Abstract
We review long-term changes that have occurred in factors affecting water quality in East Fork Poplar Creek (EFPC; in East Tennessee) over a nearly 25-year monitoring period. Historically, the stream has received wastewaters and pollutants from a major United States Department of Energy (DOE) facility on the headwaters of the stream. Early in the monitoring program, EFPC was perturbed chemically, especially within its headwaters; evidence of this perturbation extended downstream for many kilometers. The magnitude of this perturbation, and the concentrations of many biologically significant water-quality factors, has lessened substantially through time. The changes in water-quality factors resulted from a large number of operational changes and remedial actions implemented at the DOE facility. Chief among these were consolidation and elimination of many effluents, elimination of an unlined settling/flow equalization basin, reduction in amount of blow-down from cooling tower operations, dechlorination of effluents, and implementation of flow augmentation. Although many water-quality characteristics in upper EFPC have become more similar to those of reference streams, conditions remain far from pristine. Nutrient enrichment may be one of the more challenging problems remaining before further biological improvements occur.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Substantial changes in the types or amounts of effluents released to a stream, or changes in a stream’s flow regime or geomorphology over an extended period of time, can greatly alter species composition and abundances. Broadly speaking, this axiom establishes the rationale for stream biological monitoring. It is from this perspective that we summarize various water-quality changes in East Fork Poplar Creek (EFPC), an East Tennessee stream, over a 25-year period. The stream historically received pollutants from a United States (U.S.) Department of Energy (DOE) facility, located on the stream’s headwaters. Through specific examples, we attempt to show how water-quality changes related to operational changes and remedial actions that were implemented at DOE’s Oak Ridge Y-12 National Security Complex (formerly known as the Oak Ridge Y-12 Plant and referred to hereafter as the Y-12 Complex).
As reported by Loar and others (2011), the Y-12 Complex was constructed in 1942 in Oak Ridge, TN, as part of the Manhattan Project. The Complex’s primary function was that of fabricating nuclear weapons components. EFPC was a convenient recipient of wastewater discharges and chemicals from spills and operational upsets, and until the early 1980s, concerns about its environmental conditions were minimal. J. M. Googin, a chemist at the Y-12 Complex for more than 49 years, noted that during the first several years of facility operation, “on some days, the stream (EFPC) ran blue, and on some days it ran green” (pers. commun. A. J. Stewart, Oak Ridge Associated Universities). In the late 1980s, the upper reaches of EFPC received diverse types of effluents from more than 200 outfalls that dominated stream flow (Loar and others 2011). Chlorinated effluents from cooling tower blow-down, metal-enriched effluents from various machining and plating operations, mercury and nitrate from lithium-isotope enrichment operations (Southworth and others 2011), and polychlorinated biphenyls (PCBs; used on uranium in Y-12 Complex machine shops as a cutting coolant, fire retardant and heat dissipater) all found their way into the stream.
Biological conditions in the stream were degraded as a result of these inputs, but the extent of degradation was not well characterized or understood (Loar and others 2011). Water-quality monitoring of EFPC was implemented well after the Y-12 Complex began operations. The variables measured and the frequency of these measurements were chosen primarily by the requirements set forth in the effluent limits specified in the facility’s National Pollutant Discharge Elimination System (NPDES) permit issued by the U.S. Environmental Protection Agency (EPA) in 1985. More than 100 constituents have been measured at least occasionally since monitoring began, but with each permit renewal the specific requirements changed. Further, as the chemical, physical, and biological conditions became better understood, additional changes were made in the constituents that were monitored. For example, some of the monitored variables were of questionable biological significance (e.g., 237Np), and some that were initially not included but required later were deemed to be biologically important (e.g., chlorine), and thus were included to support the Y-12 Complex Biological Monitoring and Abatement Program (BMAP) (Loar and others 2011). The changes in the constituents measured, combined with changes in measurement and analytical methods for some constituents, contributed to extensive data gaps for some variables (e.g., 1993 and 1994), making analysis of temporal trends challenging. Such considerations formed our strategy of using a best professional judgment approach in selecting and reporting on EFPC water-quality trends. Here, we summarize specific water-quality changes and pollution abatement actions, some of which studies suggest have had direct, positive effects on EFPC biota (Ryon 2011; Smith and others 2011).
