Abstract
This study aims to investigate leaching characteristics of zinc slag according to leaching tests, including; TCLP (Toxicity Characteristic Leaching Procedure), SPLP (Synthetic Precipitation Leaching Procedure), ASTM-D3987 (American Society for Testing and Materials), and TS EN-12457-4 (Turkish Standards Institute) tests methods. The present study describes the adsorption potential of natural and biochar walnut shells for removing ions from the zinc leachate. TCLP leachate, with a value of 38.575 mg/L, has a high zinc (Zn+2) concentration compared to other methods. Therefore, TCLP leachate was used in the adsorption experiments. Adsorption experiments were carried out at different adsorbent dosages, pH values, and contact time conditions. In the dosage study, the highest removal efficiency was obtained as 84% and 92% in natural and biochar walnut shell adsorbents, respectively. As a result of pH study, it was observed that adsorption under alkaline conditions had a much higher removal efficiency. Moreover, adsorption studies performed against contact time were applied to four different kinetic models and both adsorbents were found to be fit with the pseudo-second-order model. This kinetic model showed that the Zn+2 adsorption mechanism of natural and biochar walnut shells is chemical adsorption. With this study, it was shown that a very high 96% zinc removal can be achieved under optimum adsorption conditions. This may be the first study of zinc removal after leaching from industrial slag in the literature. This study has shown that high removal efficiencies can be obtained by an economical adsorbent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid growth of the metallurgical industry generates large amounts of metallurgical waste. The discharge and accumulation of many metallurgical wastes are essential from both economic and environmental points of view. These wastes contain generally valuable elements that may be reduced, reused, recovered, and recycled. Zinc is one of these valueable metals (Rudnik 2019; Ng et al. 2016). Increased global zinc consumption is decreasing the amount of primary zinc resources (Song et al. 2019). Therefore, zinc should be recovered from metallurgical waste as a secondary source for the sustainability of the zinc industry (Perez et al. 2018). Smelting slag produced from the zinc industry contains a high content of zinc. The zinc smelting slag produced from pyrometallurgical and hydrometallurgical processes is not only a hazardous waste but also a potential valuable solid (Song et al. 2019; Zhang et al. 2019; Ha et al. 2015; Jha et al. 2013; Bakhtiari et al. 2011). Zinc sludges, slags, and ashes are immobilized with adsorption, stabilization-solidification (S/S), and vitrification (Kul and Topkaya 2008; Vahidi et al. 2009; Bulut et al. 2009). Metallurgical solid wastes are stored in landfills and cause serious environmental pollution problems, by creating heavy metal pollution in the ecological system (Zhang 2019; Dessouky et al. 2008). To solve this problem and control the biological activities of heavy metals in the environment, various methods such as sedimentation, electrokinetic recovery, membrane, adsorption, ion exchange and biological improvement are used (Pour and Ghaemy 2015). However, these technologies have high operational, equipmental and chemical costs. Among these methods, interest in adsorption increases due to ease of use and the possibility of regeneration (Malik et al. 2007).
Recently, the use of agricultural wastes as adsorbents in the removal of pollutants from wastewater has emerged as an economical and applicable method (Kadirvelu et al. 2003). The use of agricultural wastes as adsorbents is preferred because they are cheap, easily accessible, and they are plentiful. (Agarwal et al. 2017).
Biochar is a carbon-rich material produced by pyrolysis in a limited or completely oxygen-free environment using different biowaste, immobilizing heavy metals, and reducing their toxicity (Wang et al. 2018; Liu et al. 2015; Lehmann 2007). In the literature, there are many studies regarding the use of biochars as an adsorbent. These biochars obtained from agricultural wastes have high efficiency in terms of pollution removal (Barbosa et al. 2013; Dowlatshahi et al. 2014; Ghasemi et al. 2015; Gıraldo and Pırajan 2012).
Turkey is among the first four countries in the world walnut production. According to Turkish Statistical Institute (TUIK 2017), about 178,142 tons of walnuts are being produced in Turkey annually. Depending on the amount of production, a large amount of waste walnut shell is generated. Walnut shell is a low-cost raw material with a high constant carbon content (Song et al. 2017).
