancreatic ducts. Stricturing, obstruction, and thrombosis of the splenic or portal veins are also identified as complications. Ductal disruption and stricturing may result in (1) pseudocysts (that may, in turn, compress or obstruct the biliary and pancreatic ducts or splenic and portal veins, resulting in thrombosis and portal hypertension), (2) fistulas to the thorax (causing pleural effusions or cardiac tamponade), and (3) pancreatic ascites. Injury to the arterial wall from pancreatic enzymes and inflammation are thought to be implicated in the development of pseudoaneurysms that most often are associated with pseudocysts.
Ductal Disruptions.
Fluid collections are the most common complication of chronic pancreatitis and result from ductal disruption.
Pseudocysts.
Pseudocysts or retention cysts are present in 50% of the patients who are candidates for LR-LPJ or DPHR. Unlike the cysts associated with acute pancreatitis, these cysts are unlikely to resolve spontaneously [57]. Surgeons who are presented with a patient with chronic pancreatitis who also has a pseudocyst should evaluate the pancreas with a helical CT scan with contrast before proceeding with cyst jejunostomy or cyst gastrostomy. If the CT scan cannot adequately assess the duct patency, then an ERCP should be performed. The presence of one or multiple strictures downstream from the pseudocyst, if not bypassed, will likely result in a recurrence or the development of other pseudocysts or pain. In patients with main duct strictures and a pseudocyst, the main pancreatic duct should be opened throughout its length and drained into a Roux-en-Y limb. Cyst walls should always be biopsied to rule out a cystic tumor.
Fistulas and Ascites.
Experience has shown that 30% to 50% of pancreatic fistulas or pancreatic ascites may resolve spontaneously with conservative management. However, follow-up information on recurrence of the pancreatic fistula or ascites or the incidence of pseudocyst is meager [58 ,, 59, 60]. Forty percent of our patients with pancreaticopleural fistulas who initially responded to conservative management with resolution of their pleural fistulas developed new pseudocysts within 18 months [59]. Conservative management consists of nothing by mouth (NPO), total parenteral nutrition (TPN), and octreotide. Failure of conservative management after 6 to 8 weeks is an indication for operative intervention with the objective of decompressing the main pancreatic duct. The status of the main pancreatic duct needs to be evaluated by helical CT scan with contrast, ERCP, or a fistulogram. In patients with multiply strictured ducts, the entire main duct should be decompressed. Both LR-LPJ and DPHR are excellent options. If the fistula, pseudocyst, or ascites is the result of leakage of pancreatic juice from the main duct in the tail of the pancreas, distal resection of the pancreatic tail with preservation of the spleen is another alternative. Distal pancreatectomy mandates that the remainder of the pancreas is normal, including the main duct.
Common Bile Duct Obstruction.
Common bile duct obstruction or stricture is the second most common complication of chronic pancreatitis. It results from pancreatic fibrosis, inflammation, or pseudocyst compression of the 1.5- to 6-cm (average = 3-cm) intrapancreatic portion of the common bile duct. The stricture usually includes the entire intrapancreatic course of the common bile duct. The incidence of stricture in chronic pancreatitis is reported to be between 3.1% and 45.6% and averages 23%, as shown by data derived from 1,747 patients operated on for chronic pancreatitis [61]. Anatomic evidence of narrowing of the intrapancreatic portion of the common bile duct is common but probably not significant in the absence of proximal dilation or biochemical evidence of obstructive biliary disease [61]. In patients with proximal dilatation or biochemical evidence of obstructive biliary disease, endoscopic stenting as a short-term “crutch” is reported to be “safe,” but is not a long-term solution [62, 63, 64].
Operative indications in patients with anatomical evidence of stricture include cholangitis, morphologic evidence suggestive of biliary cirrhosis, progressive dilation of the common bile duct or intrahepatic ducts, persistent jaundice or elevation of the serum bilirubin for longer than one month, persistent elevation of the liver alkaline phosphatase three times normal for longer than one month, and the inability to rule out cancer [65].
Most patients with chronic pancreatitis complicated by a stricture of the common bile duct will require an operation on the pancreas for pain relief and for decompression of the biliary tract. When operations are directed at decompressing the biliary tract and the pancreas is ignored as a cause of pain, then pain relief is seldom achieved. None of the 21 patients reported by Yadegar et al., in whom operative intervention was limited to the biliary tract, were relieved of pain [66]. Similarly, Stabile et al. noted pain relief in only 7 (18%) of 38 patients with chronic pancreatitis in whom the biliary tract alone was decompressed [67]. The LR-LPJ procedure is one of the simpler options for both decompressing the biliary tract and providing pain relief.
Duodenal Obstruction.
Duodenal obstruction is a less common complication of chronic pancreatitis than common bile duct obstruction. Among 55 patients with chronic pancreatitis, Prinz and Greenlee found that 16 (29%) had common bile duct obstruction and 8 (14.6%) had duodenal obstruction [25]. Duodenal obstruction can result from inflammation and fibrosis or from compression by a pseudocyst. The LR-LPJ procedure may be an option in patients with duodenal obstruction, particularly when the obstruction is due to pseudocyst compression [34].
Vascular Abnormalities.
Vascular abnormalities are present in as many as 60% of all patients with chronic pancreatitis [21, 68, 69, 70]. Abnormalities include splenic vein thrombosis associated with left-sided portal hypertension with or without gastric varices; portal vein thrombosis; pseudoaneurysms of the pancreatic and peripancreatic vasculature; atherosclerotic occlusion or congenital absence of the celiac axis; and arterial anomalies. Such vascular conditions can affect the selection or outcome of operations involving the pancreas. We depend on the helical CT scan with arterial and venous phase to assess vascular anomalies associated with chronic pancreatitis. Neither LR-LPJ nor DPHR has a direct role in the management of the vascular complications of chronic pancreatitis.
Pancreatic Malignancies Can Mimic Chronic Pancreatitis
The clinical presentation of pancreatic tumors may at times mimic chronic pancreatitis. Malignancies of the head of the pancreas may obstruct the main pancreatic duct and cause pancreatitis or a pseudocyst distally in the pancreas. Cystic tumors in the absence of stippling or a wall mass on CT scan may be mistaken for pseudocysts. In 5% to 15% of patients with noncalculous obstruction of the common bile duct, it may be impossible to establish a diagnosis of chronic pancreatitis or tumor preoperatively [68, 69, 96]. In such patients pancreaticoduodenectomy is the operation of choice. Virtually all large series reporting the results of longitudinal pancreaticojejunostomy contain some patients found to have pancreatic cancer, not chronic pancreatitis [71, 72, 73]. The absence of a history of alcoholism or biliary disease in a patient with the diagnosis of pancreatitis or an elevated serum C-19-9 level should raise a red flag, suggesting the possibility of pancreatic cancer. To our knowledge, we have never mistakenly embarked on or performed LR-LPJ on a patient with pancreatic cancer. We attribute our success in avoiding an error in diagnosis to maintaining a high index of suspicion of cancer. If still in doubt, we perform pancreaticoduodenectomy or distal pancreatectomy when indicated rather than LR-LPJ.