In considering how the water-quality changes in EFPC relate to the stream’s biota, we note three points. First, mechanisms by which changes in water-quality conditions affect biota are diverse and depend upon the water-quality variable in question. Second, there can be a significant time lag between a water-quality change and the full manifestation of its biological effects. And third, the underlying geology, geomorphology, and hydrology of a watershed can temper contaminant transport and fate in streams, and thus affect water quality. Even changes in a single water-quality property are more complicated than one might initially suppose, both in terms of how they can affect biota, and how biota respond (Levandowsky 1972). For example, a change in pH can secondarily affect other biologically important water-quality factors, such as the bioavailability of metals. Additionally, the biological impacts of changes in some water-quality factors are potentiated by biotic conditions. For example, food-web structure can affect the accumulation of mercury in stream fish, even if mercury concentrations in the water remain relatively constant.
Several examples of effects of specific water-quality conditions on biota in EFPC are given, and historically, some of the changes in water-quality factors reported here have been used in creating hypotheses about long-term improvements in biological conditions in the stream, with the presumption that water-quality conditions will continue to improve due to future remedial actions or changes in best management practices. Previous studies showed that the stream was chemically altered over its entire length (≈25 km), and that the magnitude of this alteration declined through time (Stewart 2001; Stewart and others 1992). However, because the remedial actions and best management practices that affected water quality in EFPC were implemented within the boundaries of the Y-12 Complex, and because water-quality was monitored more extensively in the headwaters, the primary focus of this paper is on long-term changes in the stream’s upper reaches, where biological degradation and contaminant exposures were most pronounced.
Perhaps the most significant abatement action implemented to improve conditions in EFPC was that of flow management, which started on an intermittent basis in mid-1996 before full implementation in January 1997 (see Loar and others 2011 for further details). Water was pumped from Melton Hill Reservoir, a nearby reservoir on the Clinch River, into the headwaters of EFPC via a channel lined with large limestone rocks. The purpose of this action was to stabilize discharge at 7 mgd (2.65 × 104 m3/day) as measured at Station 17, the downstream water quality monitoring station at the Y-12 Complex. Flow management had strong effects on water temperature and various water quality constituents, and these effects are discussed in greater detail later in this paper. In addition to the influences of flow management and changes in discharges of treated effluents, water-quality changes in EFPC were driven by other factors, such as releases of wastes and chemicals from spills and operational failures, and changes in physical structures and biological conditions in the stream. For example, suspended particulate matter, water temperature and pH were altered as the stream flowed through New Hope Pond, an unlined 2.2-ha detention basin previously located ~1.5 km from the stream’s origin (Loar and others 2011), and they continued to be affected by Lake Reality, a replacement for New Hope Pond built in late 1988. In another example, Stewart and others (1996) found that daily changes in concentrations of total residual chlorine (a toxicant initially not measured routinely) in upper EFPC were driven more by in-stream conditions such as exposure to sunlight and the presence of large quantities of algal biomass than by changes in chlorine inputs. The findings of this study were factored into an environmental management decision to dechlorinate effluent discharges entering the stream.
Finally, our approach for analyzing, interpreting and describing changes in water-quality conditions in EFPC seeks to inform readers with selected specific examples and descriptions of lessons-learned. We think this approach facilitates communication of our understanding of the relationships between remedial actions and in-stream water-quality conditions. We supplement this information by integrating results from other water-quality studies of EFPC that have relevant details.