The primary purpose of this study is to investigate leaching characteristics of zinc slag waste using TCLP, SPLP, ASTM-D3987, and TS EN-12457-4, and to understand the adsorption behavior of zinc ions from aqueous leachate of zinc slag waste metal on natural and biochar walnut shell. For this goal, experiments were carried out at different pH ranges, at different contact times and at different adsorbent dosages to determine the optimum adsorption conditions. Adsorption experiments performed against contact time were applied to pseudo-first-order, pseudo-second-order, intraparticle diffusion, and Elovich kinetic models.
Materials and Methods
Materials
Zinc slag
The zinc slag used in this study was obtained from a zinc plant in Kayseri, Turkey. It is the only plant in Turkey that produces zinc from primary ore containing zinc carbonate. It is enriched slag taken from the factory’s old waste site. The chemical composition of the zinc waste was evaluated with X-Ray Fluorescence techniques using a PANalytical brand and a Minipal4 model XRF spectrophotometer. According to XRF analysis, the zinc slag contained detectable amounts of anglesite (PbSO4), gypsum (CaSO4·2H2O), and zinc sulfate hydrate (ZnSO4·2H2O). The chemical composition of zinc slag waste is provided in Table 1.
Natural and biochar walnut shell
Walnut shells collected by such means were washed three times with distilled water and dried in an oven at 105 °C for 24 h to remove the moisture content. Then, the walnut shells were crushed with the help of a mixer crusher and sieved for a particle size of 2–4 mm.
Walnut shell biochar production was conducted with a Uniterm brand high-temperature reactor. The reactor provided a stable temperature through mantle heating. The reactor chamber consisted of titanium alloyed stainless steel material. There were inlet and outlet sections that provided a nitrogen gas flow in the reactor. The gas outlet was passed through ice water and cooled. Twenty grams of natural biochar was placed in the reactor, and pyrolysis was performed under 600 °C, at a 1 h standby time, at a slow pyrolysis speed of 5 °C/min, and at nitrogen gas flow conditions of 100 mL/min. Pyrolysis conditions were determined by testing similar pyrolysis studies in the literature and selecting average values (Qiu et al. 2018; Şamdan 2013; Georgieva et al. 2020; Arslan 2018). At the end of the pyrolysis process, 4.6 g of biochar walnut shells was obtained.
According to the BET (Brunauer, Emmett and Teller Theory), specific surface area measurement results, the natural walnut shell had 2.551 m2/g. Furthermore, a thermal treatment biochar walnut shell after 600 °C has as 6.934 m2/g specific surface area. The surface area is a parameter that directly affects adsorption efficiency. There was no significant change in surface area after thermal treatment. This situation was also reflected in the removal efficiency obtained in the adsorption experiments. The removal efficiency of natural and biochar walnut shell adsorbents were close to each other. BET analysis results for natural and biochar walnut shells are depicted in Table 2.
Methods
Batch leaching experiments
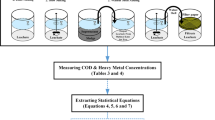
Leaching experiments were used to assess leaching in organic and inorganic contaminant potentials in liquid, solid, and the other wastes. The batch leaching experiments were conducted at varying levels of pH, adsorbent dosage, and contact time to analyze leaching behaviors of contaminants in the zinc leachate. These methods were used to supply constant contact time between the sample and the leachate (liquid/solid). At the end of the leaching period, the liquid was removed and analyzed. The most common applied batch leaching methods were TCLP, SPLP, ASTM-D3987, and TS EN-12457-4 (USEPA 2003; WTC 1991; Van et al. 1997; Tibet and Çoruh 2017).
In the TCLP method, a solution was prepared with glacial acetic acid (CH3COOH) and sodium hydroxide (NaOH). To 5 g of zinc slag was added 100 mL of TCLP solution (at a 20:1 ratio), and it was shaken at 25 °C, 165 rpm for 18 h. Sulfuric acid (H2SO4) and nitric acid (HNO3), SPLP solutions were prepared and used in the SPLP method. To 5 g of zinc slag was added 100 mL of SPLP solution (20:1 ratio), and it was shaken at 25 °C and, 165 rpm for 18 h. In the ASTM-D3987 method, 100 mL of distilled water was added to 25 g of zinc slag (4:1 ratio) and shaken at 25 °C at 165 rpm for 48 h. In the TS EN-12457-4 method, 100 mL of distilled water was added to 10 g zinc slag (1:10 ratio) and shaken at 165 rpm for 24 h (Table 3). After each of the leaching methods, samples were filtered by vacuum filtration and zinc concentrations were read by atomic adsorption spectrophotometer (AAS).