Technique of the LR-LPJ
Although the technical details of performing the LR-LPJ are covered elsewhere [29, 74, 75, 76, 77], we will give a brief summary of some of the important steps in the operation.
The ductal system is opened by means of electrocoagulation from the pancreas tail to the duodenum. All calculi encountered in the ductal tract are extracted. Often the calculi extend into the tributary ducts and are difficult to dislodge. With the duct opened from tail to duodenum, it should be possible to pass a probe freely into the duodenum through the duct of Wirsung that has been opened close to the ampulla of Vater.
In patients with biochemical and anatomical evidence of intrapancreatic common bile duct obstruction from stricture or compression, it is essential to free the intrapancreatic portion of the common bile duct during the coring out of the head of the pancreas. To avoid injury to the common bile duct, a choledochotomy is performed and a Bakes dilator is passed distally through the ampulla into the duodenum. The location of the common bile duct can then be ascertained by palpation, and injury to the bile duct can be avoided during the excision of the fibrotic inflamed tissue that surrounds the common bile duct. In the absence of preoperative evidence of common bile duct obstruction, we have not found it necessary to perform a choledochotomy and intubation of the distal common bile duct with a Bakes dilator.
In coring out the head of the pancreas, we do not remove tissue posterior to the duct of Wirsung (that is, within millimeters of the posterior surface of the gland). We prefer to remove slices of pancreatic tissue in coring out the head and uncinate process rather than removing the tissue as single specimen. By removing slices of tissue we can periodically assess the thickness of the remaining pancreas and palpate retention cysts or impacted calculi in the tributary ducts. When the coring out process is complete, the surgeon should be able to palpate a shell of pancreatic tissue between the index finger held behind the head of the pancreas and the thumb in the cored out head of the pancreas. We have employed cautery to accomplish the local resection of the head of the pancreas. An alternative method for coring out the head of the pancreas using the cavitational ultrasonic surgical aspirator (CUSA) has been employed by Dana Andersen (personal communication). Dividing the jejunum approximately 15 cm beyond the ligament of Treitz creates a Roux-en-Y limb. The limb is used to drain the main pancreatic duct in the body and tail of the pancreas and in the cored out head of the pancreas. The jejunal limb is attached to the capsule of the pancreas, and an end-to-side jejunojejunostomy is placed approximately 40 cm below the pancreaticojejunostomy.
Since our original description of the LR-LPJ in 1987 [78], we have made several modifications to our technique.
-
1.
We no longer identify the portal vein superior to the pancreas, because exposure of the superior mesenteric vein below the inferior border of the pancreas provides an adequate guide to the course of the portal vein below the neck of the pancreas.
-
2.
To maximize drainage of the uncinate process and provide an adequate cuff of uncinate to sew to, we now free the uncinate process from the superior mesenteric vein by dividing the small venous tributaries that drain the uncinate process.
-
3.
We ligate the gastroepiploic artery where it emerges from the pancreas near the neck of the gland. This maneuver allows us to place fewer hemostatic sutures along the inner aspect of the duodenum. In fact, we no longer routinely use hemostatic sutures along the inner aspect of the duodenum, but instead cauterize or suture ligate specific bleeding points.
-
4.
After coring out of the head of the pancreas, the cuff of pancreas between the cored out head and the duodenum, to which the jejunum can be attached, may be limited. In this situation we often include some duodenum in our suture bites.
Results of LR-LPJ
Because of the variety of therapeutic options for management of chronic pancreatitis, ranging from operative procedures to stent placement, there is a need to develop standards by which their efficacy can be judged. The following criteria have been proposed:
-
1.
Ease and safety of use.
-
2.
Completeness and duration of pain relief.
-
3.
Incidence and severity of resultant exocrine and endocrine insufficiency.
-
4.
Morbidity and mortality associated with the procedure.
-
5.
Impact of the procedure on the length and quality of life.
Of these proposed criteria, pain assessment has been most lacking in standardization. In a 1996 editorial in the Archives of Surgery, a plea was made to standardize the criteria by which a successful or unsuccessful outcome of therapy could be judged [79]. The editorial contained specific recommendations regarding outcome measures for assessing pain and quality of life. These included the patient’s description of pain before and after therapy through the use of a visual analog pain scale in which 0 is no pain and 10 the worst pain imaginable. The scale is usually 10-cm in horizontal length, on which the patient indicates the level of pain by marking a position on the horizontal axis. It was recommended that the assessment be made at least annually. (A daily recording in diary form is most helpful/accurate for the patient and physician.) The editorial further stated that the use of narcotics should be quantified by the type of narcotic and the frequency of use: none, minimal (hydrocodone bitartrate equivalent used 1 to 3 times a month); moderate (hydrocodone bitartrate equivalent used daily or weekly); or major (meperidine hydrochloride used daily, weekly, or monthly). The editorial recommended a quality of life assessment. Some quality of life assessments and performance scores that have been used in the evaluation of patients with chronic pancreatitis are the Visick scale, SF-36, the Karnofsky performance score, and the European Organization for Research and Treatment of Cancer’s Quality of Life Questionnaire [80].
Distinguishing the Sequelae of Chronic Pancreatitis from Those of the Therapeutic Intervention
Distinguishing sequelae of the natural history of chronic pancreatitis (and possibly alcoholism) from the results of therapeutic intervention are not always possible. Any operation that requires removal of pancreatic tissue will tend to reduce pancreatic exocrine and endocrine function. Chronic pancreatitis also tends to cause progressive deterioration in pancreatic exocrine and endocrine function over time, and its impact is greater than that of most operations commonly employed in the treatment of pain relief in chronic pancreatitis (with the exception of total pancreatectomy or more than 50% distal resection.) Slezak and Anderson reported that operations more likely to precipitate diabetes after procedures for pain relief in chronic pancreatitis included total pancreatectomy, near-total distal pancreatectomy, standard or pylorus preserving pancreaticoduodenectomy. Operations less likely to precipitate diabetes include longitudinal pancreaticojejunostomy, LR-LPJ, DPHR, and less than 50% distal pancreatectomy [81]. Malka et al. compared 231 patients who had undergone pancreatic surgery for pain relief with 222 patients with chronic pancreatitis who did not come to operation. These patients were followed on average for 7 years. Among patients followed for 25 years, 83% became diabetic and 54% were insulin dependent. There was no difference in the incidence of diabetes between the operated and non-operated groups [82]. Continued alcohol use also affects the rate of progression of exocrine and endocrine dysfunction. For example, during a 7-year period Gullo et al. [83] noted in a group of nonoperated patients with alcohol-induced chronic pancreatitis that diabetes developed in 50% of patients who continued to drink and 28% of those who abstained. Similar results were noted by Ammann et al. [84]. After operation, the effects of abstinence or continued abuse of alcohol would be expected to follow a pattern similar to the nonoperated group with regard to exocrine and endocrine function. Continued alcohol use also adversely effects long-term survival after operations for pain relief in patients with chronic pancreatitis. Five-year survival was 55% for alcoholics and 86.3% for non-alcoholics, and at 10 years the rate was 48% for alcoholics and 78% for non-alcoholics [85, 86]. Of course, determining whether a patient is continuing to imbibe alcohol can be a problem, as family members may show classic codependent behavior and may collude with the patient in denying alcohol intake.