Materials and Methods
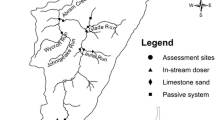
Much of the data reported in this paper were collected and supplied by the Y-12 Complex’s Environment, Safety, and Health Organization at their water-quality monitoring station referred to as Station 17, located downstream from all effluent discharges (Fig. 1). These data were collected from 1989 to 2004 primarily to meet NPDES permit requirements, so sampling and analysis methods adhered to standard EPA protocols. The sampling method that was used—grab samples, 24-h composite samples, or in-situ measurements—depended upon the constituent or property being analyzed. For many constituents, one water sample was collected and analyzed daily, and the volume of water passing Station 17 was measured continuously with a stream gauge at the monitoring station for estimates of total daily and average discharge. In brief, many water samples were analyzed for major cations and many metals (e.g., calcium, sodium, potassium, aluminum, boron, cadmium and zinc) by inductively-coupled plasma spectroscopy or in some cases by graphite-furnace atomic absorption spectroscopy. Chlorine (total residual and free) was measured amperometrically from fresh grab samples (see Stewart and others 1996). Hardness and alkalinity were measured by titrating non-filtered grab samples with a strong acid, using a potentiometric (for alkalinity) or colorimetric (for hardness) endpoint (Wetzel and Likens 1991). Nitrate was measured using the cadmium (Cd) reduction method (American Public Health Association (APHA) 2005), and total suspended solids (TSS) were determined by filtering samples through pre-weighed filters, and then drying and reweighing the filters (APHA 2005). Conductivity, dissolved oxygen, pH and temperature were measured in situ with calibrated portable water-quality meters, and continuous readings of temperature were measured with Peabody Ryan Model J90 thermographs. Turbidity of grab samples was determined by nephelometry with portable turbidity meters.
Temporal trends in selected examples of water-quality constituents are expressed as mean monthly concentrations and flux. Flux is defined as the concentration of the constituent (mg/l) multiplied by stream discharge (l/s), and is expressed as mass per unit of time (g/s). Daily flux was calculated from the daily estimates of constituent concentrations and average discharge, and then used to calculate average monthly flux. Constituent concentration and flux were chosen to express trends because they emphasize different perspectives: changes in concentration can be expected to be particularly relevant to stream biota, whereas changes in flux are particularly meaningful from an operational perspective.
A principal components analysis (PCA) was used to integrate and visualize temporal trends in a subset of water-quality metrics for upper EFPC. Annual means of samples collected monthly from the Y-12 Complex’s Station 17 from 1989 to 2004 (excluding 1993 and 1994 when data weren’t available) were used in the analysis. PCA is often preferred over other ordination methods for analyzing variables with linear responses and measured in different units, which are typical characteristics of water-quality data (McCune and Grace 2002; Quinn and Keough 2002). The appropriateness of a PCA for the data set was initially confirmed with a detrended correspondence analysis (length of gradient = 0.49; ter Braak and Šmilauer 2002). Many physiochemical variables were measured only occasionally from 1989 to 2004, but only seven—discharge, total calcium (Ca), total potassium (K), total sodium (Na), total zinc (Zn), TSS, and conductivity—provided a complete set of pair-wise measurements for the assessment period. These seven variables are considered to be reasonably sensitive to broad changes in water quality (e.g., conductivity, Ca, K, Na), indicative of specific changes in water quality (e.g., Zn, TSS), or physically important to stream biota (e.g., discharge). Because mercury (Hg) is covered in detail elsewhere (Southworth and others 2011), we focused on other potentially important constituents and pollutants. Precipitation can have strong effects on physical and chemical conditions of streams, especially discharge, TSS, ion concentrations, and fluxes. Further, under circumstances where flows are highly manipulated by human activities, such as EFPC, precipitation can confound understanding and interpretation of these manipulations. For this reason, total annual precipitation was used as a covariate in a partial PCA to remove its influence on the other variables (Quinn and Keough 2002). As with an analysis of covariance, partial ordination methods exclude the variance of covariates from the analysis (Van den Brink and others 2003). Precipitation data were from the National Oceanic and Atmospheric Administration’s meteorological station in Oak Ridge, TN, located ~1.5 km north of the Y-12 Complex. Before running the PCA, data for TSS and Na were log-transformed to reduce heterogeneity, and all variables, including precipitation, were centered to zero mean and standardized by the standard deviation (McCune and Grace 2002). The PRINCOMP procedure in the personal computer version of the Statistical Analysis System (SAS, version 9.2, Cary, NC) was used for the partial PCA and calculations of summary statistics.