Adsorption experiments
Adsorption studies were conducted with natural and biochar walnut shells under different adsorption conditions to reduce zinc concentrations, which were released after leaching. Adsorption experiments were ran to determine maximum removal conditions, at different walnut shell dosages (1.66, 3.33, 10, 16.66 and 33.33 g/L), at different contact times (1, 15, 30, 60, 90, 120, and 240 min) and different pH values (3, 5, 7, 9, and 11). General adsorption conditions were performed using 30 mL of TCLP fluid, a dosage of 16.66 g/L of walnut shell, and a 60 min contact time. Dosage and contact time studies were performed at a pH solution of 8.17. At the end of the contact time, all samples were filtered with 0.45 µm syringe filters. The obtained filtrates were read after adsorption using a hollow zinc cathode lamp, which was an AAS of UNICAM 929 brand and model.
The removal efficiency values according to the contact time, dosage, and pH after adsorption were calculated using the following formula (Eq. 1).
where E (%) is removal efficiency, C0 (mg/L) is initial concentration, and C (mg/L) is concentration after adsorption.
Furthermore, adsorption capacities (qe) were calculated in all adsorption studies. Adsorption capacity is the amount of adsorbate that the unit (mass or volume) of the adsorbent can adsorb, and it is expressed in mg/g unit. The adsorption capacity (qe) is formulated in Eq. 2;
where C0 and Ce (mg/L) are the initial concentration and equilibrium concentration of dye solution, respectively, and V (L) is the volume of dye solution and m (g) is the mass of adsorbent.
Results and Discussion
Effect of Leachate Solution on Zinc Release from Zinc Smelting Slag
Zinc concentration values of zinc slag were examined with different standard methods. For this purpose, TCLP, SPLP, ASTM-D3987, and TS EN-12457-4 tests were applied. Zinc concentrations in leachate obtained after using this standard method were analyzed with the UNICAM 929 brand and model AAS. Concentration values were read with a hollow zinc cathode lamp in acetylene flame using the direct aspiration technique. The device provides the concentration value in mg/L. Zinc concentrations obtained according to leaching methods are provided in Table 4.
As seen in Table 4, the highest zinc concentration was obtained in TCLP leachate as 38.575 mg/L. For this reason, TCLP leaching was used in the adsorption experiments.
Effect of Adsorbent Dosage
Adsorbent dosage studies were performed using the same dosages and conditions for natural and biochar walnut shells. The experiments were executed in 30 mL of TCLP solution, at 8.17 pH, 60 min contact time, and at 150 rpm shaking speed. The shaking speed of 150 rpm, was accepted as an average adsorption shaking speed, considering our previous experiments. Theoretically, when the adsorbent dosage increases, the adsorption efficiency increases because the active centers where the ions can be held increase. However, the increase in adsorbent dosage, after exceeding a certain value, does not affect the adsorption process (Dowlatshahi et al. 2014).
As depicted in Fig. 1, the result of the dosage study appears to support this theory. Zinc removal efficiency increased continuously in experiments up to 16.66 g/L adsorbent dosage. However, when the dosage is increased to 33.33 g/L, it is seen that the removal efficiency begins to decrease partially. It was observed that the effects of natural and biochar walnut shells in removal efficiency were close.
The highest removal efficiency was obtained as 84% and 92% for natural and biochar walnut shells, respectively, at 16.66 g/L dosage. Therefore, 16.66 g/L was chosen as the optimum adsorbent dosage. Adsorption capacity (qe) values decreased with increasing efficiency. Similar values were observed in the natural and biochar walnut shells, and the highest value was calculated as 11.70 mg/L and 13.08 mg/L at a concentration of 1.66 g/L, respectively. Rao et al. (2008), in their zinc (Zn+2) and lead (Pb+2) removal study of agricultural waste biochar demonstrated that, removal efficiency increased from 70% to 99% after a 10 g/L adsorbent dosage. These results were parallel to ours. Zhou et al. (2018), in their study, which included tobacco biochar, obtained increasing removal efficiency with the increasing amount of adsorbents in terms of lead (Pb+2), cadmium (Cd+2), and copper (Cu+2) removal. The yield remained stable or partially decreased after an individual dosage rate.