University of California at Davis Medical Center (UCDMC) Experience with LR-LPJ
Pain and Narcotic Results
Using the visual analog scale where 0 denotes no pain and 10 is the worst pain imaginable, and quantifying narcotic use, the follow-up of 47 of 50 patients who had undergone LR-LPJ procedures were reported in 1994 (there was no follow-up in 1 patient, and 2 patients died at 6 months) [2]. Narcotic use was quantified by type of narcotic and frequency of use. Average follow-up was 37 months. Pain relief was excellent in 35 patients (74.5%), improved in 6 patients (12.75%), and unimproved in 6 patients (12.75%) (see Table 5).
Endocrine Status
After LR-LPJ, 5 of 45 patients (11%) followed for an average of 37 months had progression of their diabetes. One patient who required oral diabetic control preoperatively needed insulin postoperatively. Another who was nondiabetic preoperatively required insulin postoperatively. Three patients who were nondiabetic preoperatively became diet controlled, oral anti-glycemic controlled, and insulin-dependent diabetics at 16, 22, and 3 months postoperatively, respectively. These results are comparable to those after an LPJ procedure [2] (Table 1).
Exocrine Status
After LR-LPJ, 19 diabetic patients (42.5%) did not have steatorrhea and 22 patients (24.4%) had steatorrhea preoperatively and postoperatively. Postoperatively five patients (11%) developed steatorrhea and 10 (22.3%) had less steatorrhea. These results compare favorably with other operative procedures used in the control of pain and the complications of chronic pancreatitis, and they most resemble the results with LPJ, an operation in which little pancreatic tissue is destroyed (Table 2).
A sophisticated assessment of nutritional status and intestinal absorption after LR-LPJ was performed at UCDMC [84]. Eleven patients were studied 3 weeks after LR-LPJ. All had abnormal digestion of fat and protein and decrease of total energy. Pancreatic enzyme supplements were given for 4 weeks, starting one month after LR-LPJ. Supplements significantly improved protein absorption and nitrogen balance. Placebo substitution did not improve the absorption of dietary fat and total energy. We concluded that long-term postoperative enzyme supplementation is both efficacious and necessary in patients with chronic pancreatitis.
Work
In our patient population, few patients who were unemployed preoperatively took on a job after LR-LPJ (Table 4).
Mortality and Morbidity
Operative mortality is low in operations for chronic pancreatitis with the exception of total pancreatectomy. The operative mortality for LR-LPJ is less than 1% [2, 78, 84, 87]. Morbidity is particularly high in patients undergoing total pancreatectomy and 80%–95% distal resection of the pancreas (see Table 6). Morbidity associated with LR-LPJ averages approximately 19%.
Late Deaths
The incidence of late deaths after operations for chronic pancreatitis is a function of the completeness and length of follow-up, the patient population studied, the incidence of alcoholism, and the percentage of patients who abstain from alcohol postoperatively. The low incidence of late deaths after LR-LPJ reflects the shorter period of follow-up, not some special immunity conferred by the operation [2, 78, 84, 94]. Most deaths after LR-LPJ result from progression of the disease and the effects of continued alcohol abuse (see Tables 7 and 8).
Prospective Randomized Trials
Determining whether one operation or therapeutic intervention is more effective than another in patients with chronic pancreatitis is a daunting task, even with standardized criteria to measure outcome, for the following reasons:
-
1.
The success of LR-LPJ or any other operation in managing the pain and complications of chronic pancreatitis reflects the surgeon’s ability to match the patient’s problem with the most appropriate solution. When there is no single operation that relieves pain and addresses all complications of chronic pancreatitis, failure of the surgeon to select the appropriate operation could result in failure to relieve pain or resolve a complication of chronic pancreatitis, not because the operation failed, but because it was not the best operation for the patient’s problem.
-
2.
Patient selection may skew the incidence of pain relief; for example, some surgeons will not operate on the narcotic- or alcoholic-addicted patient without evidence of abstinence, whereas other surgeons will proceed without such assurance. After procedures for pain relief, patients who abstain from alcohol may have a lower incidence of pain than those who continue to drink. However, the studies are not clear on this issue and most have been derived from evaluation of patients who have not been operated on [83, 84, 96, 97].
-
3.
Not all patients are easy to categorize with regard to pain relief.
-
4.
No standard method of pain assessment had been accepted or used in reports on the results of therapeutic interventions prior to 1996, when some were proposed in an Archives of Surgery editorial [79].
The problems enumerated earlier that make it difficult to evaluate competing therapies have been taken seriously by European surgeons who have taken the leadership in initiating a number of prospective randomized trials to compare the efficacy of a number of operative procedures used to relieve the pain in patients with chronic pancreatitis [28, 52, 80, 98, 99].
Pylorus-preserving pancreaticoduodenectomy has been compared with duodenum-preserving head resection (Beger procedure) in three prospective, randomized trials. These studies have been interpreted as showing the superiority of duodenum-preserving pancreatic head resection compared with pylorus-preserving pancreaticoduodenectomy with respect to pain relief and nutritional deficiency [34, 52, 99].