While most of the water-quality data reported here were collected at the Y-12 Complex’s Station 17 (see above), water-quality data also were collected from other locations in EFPC and a reference stream (Fig. 1) to meet various requirements or objectives of specific studies. The reference stream used in our assessment, Brushy Fork (BFK7; Fig. 1), did not represent pristine conditions since it was located in a watershed with rural impacts; however, BFK7 lacks the industrial effluents found in EFPC. Therefore, the water quality and biotic community at BFK7 should represent conditions had the Y-12 Complex not been constructed. Because reference stream data were limited, they are only mentioned in context when invoked to support a particular discussion point. By convention, sites are named based on their distance from the mouth of the stream. For example, we use EFK24 to designate the site within the Y-12 Complex located nearest the headwaters of EFPC: this site is approximately 24 km upstream of the confluence of EFPC and Poplar Creek. EFK23 (about 1 km downstream of EFK24) was ~75 m upstream of Station 17 (Fig. 1).
Results and Discussion
In the absence of historical data, we presume that water quality of EFPC was similar to that of other streams in the area before the Y-12 Complex was built. As noted in Loar and others (2011), the basic geology of the region is dolomite and limestone, with steep northeast-southwest trending ridges separated by valleys. Undisturbed streams in the Oak Ridge area are fed largely by springs and seeps, and they are well shaded by riparian vegetation in spring and summer (Hill and others 2001). As a result, mean monthly water temperatures in non-disturbed streams in the Oak Ridge area rarely exceed 18°C and rarely drop below 10°C (Hill and others 2010a). The pH of these streams is slightly basic, and moderately hard (typical hardness ~130 mg/l as CaCO3) with a hardness-to-alkalinity ratio of ~0.8 to 1.0. Conductivity typically ranges from about 80 to 300 μS/cm, with highest values normally in late summer and early fall as the proportion of groundwater increases in the flow (Stewart 2001).
Since biological monitoring of EFPC started in 1985, EFPC has been distinctive from other area streams based on various water-quality factors: it has a higher discharge per unit watershed area, warmer temperatures, elevated concentrations of dissolved salts (based on measurements of conductivity), and is enriched with nitrogen and phosphorus. In short, the headwaters of EFPC show symptoms of urban stream syndrome (sensu Walsh and others 2005), but the main source of these symptoms is the Y-12 Complex. The source of factors contributing to these symptoms becomes increasingly ambiguous as the stream flows through the city of Oak Ridge. This is clearly evident from spatial trends in water quality, with maximum degradation within the Y-12 Complex, and a tendency for these conditions to attenuate with distance downstream (Stewart and others 1996; Stewart 2001).
Mean monthly discharge in EFPC at Station 17 from 1986 to 2005 and estimates of discharge at BFK7 from 1986 to 1989 are shown in Fig. 2. The two locations differ with respect to upstream watershed area (~3 km2 at Station 17 and ~40.3 km2 at BFK7). The greater watershed area at BFK7 accounts for the higher discharge values in winter and spring when evapotranspiration is minimal, while changes in EFPC primarily reflect changes in discharges from the Y-12 Complex and occasional peaks from storms in the winter and spring at Station 17. Figure 2 also shows that base-flow discharge at Station 17 was greater than base-flow discharge at BFK7 from 1986 to 1989, and that base flow at Station 17 declined from 1986 to 1993. The greater base flow at Station 17 compared to BFK7 from 1986 to 1989 was due to effluent discharges. The decline in base flow at Station 17 from 1986 through 1993 was due to a reduction in the volume of effluents discharged by the Y-12 Complex.
Mean monthly discharge at Station 17 in upper EFPC, 1986–2004, and estimated discharge for Brushy Fork at reference site BFK7. Broken lines indicate missing or unavailable data. Flow for BFK7 estimated as Q BF = A BF/A PC * (Q PC), where A BF and A PC are the watershed areas for BFK7 and Poplar Creek at USGS gauge 03538225, and Q PC is the discharge as recorded at the Poplar Creek gauge
In late summer and early fall, flows in most East Tennessee streams normally decline due to a high rate of evapotranspiration and less precipitation. At Station 17, in contrast, seasonal fluctuations in flow were reduced by effluents, and other than periodic spikes associated with spates, flow remained relatively stable. For example, from mid-1986 to mid-1989, discharge at Station 17 (unadjusted for upstream watershed area) averaged 350.5 l/s with a coefficient of variation of 20.9%, while at BFK7, mean discharge (unadjusted for upstream watershed area) and its coefficient of variation were 652.6 l/s and 107.0% respectively. In 1982 about 52% of the watershed above Station 17 was comprised of impervious surfaces (Loar and others 2011), a condition that has changed little since (J. G. Smith personal observation). The amount of impervious surface undoubtedly plays a major role in extreme discharge fluctuations that occur in EFPC after intense spates: the water level rises rapidly, but flow returns to near normal within 24–48 h (J.G. Smith personal observation). Because discharge data are summarized as monthly means in Fig. 2, these rapid fluctuations in discharge from intense spates are not evident.