Effect of pH
Solution acidity has a significant effect on adsorption capacity, because the surface of the adsorbent is formed according to the pH of the solution (Barbosa et al. 2013). The pH of the solution also contributes to the binding of heavy metal ions on the adsorbent surface to the adsorption zones (Ghasemi et al. 2015). In this study, we investigated the effect of pH on removal efficiency. Experiments were performed with at 30 mL of TCLP solution, 16.66 g/L of walnut shell dosage, 60 min contact time, and a 150 rpm shaking speed at 3, 5, 7, 9, and 11 pH values. The effect of pH on zinc adsorption in the TCLP leachate is depicted in Fig. 2. When the pH value is lower than 5, the decisive regions of the adsorbent surface increases. This causes repulsion between the active adsorbent points and the metal cation, therefore, low adsorption occurs. As the pH increases to between 7 and 9, the H+ ions attached to the surface are released from the active areas, allowing the empty areas to adsorb more metal ions.
Furthermore, close removal efficiency values of natural and biochar walnut shells were obtained at each pH value. Biochar walnut shell provides a 2–8% higher yield on average. The removal efficiency at pH 9 and 11 reached by natural and biochar walnut shells were 93% and 96%, respectively. Adsorption capacity (qe) values increased in parallel with removal efficiency. The highest values were calculated as 2.23 mg/g and 2.19 mg/g for natural and biochar walnut shells, respectively. Rao et al. (2008) studied Pb+2 and Zn+2 removal of agricultural waste biochar. In their study, as the pH value increased for both heavy metals, the removal efficiency increased, and they obtained 99% efficiency at high pH values. Depci et al. (2006) obtained 90% zinc removal efficiency at pH ≤ 7 with oak wood biochar adsorbent. The removal efficiency was ~20% at pH 2–3 values. They explained this by the tendency of zinc to precipitate at very low pH values. Similarly, in Şamdan’s study, which utilized pumpkin seed shell to remove lead, she obtained increasing heavy metal removal efficiencies with increasing pH values.
Effect of Contact Time
Adsorption continues until there is a balance between the solute concentration and the concentration held on the adsorbent surface. Therefore, the adsorption efficiency of the samples taken in specific time intervals can be determined to find the equilibration time and optimum contact time (Kadirvelu et al. 2003).
In this study, contact time experiments were conducted using 30 mL of TCLP solution, 16.66 g/L of adsorbent dosage, an 8.17 pH value, and a 150 rpm shaking speed at 1, 15, 30, 60, 90, 120 and 240 minutes. Figure 4 depicts the results of zinc removal efficiency versus contact time. As indicated in Fig. 3, it can be said that the adsorption attained equilibrium between 15 and 60 min. Regarding the decline in the curve after reaching equilibrium, this might be explained by the change in the pH of the solution which might reverse the process from desorption to adsorption. Yu et al. (2000) interpreted this effect as being linked to the, occurrence of saturation in the adsorbent, which leads to adsorption taking place in the inner surface instead of the outer surface. Due to the smaller inner surface area, the increased contact time causes efficiency to decrease. Rapid absorption and balance in a short time is an important parameter that demonstrates the effectiveness of the adsorbent.
However, it was observed that removal efficiency obviously decreaseds continuously in the 90th minute and afterward both for natural and biochar walnut shells. The excessive amount of active free space required for adsorption on the adsorbent surface, in the beginning, causes the adsorption to occur faster; however, as the time approaches the balance, the interaction between adsorbent and adsorbate decreases and this interaction ends in balance (Changmai et al. 2018), as balance mainly depends on the saturation of active areas on the adsorbent surface and slow pore diffusion (Lemraski and Sharafinia 2016). The highest removal efficiencies were 84% in natural walnut shell and 92% in biochar walnut shell. These efficiencies were obtained in 60 min, which was selected as the optimum time. Adsorption capacity (qe) results were in proportional to removal efficiency. The highest qe values were recorded as 2.15 mg/g and 2.10 mg/g for natural and biochar walnut shells at 60 min, respectively.
Adsorption Kinetics
Adsorption kinetic demonstrates a significant dependence on the adsorption material’s physical and/or chemical characteristics, which also influences the adsorption mechanism. Kinetic models are used to describe the ions changes in the adsorption studies with time.