In 1995 Izbicki and associates reported the results of a prospective, randomized trial comparing the results of LR-LPJ (22 patients) and DPHR (20 patients) in the management of pain and the complications of chronic pancreatitis (average follow-up of 18 months) [80] (Table 9). In 1997 Izbicki et al. extended their series and reported a 30-month average follow-up on 30 patients after LR-LPJ and 38 patients after DPHR [98]. There was no mortality in either group. Postoperative morbidity was 22% after LR-LPJ and 32% after DPHR. The postoperative pain score decreased in 93% of patients after LR-LPJ and in 95% after DPHR. The patients’ quality of life improved 67% following both procedures. There was no difference in exocrine or endocrine function between the two groups. In summary, Izbicki and colleagues found no difference between the two procedures with regard to pain relief, quality of life, and exocrine and endocrine function (Table 10). This result is not surprising considering that both procedures involve removal of a portion of the head of the pancreas and effectively decompress the ducts of Wirsung, Santorini, and uncinate, and their tributary ducts by either resecting the ducts or resecting the overlying tissue. Whether the ensuing pain relief is due to removal of damaged nerves in the head of the pancreas or by decompression or resection of the ducts is unknown. The only apparent major differences between the operations are dividing the pancreas at its neck (a requirement of the duodenum-preserving head resection) and the Roux-en-Y drainage of the neck, body, and tail of the pancreas (a requirement of the LR-LPJ). Although major duct decompression in the body and tail of the pancreas can also be accomplished in the duodenum-preserving head resection, Beger’s group in Ulm has only used this option in 10% of their patients, i.e., a longitudinal pancreaticojejunostomy involving the neck, body, and tail of the pancreas [28]. In patients with chronic pancreatitis, the need for decompression of the main duct in the neck, body, and tail of the pancreas by longitudinal pancreaticojejunostomy needs to be explored further. In many patients in whom the major pancreatic duct in the body and tail of the pancreas is patent (even though abnormal in appearance and diseased) it may be necessary only to perform local resection of the head of the pancreas with Roux-en-Y drainage of the head of the pancreas, leaving the body and tail undrained. Perhaps only longitudinal pancreaticojejunostomy of the body and tail of the pancreas is needed in patients with multiple or complete strictures in the main duct in the neck, body, and tail of the pancreas. Technically, the LR-LPJ is easier to perform than the DPHR, because the LR-LPJ procedure does not require that the neck of the pancreas be divided.
When the results of the LR-LPJ experience at UCDMC were compared directly with those of the duodenum-preserving head resection at the University of Ulm, Germany [99], the results were similar with regard to pain relief and mortality; however, there seemed to be a greater likelihood patients would return to work and gain more weight in Ulm. Inasmuch as these differences were not noted in the randomized studies of Izbicki et al. [80, 98] in the same patient population comparing these two operations, it is likely these reported differences in results between Sacramento and Ulm were attributable to differences in patient population.
In 1998 the LR-LPJ in another prospective randomized study by Izbicki et al. was compared with the pylorus-preserving pancreaticoduodenectomy (PPPD) [34]. There was one operative death (from myocardial infarction) among the 31 patients with LR-LPJ (3.2%) and none among the 30 patients with PPPD. The operating time and blood loss were significantly less in the LR-LPJ group. Additionally, the postoperative morbidity rate was also less, 19.4% versus 53.3% for LR-LPJ versus PPPD, respectively. Delayed gastric emptying occurred only in the PPPD group (30%). The pain score was similarly improved in both groups of patients, 94% in LR-LPJ patients versus 95% in other pancreaticoduodenectomy patients. There was a significantly better improvement in the global quality of life assessment in the LR-LPJ patients, 71% versus 43% for the PPPD (Tables 5, 6, 11, 12, 13).Table 10 summarizes both trials reported by Izbicki et al. in terms of the endocrine status after LR-LPS, DPHR, and PPPD.
In summary, the European prospective, randomized, controlled trials of the operative management of patients with chronic pancreatitis have shown that the LR-LPJ and the duodenum-preserving head resection have some advantages over PPPD. The LR-LPJ and the DPHR provide similar degrees of pain relief and overall improvement in quality of life. The LR-LPJ may be technically easier to perform than DPHR in patients with chronic pancreatitis.
Résumé.
L’étiologie de la douleur de la pancréatite chronique peut être une hyperpression canalaire et/ou des pressions intra parenchymateuses augmentées ou encore des lésions nerveuses. Il est difficile d’évaluer la sévérité de la douleur dans cette population de patients, un problème rendu encore plus ardu par la fréquence d’addiction aux narcotiques. Les interventions visant à soulager la douleur de la pancréatite chronique comprennent la dénervation du pancréas, la décompression du canal principal, la résection d’une partie ou de tout le pancréas malade et la réduction de la sécrétion pancréatique. La chirurgie est indiquée en cas de douleur chronique lorsque cette dernière est sévère, compliquée ou potentiellement d’origine maligne. Les interventions qui produisent un soulagement durable de façon reproductible ont en commun la résection de toute ou une partie de la tête du pancréas. Les effets secondaires non désirables sur la fonction exocrine et endocrine, la nutrition, et la qualité de vie sont en rapport avec le volume du pancréas réséqué. Le procédé idéal devrait être facile à réaliser, avoir une morbidité et une mortalité peu élevées, produire un soulagement prolongé de la douleur et n’augmenter ni l’insuffisance exocrine ni endocrine du pancréas. Aucune intervention n’est idéale. La résection de la tête du pancréas associée à l’anastomose pancréaticojéjunale longitudinale (LR-LPJ), proposée par Frey et la résection céphalique avec conservation du duodénum (DPHR), proposée par Beger, seront discutées dans ce chapitre. La conceptualisation, le développement et la technique de la LR-LPJ sont discutés et l’évolution des patients opérés selon ces techniques sera comparée à celles d’autres techniques.
Resumen.
En la pancreatitis crónica el dolor puede ser debido a: hipertensión ductal, aumento de la presión del parénquima o a alteraciones del plexo nervioso peripancreático. El difícil averiguar la intensidad del dolor en estos pacientes tanto más cuanto que presentan, con frecuencia, una drogodependencia. Las intervenciones terapéuticas para aliviar el dolor incluyen: la denervación pancreática, la descompresión del Wirsung, la resección parcial o total del páncreas y la reducción de la secreción pancreática. Las indicaciones operatorias para el tratamiento del dolor crónico vienen dadas por: la intensidad del dolor, complicaciones relacionadas con el mismo y la posible malignización del proceso pancreático. Las operaciones que consiguen aliviar durante más tiempo el dolor tienen en común, que en todas ellas se efectúa una resección total o parcial (de la cabeza) del páncreas. Los efectos adversos de dichas intervenciones tales como alteraciones: de la función endocrina y exocrina, nutricionales y los que se refieren a la calidad de vida del paciente dependen de la amplitud de la resección pancreática. La terapia ideal sería aquella que fuera fácil de realizar técnicamente, con escasa morbilidad-mortalidad, produciendo un alivio prolongado del dolor, sin incrementar la insuficiencia del páncreas endo y exocrino. Ninguna técnica operatoria cumple estos ideales. En este artículo se discute la bondad de la pancreaticoyeyunostomía longitudinal (LR – LPJ) propuesta por Frey y la de la duodenopancreatectomia conservando el píloro (DPHR) propuesta por Berger. Se describen las bases conceptuales, el desarrollo y la técnica de la LR-LPJ, comparando sus resultados con los obtenidos con otras técnicas en el tratamiento de la pancreatitis crónica.