As discussed in Loar and others (2011), effluent discharges to EFPC and removal of riparian vegetation have greatly altered the stream’s thermal regime. The combination of warm effluent discharges and lack of riparian vegetation resulted in warmer water temperatures in EFPC than in Brushy Fork, especially during the winter. While average summer temperatures adjacent to Station 17 (at EFK23) became more similar to those of the BFK7 reference site after full implementation of flow management in January 1997, average temperatures during the winter continued to be ~5°C higher in EFPC (Loar and others 2011).
Conductivity in EFPC generally declined with distance from the Y-12 Complex, but temporal trends were similar at all sites. Thus, for EFPC, only results for EFK23 and EFK14 are compared with BFK7 to demonstrate spatial and temporal variation; summary statistics for other locations are given in Loar and others (2011). Except when measured during elevated flows associated with spates, conductivity at EFK23 was typically two to three times higher than at BFK7, and 50–100 μS/cm higher than at EFK14, before flow management was implemented. However, after flow management started, conductivity at EFK23 was similar to that at EFK14 and typically about 50 μS/cm higher than at BFK7.
The water-quality information summarized above and discussed in Loar and others (2011) provides a general perspective on how water quality conditions in EFPC compared to BFK7, but it does not necessarily provide insight on causes of biological degradation. To add value to the EFPC water-quality information, we present data for several measured water-quality constituents or properties by invoking specific examples supporting three perspectives: (1) the importance of concentration versus flux; (2) the importance of temporal scale; and (3) the significance of spatial scale. We use the examples and perspectives as a basis for recommending particular water-quality monitoring activities for other biological monitoring programs.
The Importance of Concentration Versus Flux
Some of the complexities of water-quality changes that have occurred in EFPC since 1986 are illustrated by examples of temporal trends in concentrations and flux of Ca, Na, Zn, U, nitrate and TSS, at Station 17 adjacent to EFK23 (Figs. 3, 4, 5, 6, 7, 8, 9, respectively). We then describe how evaluation of such trends can be used to inform and direct environmental management decisions.
Mean monthly concentration and flux rate (flux = concentration multiplied by discharge) for total uranium at Station 17 in upper EFPC, 1995–2004. Horizontal lines show concentrations for toxicological benchmark for Tier II chronic toxicity (0.0026 mg/l; Suter 1996) and the toxicity “trigger value” for aquatic life (0.0053 mg/l; Fox 2006)
Calcium
Mean monthly concentrations of Ca at Station 17 were 50–70 mg/l in 1989–1991, and 45–50 mg/l by 1995–1996 (Fig. 4); they declined further (to about 40 mg/l), and became less variable, after implementation of flow management (Fig. 4). The high concentrations of Ca during 1989–1991 resulted largely from discharge of water from numerous cooling towers that were in operation at the Y-12 Complex. The cooling towers removed excess heat from coolant within a closed-loop system, by evaporating water used as a coolant. Ions in water (e.g., Ca, Mg, Na and K) do not evaporate, so this process increases their concentrations. When the residual water became too ion-rich for additional use, it was discharged to EFPC as cooling tower “blow-down.” Water used for evaporative cooling in cooling towers can contain low concentrations of microbiocides, anti-scaling compounds and corrosion inhibitors. When the use of the cooling towers declined at the Y-12 Complex, Ca concentrations in EFPC also declined. From 1995 to 1996, Ca concentrations declined further, from about 45 to 35 mg/l. Ca concentrations then stabilized at ~35 to 40 mg/l when flow management was fully implemented (Fig. 4).