To evaluate and compare the zinc adsorption of natural walnut shell and biochar walnut shell, pseudo-first-order, pseudo-second-order, intra-particle diffusion, and Elovich models (whose linear forms are expressed in Table 5) were used to examine adsorption kinetics. The most suitable model was chosen based on the linear regression and correlation coefficient (R2) values. An R2 value close to 1 indicates model suitability. The regression and correlation coefficients calculated for these four models are provided in Table 5.
Adsorption kinetic data were well-fitted with the pseudo-second-order kinetic model for natural and biochar walnut shells (R2 = 0.98 and 0.95, respectively) for Zn+2 compared to the other three models. These results suggest that the Zn+2 adsorption in the single and binary systems on natural and biochar walnut shells follows the second-order kinetic reaction. A pseudo-second-order equation is based on solid-phase sorption and rate expression based on sorption equilibrium capacity (Ho and McKay 1999; Shahwan 2014). The main hypothesis for this kinetic model is that the rate-limiting step is chemical adsorption or physicochemical adsorption, which includes the valence forces by sharing or exchanging electrons between the adsorbent and the solute (Ngulube et al. 2018; Malakootian et al. 2018). Physisorption or physical adsorption is a type of adsorption in which the adsorbate adheres to the surface only through Van der Waals (weak intermolecular) interactions, which are also responsible for the non-ideal behavior of real gases (Shen et al. 2009). On the other hand, chemisorption is a type of adsorption whereby a molecule adheres to a surface through the formation of a chemical bond, as opposed to Van der Waals forces that, cause physisorption. The Zn+2 adsorption mechanism for natural and biochar walnut shells was characterized by chemical adsorption.
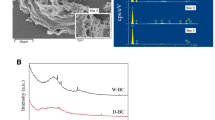
In this study, Fourier transform infrared spectroscopy (FTIR) analysis was performed to recognize the functional groups on the adsorbent surface. Such an approach, takes into account the possible chemical interaction between the solute and adsorbent. FTIR has been utilized to analyze the surface functional groups of samples in recent decades. The FTIR spectra of natural and biochar walnut shell was recorded using Perkin Elmer USA FTIR spectrometer between 400 cm−1 and 4000 cm−1.
Figure 4, provides the combined FTIR spectrum of natural and biochar walnut shell adsorbents. As depicted in Fig. 4, the FTIR spectrum of natural walnut shell clearly demonstrated six peaks. The spectrum includeds the peaks, corresponding to the stretching vibration of C–O–C (at ~1050 cm−1), –CH2 (at ~1150 cm−1), N–H (at ~1600 cm−1), C=O (at ~1730 cm−1), C–H (at approximately 2920 cm−1), –OH (at approximately 3420 cm−1). Furthermore, the FTIR spectrum of biochar walnut shell displayed peaks corresponding to the stretching vibration of carbohydrate C–O at approximately 990 cm−1, C–O–C functional groups of cellulose at 1043 cm−1, aromatic C=O, C=C at 1616 cm−1, C–H functional groups of alkane at 3000 cm−1. (Uchimiya et al. 2010; Kumar et al. 2011; Zheng et al. 2013; Godfred et al. 2019).
Conclusions
The disposal of zinc wastes through landfills poses a threat to the environment and human health. In order to reduce this harmful effect, researchers have sought to determine the levels of zinc leakage from waste zinc slag and to decrease zinc concentrations with natural adsorbents. First, zinc leaching tests were performed according to TCLP, SPLP, ASTM-D3987, and TS EN standards. TCLP-12457-4 leachate with the highest zinc concentration (38.575 mg/L) was selected for adsorption study. For zinc removal, adsorption experiments were applied under different conditions using natural and biochar walnut shells. Optimum adsorption conditions were performed with 30 mL of TCLP solution. An adsorbent dosage of 16.66 g/L, an approximate pH value 9, 60 min contact time, and a 150 rpm shaking speed were chosen for the experiments. Under these conditions, the highest zinc removal efficiencies were obtained in natural and biochar walnut shells as 86% and 92%, respectively. Furthermore, the removal efficiency was very low in the acidic environment, whereas it was extremely high in the primary environment. When the pH of the environment rose to 9 and above, a removal efficiency of up to 96% was obtained with the biochar walnut shell. The zinc removal efficiencies of natural and biochar walnut shell adsorbents were close to each other. Such a result may appear to portray thermal treatment of walnut shell as being an unnecessary step. However, in the purified sample obtained after the adsorption with a natural walnut shell, the natural walnut shell left a little yellow–green color and caused turbidity. This situation required a separate treatment step and led to extra cost and time loss. Thermally treated biochar walnut shell has high zinc removal efficiency without causing any negative changes in the sample structure. A recovery study was not performed, since the levels of zinc concentrations released were not considered as worth recovering. Time studies have been applied to kinetic models, and the results of this study were compatible with the pseudo-second-order kinetic model. Leaching properties, behaviors, and characteristics of waste materials have been generally examined in the literature. As in this study, no heavy metal removal study was found after leaching from waste zinc slag. In this respect, the study contributed to the literature in its demonstration that the heavy metal content of industrial waste slag containing heavy metals with natural adsorbents after leaching could be reduced. These results suggest that walnut shells, can be effectively used to reduce the heavy metal content of zinc leachate.