References
ND Karanjia AL Widdison FW Leung et al. (1994) ArticleTitleCompartment syndrome in experimental chronic obstructive pancreatitis: effect of decompressing the main pancreatic duct Br. J. Surg. 81 259–264 Occurrence Handle1:STN:280:ByuB3cjnsFQ%3D Occurrence Handle8156353
CF Frey K Amikura (1994) ArticleTitleLocal resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy in the management of patients with chronic pancreatitis Ann. Surg. 220 492–504 Occurrence Handle1:STN:280:ByqD3s7jsVw%3D Occurrence Handle7524454
EL Bradley Suffix3rd (1982) ArticleTitlePancreatic duct pressure in chronic pancreatitis Am. J. Surg. 144 313–316 Occurrence Handle7114368
CF Frey (1973) 95% pancreatectomy LC Carey (Eds) The Pancreas Mosby Year Book St. Louis 202–229
DE Bockman M Buchler P Malfertheiner et al. (1988) ArticleTitleAnalysis of nerves in chronic pancreatitis Gastroenterology 94 1459–1469
AL Widdison C Alvarez ND Karanjia et al. (1991) ArticleTitleExperimental evidence of beneficial effects of ductal decompression in chronic pancreatitis Endoscopy 23 151–154 Occurrence Handle1:STN:280:By6A3MjhsFM%3D Occurrence Handle1860444
E Weihe M Büchler S Müller et al. (1990) Peptidergic innervation in chronic pancreatitis HG Beger M Buchler H Ditschuneit et al (Eds) Chronic Pancreatitis Springer-Verlag Berlin 83–105
R Amman (2001) ArticleTitleThe natural history of alcoholic chronic pancreatitis Ann. Intern. Med. 40 368–375
DM Proca EC Ellison D Hibbert et al. (2001) ArticleTitleMajor pancreatic resections for chronic pancreatitis Arch. Pathol. Lab. Med. 125 1051–1054 Occurrence Handle1:STN:280:DC%2BD3Mvltlahuw%3D%3D Occurrence Handle11473456
IP Linehan MA Lambert DC Brown et al. (1988) ArticleTitleTotal pancreatectomy for chronic pancreatitis Gut 29 358–365 Occurrence Handle1:STN:280:BieC1crovVc%3D Occurrence Handle3356368
A Mannell MA Adson DC McIlrath et al. (1988) ArticleTitleSurgical management of chronic pancreatitis: long-term results in 141 patients Br. J. Surg. 75 467–472 Occurrence Handle1:STN:280:BieB28bpvVc%3D Occurrence Handle3390681
JW Braasch L Vito FW Nugent (1978) ArticleTitleTotal pancreatectomy of end-stage chronic pancreatitis Ann. Surg. 188 317–322 Occurrence Handle1:STN:280:CSeB2Mrot1U%3D Occurrence Handle686897
CF Frey CG Child WF Fry (1976) ArticleTitlePancreatectomy for chronic pancreatitis Ann. Surg. 184 403–414 Occurrence Handle1:STN:280:CSiC3M%2Fos1A%3D Occurrence Handle1015887
HG Beger W Krautzberger R Bittner et al. (1985) ArticleTitleDuodenum-preserving resection of the head of the pancreas in patients with severe chronic pancreatitis Surgery 97 467–473 Occurrence Handle1:STN:280:BiqC28fnsFM%3D Occurrence Handle3983823
FP Gall C Gebhardt H Zirngibl (1982) ArticleTitleChronic pancreatitis -- results of 116 consecutive, partial duodenopancreatectomy combined with pancreatic duct occlusion Hepatogastroenterology. 29 115–119 Occurrence Handle1:STN:280:Bi2B28zisFY%3D Occurrence Handle7106696
LW Traverso RA Kozarek (1993) ArticleTitleThe Whipple procedure for severe complications of chronic pancreatitis Arch. Surg. 128 1047–1053 Occurrence Handle1:STN:280:ByyA2sfltlM%3D Occurrence Handle8368923
FP Gall C Gebhardt R Meister et al. (1989) ArticleTitleSevere chronic cephalic pancreatitis: use of partial duodenopancreatectomy with occlusion of the pancreatic duct in 289 patients World J. Surg. 13 809–817 Occurrence Handle1:STN:280:By%2BC28zkt1I%3D Occurrence Handle2623892
MJ Cooper RC Williamson IS Benjamin et al. (1987) ArticleTitleTotal pancreatectomy for chronic pancreatitis Br. J. Surg. 74 912–915 Occurrence Handle1:STN:280:BieD38ngslE%3D Occurrence Handle3664222
S Miyakawa M Hayakawa A Horiguchi et al. (1996) ArticleTitleEstimation of fat absorption with the 13C-trioctanoin breath test after pancreatoduodenectomy or pancreatic head resection World J. Surg. 20 1024–1028 Occurrence Handle10.1007/s002689900156 Occurrence Handle1:STN:280:BymA1MvntVI%3D Occurrence Handle8798360
RP Kerremans FM Penninckx J Groote ParticleDe et al. (1987) ArticleTitleSubtotal resection of the head of the pancreas combined with ductal obliteration of the distal pancreas in chronic pancreatitis Ann. Surg. 205 240–245 Occurrence Handle1:STN:280:BiiC2Mrlt10%3D Occurrence Handle3827358
MS Woods LW Traverso RA Kozarek et al. (1995) ArticleTitleSuccessful treatment of bleeding pseudoaneurysms of chronic pancreatitis Pancreas 10 22–30 Occurrence Handle1:STN:280:ByqB3cjjsFQ%3D Occurrence Handle7899456
WM Stone MG Sarr DM Nagorney et al. (1998) ArticleTitleChronic pancreatitis. Results of Whipple’s resection and total pancreatectomy Arch. Surg. 123 815–819
JM Howard Z Zhang (1990) ArticleTitlePancreaticoduodenectomy (Whipple resection) in the treatment of chronic pancreatitis World J. Surg. 14 77–82 Occurrence Handle1:STN:280:By%2BC28zksVM%3D Occurrence Handle2305589
WH Nealon CM Townsend SuffixJr JC Thompson (1988) ArticleTitleOperative drainage of the pancreatic duct delays functional impairment in patients with chronic pancreatitis. A prospective analysis Ann. Surg. 208 321–329 Occurrence Handle1:STN:280:BieA3sbkt1E%3D Occurrence Handle3421756
RA Prinz HB Greenlee (1990) ArticleTitlePancreatic duct drainage in chronic pancreatitis Hepatogastroenterology. 37 295–300 Occurrence Handle1:STN:280:By%2BA38zns1A%3D Occurrence Handle1695604
A Escallon SuffixJr JS Aldrete (1986) ArticleTitleLateral pancreaticojejunostomy for pain relief in chronic pancreatitis: analysis of effectiveness in 19 patients South. Med. J. 79 936–940 Occurrence Handle2426795