Flux for Ca was more variable than concentration, but like concentration, it generally trended downward from 1989 to 1996. Ca flux then increased with the implementation of flow management (Fig. 4). Even when “stabilized” by flow management, Ca flux was more variable than concentration; flux varied by a factor of about two, ranging from about 13 to 25 g/s (Fig. 4). Much of the temporal variation in Ca flux from 1997 to 2005 could be attributed to variation in precipitation events: Ca inputs to the stream occur more or less in direct proportion to the amount of runoff. By then, most cooling tower operations at the Y-12 Complex had converted to ozone-based, zero-discharge systems that eliminated blow-down, and Ca inputs to EFPC from other operations at the Y-12 Complex were minor.
Two studies have linked Ca dynamics to ecological conditions in upper EFPC. First, the combination of moderately high levels of Ca and nutrient enrichment established opportunity for in-stream precipitation of calcium carbonates. The process of carbonate precipitation, which was driven primarily by algal photosynthesis, converted soluble Ca to particulates that could cause co-precipitation of various metals including Cd, Ni, and Zn (Cicerone and others 1999). Second, carbonate particles that formed in Lake Reality were exported to EFPC sites downstream, where they settled on substrates and became integrated into the periphyton matrix. At Station 17, located 0.5 km downstream of Lake Reality, chironomid larvae (Cricotopus spp.) were abundant (96% of all macroinvertebrates collected at the site), but had lower production (by about 45%, compared to a site upstream of Lake Reality). The lower production of chironomids at Station 17 was hypothesized to be due to the dilution of food quality by inorganic particulate matter (Runck 2007). It also was hypothesized that the production difference could affect the flux of mercury through the food web and the export of Hg via Cricotopus emergence (Runck 2007).
Sodium
Mean monthly concentrations of Na at Station 17 ranged from about 10 to 33 mg/l and varied considerably from 1989 to 1996 (Fig. 5). After flow management was implemented, Na concentrations generally ranged from about 6 to 17 mg/l. Higher concentrations of Na before flow management were due to the combined effects of discharges of cooling tower blow-down and reductions in stream discharge, as noted previously for Ca.
Flux rates for Na declined from a high of about 11 g/s in 1989 to 1–5 g/s during 1995–1996 (Fig. 5). With implementation of flow management, Na flux rates increased slightly to 3–6 g/s. Na concentration and flux both became less variable with flow management. Occasional spikes in Na concentrations and flux rates continued after flow management, but most of these spikes occurred in winter and were probably caused by runoff of salt (NaCl) applied to paved surfaces to melt snow and ice.
Sodium is not very toxic compared to metals such as copper or Zn, but an influx of Na to EFPC was initially hypothesized to have caused a fish kill (Ryon and others 1995). Following an ice storm on January 6, 1995, salt was applied to road and sidewalk surfaces within the Y-12 Complex. This caused the ice to melt rapidly, and large amounts of cold water and salt entered EFPC as runoff. The influx of cold water caused the stream’s temperature to drop 21°C (over 7.75 h) near the stream’s origin and 12.8°C (over 5.25 h) ~1 km further downstream. In-stream concentrations of Na were elevated during the event (1.9–3.0 g/l), but laboratory toxicity tests conducted later showed that the abrupt decline in water temperature, not the increase in salt (24- and 48-h LC50s were 9.6 and 8.9 g/l respectively), resulted in the kill.
Zinc
Mean monthly concentrations and flux rates of Zn are shown in Fig. 6, as well as, the 4-day and 1-h EPA criteria concentrations for Zn, based on the typical water hardness of 120 mg/l (as CaCO3) of streams in this geographic region (McMaster 1967). Mean monthly concentrations of Zn at Station 17 commonly ranged from 0.07 to 0.12 mg/l, and occasionally were greater than both criteria, both before and after flow management (Fig. 6). For comparison, Zn concentrations at a site in Beaver Creek (occasionally used as a reference for some Y-12 Complex BMAP studies) were 0.006–0.007 mg/l (Hill and others 2010a). Further, toxicity identification/reduction tests with Ceriodaphnia dubia (LA Kszos, ORNL, unpublished data) identified Zn as a likely toxicant in EFPC at the North-South Pipes. These observations indicate that intermittently high levels of Zn might warrant additional attention as a potential stressor in EFPC (e.g., Griffith and others 2004). The sources of Zn are unknown, but Zn in paints used as a corrosion inhibitor for cylinders at other DOE facilities has been linked to toxicity (Kszos and others 2004). Hill and others (2010a) found elevated levels of Zn in periphyton in upper EFPC (about 15× greater than in periphyton from reference streams). This led them to hypothesize that aqueous concentrations of Zn may be affected by periphyton.