References
Agarwal RM, Singh K, Upadhyaya H, Dohare RK (2017) Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater Today 4:10534–10538. https://doi.org/10.1016/j.matpr.2017.06.415
Arslan F (2018) Production of biochar with pyrolysis of hazelnut shell and walnut shell and investigation of their adsorption characteristics in removal of heavy metal ions from aqueous solutions. Dissertation, Hitit University, Çorum, Turkey
Bakhtiari F, Atashi H, Zivdar M, Seyedbagheri S, Fazaelipoor MH (2011) Bioleaching kinetics of copper from copper smelters dust. J Ind Eng Chem 17:29–35. https://doi.org/10.1016/j.jiec.2010.10.005
Barbosa JJM, Velandia CL, Maldonado AP, Giraldo L, Pirajan JCM (2013) Removal of lead(II) and zinc(II) ions from aqueous solutions by adsorption onto activated carbon synthesized from watermelon shell and walnut shell. Adsorption 19:675–685. https://doi.org/10.1007/s10450-013-9491-x
Bulut U, Ozverdi A, Erdem M (2009) Leaching behavior of pollutants in ferrochrome arc furnace dust and its stabilization/solidification using ferrous sulphate and Portland cement. J Hazard Mater 162:893–898. https://doi.org/10.1016/j.jhazmat.2008.05.114
Changmai M, Banerjee P, Nahar K, Purkait MK (2018) A novel adsorbent from carrot, tomato and polyethylene terephthalate waste as a potential adsorbent for Cu(II) from aqueous solution: Kinetic and equilibrium studies. Chem Eng 6:246–157. https://doi.org/10.1016/j.jece.2017.12.009
Depci T, Kul AR, Önal Y (2006) Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from van apple pulp: study in single- and multi-solute systems. Chem Eng J 202:224–236. https://doi.org/10.1016/j.cej.2012.06.077
Dessouky SIE, El-Nadi YA, Ahmed IM, Saad EA, Daoud JA (2008) Solvent extraction separation of Zn(II). Fe(II). Fe(III) and Cd(II) using tributylphosphate and CYANEX 921 in kerosene from chloride medium. Chem Eng Process 47:177–183. https://doi.org/10.1016/j.cep.2007.03.002
Dowlatshahi S, Torbati ARH, Loloei M (2014) Adsorption of copper, lead and cadmium from aqueous solutions by activated carbon prepared from saffron leaves Environ Health Eng Manag J 1:37–44. http://ehemj.com/files/site1/user_files_cb3efc/mahan-A-10-27-6-75ca8eb.pdf Accessed 14 Apr 2019
Georgieva VG, Gonsalvesh L, Tavlieva M (2020) Thermodynamics and kinetics of the removal of nickel (II) ions from aqueous solutions by biochar adsorbent made from agro-waste walnut shells. J Mol Liq 312:112788. https://doi.org/10.1016/j.molliq.2020.112788
Ghasemi M, Ghoreyshi AA, Younesi H, Khoshhal S (2015) Synthesis of a high characteristics activated carbon from walnut shell for the removal of Cr(VI) and Fe(II) from aqueous solution:single and binary solutes adsorption. Iran J Chem Eng 12:28–51. http://www.ijche.com/article_11697.html Accessed 18 Apr 2019
Gıraldo L, Pırajan JCM (2012) Synthesis of activated carbon mesoporous from coffee waste and ıts application in adsorption zinc and mercury ıons from aqueous solution. Eur J Chem 9:938–948. https://doi.org/10.1155/2012/120763
Godfred OB, Divine DS, Seung HW (2019) Preparation and characterization of alginate-kelp biochar composite hydrogel bead for dye removal. Environ Sci Pollut Res 26:33030–33042. https://doi.org/10.1007/s11356-019-06421-2
Ha TK, Kwon BH, Park KS, Mohapatra D (2015) Selective leaching and recovery of bismuth as Bi2O3 from copper smelter converter dust. Sep Purif Technol 142:116–122. https://doi.org/10.1016/j.seppur.2015.01.004
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Jha MK, Kumari A, Jha AK (2013) Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag 33:1890–1897. https://doi.org/10.1016/j.wasman.2013.05.008