DH Drake WJ Fry (1989) ArticleTitleDuctal drainage for chronic pancreatitis Surgery 105 131–140 Occurrence Handle1:STN:280:BiaC3s%2FjsVw%3D Occurrence Handle2916177
MW Buchler H Friess R Bittoner et al. (1997) ArticleTitleDuodenum-preserving pancreatic head resection: long-term results J. Gastrointest. Surg. 1 13–19 Occurrence Handle10.1016/S1091-255X(97)80004-2 Occurrence Handle9834325
CF Frey (1998) ArticleTitleThe surgical management of chronic pancreatitis: the Frey procedure Adv. Surg. 32 41–85
CE Morrow JI Cohen DE Sutherland et al. (1984) ArticleTitleChronic pancreatitis: long-term surgical results of pancreatic duct drainage, pancreatic resection, and near-total pancreatectomy and islet autotransplantation Surgery 96 608–616 Occurrence Handle1:STN:280:BiqD3MjhtFw%3D Occurrence Handle6435270
FE Eckhauser WE Strodel JA Knol et al. (1984) ArticleTitleNear-total pancreatectomy for chronic pancreatitis Surgery 96 599–607 Occurrence Handle1:STN:280:BiqD3MjhtFM%3D Occurrence Handle6484804
FP Gall H Zirngibl C Gebhardt et al. (1990) ArticleTitleduodenal pancreatectomy with occlusion of the pancreatic duct Hepatogastroenterology. 37 290–294 Occurrence Handle1:STN:280:By%2BA38zns1c%3D Occurrence Handle2373461
JA Pain MJ Knight (1988) ArticleTitlePancreaticogastrostomy: the preferred operation for pain relief in chronic pancreatitis Br. J. Surg. 75 220–222 Occurrence Handle1:STN:280:BieC28vislw%3D Occurrence Handle3349329
JR Izbicki C Bloechle DC Broering et al. (1998) ArticleTitleExtended drainage versus resection in surgery for chronic pancreatitis. A prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy Ann. Surg. 228 771–779 Occurrence Handle10.1097/00000658-199812000-00008 Occurrence Handle1:STN:280:DyaK1M%2FnsFyiuw%3D%3D Occurrence Handle9860476
PF Partington RL Rochelle (1960) ArticleTitleModified Puestow procedure for retrograde drainage of the pancreatic duct Ann. Surg. 152 1037–1043 Occurrence Handle1:STN:280:CC%2BD2M3mvVY%3D Occurrence Handle13733040
M Adolff M Schloegel JP Arnaud et al. (1991) ArticleTitleRole of pancreatojejunostomy in the treatment of chronic pancreatitis. A study of 105 operated patients Chirurgie 117 251–256 Occurrence Handle1817818
TG Wilson MJ Hollands JM Little (1992) ArticleTitlePancreaticojejunostomy for chronic pancreatitis Aust. N. Z. J. Surg. 62 111–115 Occurrence Handle1:STN:280:By2B28vpt1A%3D Occurrence Handle1586299
RH Taylor FH Bagley JW Braasch et al. (1981) ArticleTitleDuctal drainage or resection for chronic pancreatitis Am. J. Surg. 141 28–33 Occurrence Handle1:STN:280:Bi6C3cjht1I%3D Occurrence Handle7457724
EL Bradley Suffix3rd (1987) ArticleTitleLong-term results of pancreatojejunostomy in patients with chronic pancreatitis Am. J. Surg. 153 207–217 Occurrence Handle3812895
L Leger JP Lenriot G Lemaigri (1974) ArticleTitleFive to twenty year followup after surgery for chronic pancreatitis in 148 patients Ann. Surg. 180 185–192 Occurrence Handle1:STN:280:CSuB2snovFA%3D Occurrence Handle4842980
JT Holmberg G Isaksson I Ihse (1985) ArticleTitleLong term results of pancreaticojejunostomy in chronic pancreatitis Surg. Gynecol. Obstet. 160 339–346 Occurrence Handle1:STN:280:BiqC28fntFQ%3D Occurrence Handle2580360
MK DuVal (1954) ArticleTitleCaudal pancreatico-jejunostomy for chronic relapsing pancreatitis Ann. Surg. 140 775–785 Occurrence Handle13208131
H Doubilet JH Mulholland (1951) ArticleTitleThe surgical treatment of acute pancreatitis by endocholedochal sphincterotomy Surg. Gynecol. Obstet. 86 295
EL Bradley Suffix3rd JA Reynhout GL Peer (1998) ArticleTitleThoracoscopic splanchnicectomy for “small duct” chronic pancreatitis: case selection by differential epidural analgesia J. Gastrointest. Surg. 2 88–94 Occurrence Handle10.1016/S1091-255X(98)80108-X Occurrence Handle9841973
JW Maher FC Johlin D Pearson (1996) ArticleTitleThoracoscopic splanchnicectomy for chronic pancreatitis pain Surgery 120 603–610 Occurrence Handle1:STN:280:ByiD3MrntF0%3D Occurrence Handle8862367
I Ihse E Zoucas E Gyllstedt et al. (1999) ArticleTitleBilateral thoracoscopic splanchnicectomy: effects on pancreatic pain and function Ann. Surg. 230 785–790 Occurrence Handle10.1097/00000658-199912000-00007 Occurrence Handle1:STN:280:DC%2BD3c%2FotFKkuw%3D%3D Occurrence Handle10615933
J Moodley B Singh AS Shaik et al. (1999) Thoracoscopic. World J. Surg. 23 608–692
TJ Howard JB Swofford et al. (2002) ArticleTitleQuality of life after bilateral thorascopic splanchnicectomy: long term evaluation in patients with chronic pancreatitis J. Gastrointest. Surg. 6 845–852 Occurrence Handle10.1016/S1091-255X(02)00123-3 Occurrence Handle12504223
A Malesci E Gaia A Floretta et al. (1995) ArticleTitleNo effect of long-term treatment with pancreatic extract on recurrent abdominal pain in patients with chronic pancreatitis Scand. J. Gastroenterol. 30 392–398 Occurrence Handle1:STN:280:ByqA3svmvFY%3D Occurrence Handle7610357
PP Toskes CE Forsmark MT DeMeo et al. (1997) ArticleTitleOctreotide for the pain of chronic pancreatitis: results of a multicenter, placebo-controlled, dose-ranging pilot study Pancreas 00 000–000
RG Keith FG Saibil RH Sheppard (1989) ArticleTitleTreatment of chronic alcoholic pancreatitis by pancreatic resection Am. J. Surg. 157 156–162 Occurrence Handle1:STN:280:BiaD1cnmtFE%3D Occurrence Handle2910121