Uranium
Concentration and flux plots for U are shown in Fig. 7. The concentration of U almost certainly declined after flow management started, but relatively few measurements were available before flow management began, so we unable to confirm this supposition. Particularly notable for U were the unusual seasonal trends for both concentration and flux. Uranium concentrations and flux rates were consistently elevated during winter but were much lower during summer and autumn. Concentrations of various metals in streams have been shown to vary both diurnally and seasonally with calcite production and dissolution (Cicerone and others 1999; Beck and others 2009). Cicerone and others (1999) observed peaks in calcite production during warmer months and lowest calcite production during cooler months, which might explain the seasonal trends for U. The concentrations of U measured at EFPC Station 17 after 1997, shown in Fig. 7 as mean monthly values, were lower than those typically reported as toxic to aquatic life. However, U concentrations routinely exceeded 0.0026 mg/l, which is the toxicological benchmark concentration for Tier II chronic toxicity (Suter 1996), and U concentrations often exceeded 0.0053 mg/l, the calculated “trigger value” for U toxicity to aquatic life (Fox 2006). Thus, U may be considered as a potential stressor in upper EFPC, from an ecological risk perspective (Suter 1996).
Nitrate
During 1989–1993, mean monthly concentrations of nitrate in upper EFPC at Station 17 often exceeded 3.5 mg/l, and sometimes were as high as 6.5 mg/l (Fig. 8). From 2001 to 2005, concentrations of nitrate were typically 0.5–1.0 mg/l (Fig. 8). Flux rates for nitrate from 1989 to 1993 varied nearly 20-fold (from ~0.5 to ~9.5 g/s). From 2000 to 2005, flux rates varied much less, and except for a spike in early 2003 (1.15 g/s) flux rates ranged from about 0.1 to 0.7 g/s. Both concentration and flux were higher in winter and spring and lower in summer and fall from 2000 to 2005. Uptake of nitrate by algae and denitrification by bacteria could account for this pattern.
Nitrate wastes have been a significant concern for Y-12 Complex operations. Historical disposal of nitric acid wastes resulted in a nitrate-rich groundwater plume in Bear Creek Valley watershed, just west of the headwaters of EFPC (cf. Luo and others 2005; Fig. 1). Most of the Y-12 Complex’s nitrate wastes have been produced and treated on the west end of the facility; nitrate problems on the east end of the Y-12 Complex are not as severe.
Synoptic sampling at numerous locations in upper EFPC in 1989 revealed another source of nitrogen to the stream. Bags of urea ((NH2)2CO2), used as a fertilizer, were improperly stored in the open on a hillside near the stream. This allowed urea to leach into the soil, where it hydrolyzed to ammonia and CO2. As a result, high concentrations of ammonia from the shallow groundwater reached EFPC via a buried culvert. Subsequent tracking of ammonia in EFPC showed that while about 1 mg/l ammonia-nitrogen (NH3-N) was entering Lake Reality from this source, about 90% of the ammonia was gone (presumed uptake by algae) by the time the water had passed through the pond (A. J. Stewart, unpublished data). Elevated ammonia was not detected in EFPC at Station 17. Best management practices were subsequently invoked to change fertilizer storage practices and remediate the urea-contaminated soil.
Total Suspended Solids
Mean monthly concentrations and flux of total TSS in EFPC at Station 17 are shown in Fig. 9. TSS concentrations typically ranged from ~10 to ~30 mg/l, with no clear long-term trend. Mean monthly TSS flux rates often were between 1 g/s and 10 g/s, but precipitation events triggered large spikes in both TSS concentration and flux throughout the 14-year record (Fig. 9). Mean TSS concentrations in wadeable streams in East Tennessee typically range from about 5.9 to 11.3 mg/l (Graf and Arnwine 2009), indicating that TSS concentrations in EFPC may be somewhat elevated compared to other streams in the ecoregion. But eutrophic conditions drive high rates of algal production in upper EFPC (Hill and others 2010b), and algal filaments, cells and cell fragments account for at least some of the excess TSS at Station 17. Further, before bypass of Lake Reality, both New Hope Pond and Lake Reality were sources of particulate matter to EFPC (Stewart and others 1992; Cicerone and others 1999). In 1997, particulate matter exported from Lake Reality was shown to be nutritionally satisfactory as food for the filter-feeding microcrustacean, Ceriodaphnia dubia (Stewart and Konetsky 1998). We conclude that in-stream processes probably accounted for a significant fraction of the excess TSS, and that the amounts and types of TSS are probably important to the stream’s biota.