Kadirvelu K, Kavipriya M, Karthika C, Radhika M, Vennilamani N, Pattabhi S (2003) Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dye sand metal ions from aqueous solutions. Bioresour Technol 87:129–132. https://doi.org/10.1016/s0960-8524(02)00201-8
Kul M, Topkaya Y (2008) Recovery of germanium and other valuable metals from zinc plant residues. Hydrometallurgy 92:87–94. https://doi.org/10.1016/j.hydromet.2007.11.004
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) An assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manag 92:2504–2512. https://doi.org/10.1016/j.jenvman.2011.05.013
Lehmann J (2007) A handful of carbon. Nature 447:143–144. https://doi.org/10.1038/447143a
Lemraski EH, Sharafinia S (2016) Kinetics, equilibrium and thermodynamics studies of Pb2+ adsorption onto new activated carbon prepared from Persian mesquite grain. J Mol Liq 219:482–492. https://doi.org/10.1016/j.molliq.2016.03.031
Liu W, Jiang H, Yu H (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285. https://doi.org/10.1021/acs.chemrev.5b00195
Malakootian M, Hossaini H, Asadipour A, Daneshkhah M (2018) Preparation and characterization of modified sepiolite for the removal of Acid green 20 from aqueous solutions: isotherm, kinetic and process optimization. Appl Water Sci 8:174. https://doi.org/10.1007/s13201-018-0813-8
Malik R, Ramteke DS, Wate SR (2007) Adsorption of malachite green on ground nut shell waste based powdered activated carbon. Waste Manag 27:1129–1138. https://doi.org/10.1016/j.wasman.2006.06.009
Ng KS, Head I, Premier GC, Scott K, Yu E, Lloyd J, Sadhukhan J (2016) A multilevel sustainability analysis of zinc recovery from wastes. Resour Conserv Recycling 113:88–105. https://doi.org/10.1016/j.resconrec.2016.05.013
Ngulube T, Gumbo JR, Masindi V, Maity A (2018) Calcined magnesite as an adsorbent for cationic and anionic dyes: characterization, adsorption parameters, isotherms and kinetics study. Heliyon 4:10. https://doi.org/10.1016/j.heliyon.2018.e00838
Perez MSM, Gazquez MJ, Rios G, Ruiz-Oria I, Bolivar JP (2018) Diagnose for valorisation of reprocessed slag cleaning furnace flue dust from copper smelting. J Clean Prod 194:383–395. https://doi.org/10.1016/j.jclepro.2018.05.090
Pour ZS, Ghaemy M (2015) Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinylalcohol)/carboxymethyl starch-g-poly(vinylimidazole). RSC Adv 5:64106–64118. https://doi.org/10.1039/C5RA08025H
Qiu Z, Chen J, Tanga J, Zhang Q (2018) A study of cadmiumremediation andmechanisms: Improvements in the stability of walnut shell-derived biochar. Sci Total Environ 636:80–84. https://doi.org/10.1016/j.scitotenv.2018.04.215
Rao M, Rao GPC, Seshaiah K, Choudary NV, Wang MC (2008) Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Manag 28:849–858. https://doi.org/10.1016/j.wasman.2007.01.017
Rudnik E (2019) Recovery of zinc from zinc ash by leaching in sulphuric acid and electrowinning. Hydrometallurgy 188:256–263. https://doi.org/10.1016/j.hydromet.2019.07.006
Shahwan T (2014) Sorption kinetics: obtaining a pseudo-second order rate equation based on a mass balance approach. J Environ Chem Eng 2:1001–1006. https://doi.org/10.1016/j.jece.2014.03.020
Shen D, Fan J, Zhou W, Gao B, Yue Q, Kang Q (2009) Adsorption kinetics and isotherm of an ionic dyes onto organo-bentonite from single and multi solute systems. J Hazard Mater 172:99–107. https://doi.org/10.1016/j.jhazmat.2009.06.139
Song S, Sun W, Wang L, Liu R, Han H, Hu Y, Yang Y (2019) Recovery of cobalt and zinc from the leaching solution of zinc smelting slag. J Environ Chem Eng 7:1. https://doi.org/10.1016/j.jece.2018.11.022