I Klempa M Spatny J Menzel et al. (1995) ArticleTitlePancreatic function and quality of life after resection of the head of the pancreas in chronic pancreatitis. a prospective, randomized, comparative study of the duodenum preserving resection of the head of the pancreas versus Whipple’s operation Chirurg 66 350–359 Occurrence Handle1:STN:280:ByqA287msFQ%3D Occurrence Handle7634946
GA Rios DB Adams (2001) ArticleTitleDoes intraoperative electrohydraulic lithotripsy improve outcome in the surgical management of chronic pancreatitis? Am. Surg. 67 533–537 Occurrence Handle1:STN:280:DC%2BD3MzkslSntA%3D%3D Occurrence Handle11409800
C Chan M Vilatoba A Bartolucci et al. (2001) ArticleTitleImproved reduction in pain in chronic pancreatitis with combined intraoperative celiac axis plexus block and lateral pancreaticojejunostomy Curr. Surg. 58 220–222 Occurrence Handle10.1016/S0149-7944(00)00446-3 Occurrence Handle11275249
CC Frey (1998) ArticleTitleThe surgical management of chronic pancreatitis: the Frey Procedure Adv. Surg. 32 41–85
D Malka P Hammel A Sauvanet et al. (2000) ArticleTitleRisk factors for diabetes mellitus in chronic pancreatitis Gastroenterology 119 1324–1332 Occurrence Handle1:STN:280:DC%2BD3M%2Fkt1yrsw%3D%3D Occurrence Handle11054391
M Bourliere H Sarles (1989) ArticleTitlePancreatic cysts and pseudocysts associated with acute and chronic pancreatitis Dig. Dis. Sci. 34 343–348 Occurrence Handle1:STN:280:BiaC2M3ntVA%3D Occurrence Handle2646086
JL Cameron RS Kieffer WJ Anderson et al. (1976) ArticleTitleInternal pancreatic fistulas: pancreatic ascites and pleural effusions Ann. Surg. 184 587–593 Occurrence Handle1:STN:280:CSiD2cznt1M%3D Occurrence Handle984927
EW Pottmeyer SuffixIII CF Frey S Matsuno (1987) ArticleTitlePancreaticopleural fistulas Arch. Surg. 122 648–654 Occurrence Handle3579578
DW Weaver AJ Walt C Sugawa et al. (1982) ArticleTitleA continuing appraisal of pancreatic ascites Surg. Gynecol. Obstet. 154 845–848 Occurrence Handle1:STN:280:Bi2C1MbptFE%3D Occurrence Handle6177058
CF Frey (1993) The surgical treatment of chronic pancreatitis VLW Go EP DiMagno JD Gardner (Eds) The Pancreas: Biology, Pathobiology, and Disease EditionNumber2nd edition Raven Press New York 707–740
A Eickhoff R Jacobs A Leonhardt et al. (2001) ArticleTitleEndoscopic stenting for common bile duct stenoses in chronic pancreatitis: results and impact on long- term outcome Eur. J. Gastrointest. Hepatol. 13 1161–1167 Occurrence Handle10.1097/00042737-200110000-00007 Occurrence Handle1:STN:280:DC%2BD3MnmsVantQ%3D%3D
MJ Farnebecker et al. (2000) ArticleTitle. Am. J. Gastroenterol. 95 1466–1471 Occurrence Handle10.1016/S0002-9270(00)00870-4 Occurrence Handle10894580
W Schlosser B Poch HG Beger (2002) ArticleTitleDuodenum-preserving head resection leads to relief of common bile duct stenosis Am. J. Surg. 183 37–41 Occurrence Handle10.1016/S0002-9610(01)00713-9 Occurrence Handle11869700
CF Frey M Suzuki S Isaji (1990) ArticleTitleTreatment of chronic pancreatitis complicated by obstruction of the common bile duct or duodenum World J. Surg. 14 59–69 Occurrence Handle1:STN:280:By%2BC28zksVE%3D Occurrence Handle2407039
J Yadegar RA Williams E Passaro SuffixJr et al. (1980) ArticleTitleCommon duct stricture from chronic pancreatitis Arch. Surg. 115 582 Occurrence Handle1:STN:280:Bi%2BC1MjivFU%3D Occurrence Handle7377960
BE Stabile R Calabria SE Wilson et al. (1987) ArticleTitleStricture of the common bile duct from chronic pancreatitis Surg. Gynecol. Obstet. 165 121–126 Occurrence Handle1:STN:280:BiiB28vgtVc%3D Occurrence Handle3603341
DW Rattner C Fernandez-del Castillo AL Warshaw (1996) ArticleTitlePitfalls of distal pancreatectomy for relief of pain in chronic pancreatitis Am. J. Surg. 171 142–146 Occurrence Handle10.1016/S0002-9610(99)80089-0 Occurrence Handle1:STN:280:BymC2c3htVY%3D Occurrence Handle8554129
TT White AH Slavotinek (1979) ArticleTitleResults of surgical treatment of chronic pancreatitis. Report of 142 cases Ann. Surg. 189 217–224 Occurrence Handle1:STN:280:CSaC2czosFU%3D Occurrence Handle426554
C L’Hermine G Lemaître JP Maillard et al. (1971) ArticleTitleSegmental portal hypertension in pancreatitis. Angiographic aspects Lille Med. 16 928–932 Occurrence Handle1:STN:280:CS2D3s%2FnsFU%3D Occurrence Handle5115738
MJ Hart H Miyashita TT White (1983) ArticleTitlePancreaticojejunostomy. Report of a 25 year experience Am. J. Surg. 145 567–570 Occurrence Handle1:STN:280:BiyB3c7otVA%3D Occurrence Handle6846692
JS Markowitz DW Rattner AL Warshaw (1994) ArticleTitleFailure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage Arch. Surg. 129 374–379 Occurrence Handle1:STN:280:ByuB3cnhvFQ%3D Occurrence Handle8154964
CE Lucas B McIntosh D Paley et al. (1999) ArticleTitleSurgical decompression of ductal obstruction in patients with chronic pancreatitis Surgery 126 790–795 Occurrence Handle10.1067/msy.2099.99954 Occurrence Handle1:STN:280:DyaK1MvkvFansg%3D%3D Occurrence Handle10520930
CP Frey HS Ho (1995) Chronic pancreatitis: Frey operation L Spitz AG Coran (Eds) Rob and Smith’s Operative Surgery Chapman and Hall London 497–503
CF Frey (1992) Partial and subtotal pancreatectomy for chronic pancreatitis LM Nyhus RJ Baker (Eds) Mastery of Surgery EditionNumber2nd edition, vol 2 Little, Brown and Company Boston 1029–1048
RH Bell SuffixJr (1996) Atlas of pancreatic surgery RH Bell LF Rikkers MW Mulholland, (Eds) Digestive Tract Surgery: A Text and Atlas Lippincott-Raven Philadelphia 963–115
HS Ho CF Frey (2001) ArticleTitleThe Frey procedure—local resection of pancreatic head combined with lateral pancreaticojejunostomy Arch. Surg. 136 1353–1358 Occurrence Handle10.1001/archsurg.136.12.1353 Occurrence Handle1:STN:280:DC%2BD38%2FivVyhsA%3D%3D Occurrence Handle11735858
CF Frey GJ Smith (1987) ArticleTitleDescription and rationale of a new operation for chronic pancreatitis Pancreas 2 701–707 Occurrence Handle1:STN:280:BieC2cvkt1M%3D Occurrence Handle3438308
CF Frey HA Pitt CJ Yeo et al. (1996) ArticleTitleA plea for uniform reporting of patient outcome in chronic pancreatitis Arch. Surg. 131 233–234 Occurrence Handle1:STN:280:BymC1MjptFw%3D Occurrence Handle8611085
JR Izbicki C Bloechle WT Knoefel et al. (1995) ArticleTitleDuodenum-preserving resection of the head of the pancreas in chronic pancreatitis. A prospective, randomized trial Ann. Surg. 221 350–358 Occurrence Handle1:STN:280:ByqB2cznsFM%3D Occurrence Handle7726670
LA Slezak DK Andersen (2001) ArticleTitlePancreatic resection: effects on glucose metabolism World J. Surg. 25 452–460 Occurrence Handle10.1007/s002680020337 Occurrence Handle1:STN:280:DC%2BD3M3kt1ertA%3D%3D Occurrence Handle11344398
D Malka P Hammel A Sauvanet et al. (2000) ArticleTitleRisk factors for diabetes mellitus in chronic pancreatitis Gastroenterology 119 1324–1332 Occurrence Handle1:STN:280:DC%2BD3M%2Fkt1yrsw%3D%3D Occurrence Handle11054391
L Gullo L Barbara G Labo (1988) ArticleTitleEffect of cessation of alcohol use on the course of pancreatic dysfunction in alcoholic pancreatitis Gastroenterology 95 1063–1068 Occurrence Handle1:STN:280:BieA3MzltVA%3D Occurrence Handle3410221
RW Ammann A Akovbiantz F Largiader et al. (1984) ArticleTitleCourse and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients Gastroenterology 86 820–828
T Berney T Rudisuhli J Oberholzer et al. (2000) ArticleTitleLong term metabolic results after pancreatic resection for severe chronic pancreatitis Arch. Surg. 135 1106–1111 Occurrence Handle10.1001/archsurg.135.9.1106 Occurrence Handle1:STN:280:DC%2BD3cvmtF2rsw%3D%3D Occurrence Handle10982519
P Massucco M Calgaro F Bertolino et al. (2001) ArticleTitleOutcome of surgical treatment for chronic calcifying pancreatitis Pancreas 4 378–382 Occurrence Handle10.1097/00006676-200105000-00007
P Aeberhard A Karli (1992) ArticleTitleThe Frey operation: a valuable enrichment of therapeutic possibilities of chronic calcifying pancreatitis Helv. Chir. Acta 58 633–636 Occurrence Handle1:STN:280:By2B2sngt10%3D Occurrence Handle1592627
P Morel A Rohner (1987) ArticleTitleSurgery for chronic pancreatitis Surgery 101 130–135 Occurrence Handle1:STN:280:BiiC38%2FotlI%3D Occurrence Handle3810483
RL Rossi (1990) ArticleTitlePancreatic resection for chronic pancreatitis Hepatogastroenterology. 37 277–282 Occurrence Handle1:STN:280:By%2BA38zns1U%3D Occurrence Handle2197205
A Carlin D Fromm (1993) ArticleTitlePancreaticoduodenectomy for recurrent alcoholic pancreatitis associated with microabscesses Surg. Gynecol. Obstet. 176 315–318 Occurrence Handle1:STN:280:ByyB3M3jtl0%3D Occurrence Handle8096346
WR Fleming RC Williamson (1995) ArticleTitleRole of total pancreatectomy in the treatment of patients with end-stage chronic pancreatitis Br. J. Surg. 82 1409–1412 Occurrence Handle1:STN:280:BymD1MjosVQ%3D Occurrence Handle7489180
L Fernandez-Cruz E Saenz E Astudillo et al. (1993) Comprehensive treatment: resection and drainage in the management of patients with chronic pancreatitis HG Beger M Buchler P Malfertheiner (Eds) Standards in Pancreatic Surgery Springer-Verlag New York 372–384
Russel C. Duodenum-preserving pancreatectomy. Presented at the Standards in Pancreatic Surgery meeting, Merseburg, Germany, July 1–4, 1991
CM Hoozen ParticleVan PG Peeke M Taubeneck et al. (1997) ArticleTitleEfficacy of enzyme supplementation after surgery for chronic pancreatitis Pancreas 14 174–180 Occurrence Handle9057190
WH Nealon JC Thompson (1993) ArticleTitleProgressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression Ann. Surg. 217 458–468 Occurrence Handle1:STN:280:ByyB2Mnmtlw%3D Occurrence Handle8489308
PG Lankisch A Lohr-Happe J Otto et al. (1993) ArticleTitleNatural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease Digestion 54 148–155 Occurrence Handle1:STN:280:ByyA28rpslM%3D Occurrence Handle8359556
P Layer H Yamamoto L Kalthoff et al. (1994) ArticleTitleThe different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis Gastroenterology 107 1481–1487
JR Izbicki C Bloechle WT Knoefel et al. (1997) ArticleTitleDrainage versus resection in surgical therapy of chronic pancreatitis of the head of the pancreas: a randomized study Chirurg 68 369–377 Occurrence Handle1:STN:280:ByiB2sjpt1A%3D
MW Buchler H Friess MW Muller et al. (1995) ArticleTitleRandomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis Am. J. Surg. 169 65–69 Occurrence Handle10.1016/S0002-9610(99)80111-1 Occurrence Handle1:STN:280:ByqC3Mjns1c%3D Occurrence Handle7818000
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frey, C., Mayer, K. Comparison of Local Resection of the Head of the Pancreas Combined with Longitudinal Pancreaticojejunostomy (Frey Procedure) and Duodenum-Preserving Resection of the Pancreatic Head (Beger Procedure). World J. Surg. 27, 1217–1230 (2003). https://doi.org/10.1007/s00268-003-7241-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-003-7241-z