Song X, Li K, Wang C, Sun X, Ning P, Tang L (2017) Regeneration performance and mechanism of modified walnut shell biochar catalyst for low temperature catalytic hydrolysis of organic sulfur. Chem Eng J 330:727–735. https://doi.org/10.1016/j.cej.2017.08.016
Şamdan CA (2013) Preparation of activated carbon from pumpkin seed shell by chemical activation; using for removal of dye and heavy metal. Dissertation, Eskişehir Osmangazi University, Eskişehir, Turkey
Tibet Y, Çoruh S (2017) Immobilisation and leaching performance of lead-acid batteries smelting slag using natural and waste materials. Glob Nest J 19:562–573. https://www.researchgate.net/profile/Yusuf_Tibet/publication/322940152_Immobilisation_and_leaching_performance_of_leadacid_batteries_smelting_slag_using_natural_and_waste_materials/links/5b289ff1a6fdcca0f09c605e/Immobilisation-and-leaching-performance-of-lead-acid-batteries-smelting-slag-using-natural-and-waste-materials.pdf. Accessed 22 July 2019
TUIK (2017) Annual walnut production quantity data. https://biruni.tuik.gov.tr/medas/?kn=104&locale=tr. Accesed 21 Jan 2020
Uchimiya M, Lima IM, Thomas Klasson K, Chang C, Wartelle LH, Rodgers JE (2010) Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J Agric Food Chem 58:5538–5544. https://doi.org/10.1021/jf9044217
USEPA (2003) United States Environmental Protection Agency Method 1311: Toxicity characteristic leaching procedure. SW846 On-line test methods for evaluation of solid wastes. Physical chemical methods. https://www.epa.gov/sites/production/files/2015-12/documents/1311.pdf. Accesed 18 Jan 2020
Vahidi E, Rashchi F, Moradkhani D (2009) Recovery of zinc from an industrial zinc leach residue by solvent extraction using D2EHPA. Miner Eng 22:204–206. https://doi.org/10.1016/j.mineng.2008.05.002
Van SH, Heasman L, Quevauviller P (1997) Harmonisation of leaching/extraction tests. Brussels, Belgium
Wang B, Gao B, Wan Y (2018) Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd(II). J Ind Eng Chem 61:161–168. https://doi.org/10.1016/j.jiec.2017.12.013
WTC (1991) Wastewater Technology Centre. Proposed evaluation protocol for cement-based solidified wastes. Report EPS 3/HA/9. Environment Canada
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption-removal of copper. J Hazard Mater 80:33–42. https://doi.org/10.1016/S0304-3894(01)00198-4
Zhang Y, Jin B, Huang Y, Song Q, Wang C (2019) Two-stage leaching of zinc and copper from arsenic-rich copper smelting hazardous dusts after alkali leaching of arsenic. Sep Purif Technol 220:250–258. https://doi.org/10.1016/j.seppur.2019.03.067
Zhang Y (2019) Utilization status of metallurgical solid waste resource. Metall Solid Waste 214:33–37. https://doi.org/10.5772/intechopen.83587
Zheng Z, Song-da Z, Ting-qiang L, Feng-liang Z, Zhen-li H, He-ping Z, Xiao-e Y, Hai-long W, Jing Z, Muhammad TR (2013) Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants. J Zhejiang Univ 14:1152–1161. https://doi.org/10.1080/00380768.1998.10414483
Zhou Q, Liao B, Lin L, Qiuc W, Song Z (2018) Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci Total Environ 615:115–122. https://doi.org/10.1016/j.scitotenv.2017.09.220
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Darama, S.E., Gürkan, E.H., Terzi, Ö. et al. Leaching Performance and Zinc Ions Removal from Industrial Slag Leachate Using Natural and Biochar Walnut Shell. Environmental Management 67, 498–505 (2021). https://doi.org/10.1007/s00267-020-01390-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-020-01390-6