The Significance of Temporal Scale
Water quality in upper EFPC was, and continues to be, influenced strongly by activities at the Y-12 Complex. In addition to point-source discharges from various water treatment facilities, the stream receives runoff from parking lots, roads and the roofs of buildings, and diffuse inputs of pollutants from legacy contamination in soils and groundwater. When biological monitoring started in 1985, elevated levels of contaminants such as oxidants (e.g., hypochlorite in cooling-tower blow-down and in drinking water used as once-through coolant), Hg, PCBs, arsenic, Cd, copper, lead, lithium, silver, nickel, Zn and U occurred in upper EFPC, along with elevated levels of nitrate, ammonia, phosphorus and sulfate (Hill and others 2010b; Hinzman 1998; Southworth and others 2011; Stewart and others 1992, 1993). Changes in some of these water-quality factors occurred over very different time-scales.
Some Y-12 Complex operations released wastewaters to EFPC continuously, while other wastewaters, such as those from cooling towers, were released intermittently, with recurrence intervals on the order of minutes to hours. Cooling tower loads also varied seasonally, so the frequency of blow-down events varied seasonally, as well. Still other processes released effluents occasionally, either by design (e.g., batch discharges) or as a result of chemical spills or “off-normal” operations (Etnier and others 1996; Kszos and others 1992; Ryon and others 2002). Concentrations of TSS generally increased in response to storm events, and then returned to baseline, usually within a 3- to 5-day period. Collectively, pollution control activities and flow management implementation had strong effects not only on factors typically considered to be biologically significant pollutants, but also on relationships among factors generally considered to be more innocuous, such as conductivity, alkalinity and hardness.
Most of the variation in the water quality parameters included in the PCA were explained by the first two principal components (PC1 = 58.6% and PC2 = 20.3%; Table 1 and Fig. 10), with PC3 explaining an additional 11.9% (not shown in Table 1). A strong temporal trend was obvious from right to left along PC1 of the ordination plot (r 2 = 0.72, P < 0.01, regression analysis on independent variable year versus dependent variable PC1), with Ca, Na, discharge, and conductivity contributing most to the loadings. However, these parameters contributed little to the variation explained by PC2 (Table 1). The loading for Zn on PC1, on the other hand, was more modest and the loading for TSS was very low, but they were the most important contributors to loadings on PC2. These results suggest that changes in discharge and the major ions occurred throughout the study, whereas, the largest changes in Zn and TSS occurred over a much narrower period of time. Furthermore, these results suggest that the overall trends that occurred in discharge and major ions were possibly some of the most significant water quality changes through 2004, and in part, support the idea that deviations in relationships among semi-conservative properties of water can be sensitive indicators of changes in water-quality conditions (Stewart 2001).
Plot of partial PCA on annual averages for water-quality variables at Station 17 in upper EFPC. The dashed line indicates data were unavailable for 1993 and 1994. Solid circles represent the pre-flow management period; the mixed open/solid circle represents the transition period in 1996 before flow management was fully implemented; and open circles represent the period after flow management was fully implemented. Values in parentheses after axis labels are the percent of the total variation explained by each axis. See Table 1 for additional information
As suggested by the separation of sample points on the ordination plot (Fig. 10), some of the largest changes in water quality occurred from 1996 to 1998. This suggests that flow management, even when not fully implemented (for an explanation see Loar and others 2011), had very strong effects on the water-quality variables included in analysis. The effect was more than dilution per se, because the water used to augment flow in EFPC was derived from the Clinch River, which has its own compliment of ions.
We suggest that some of the biological changes reported in other papers in this series were caused by long-term changes in EFPC water-quality factors. The presumption of causality in this case can be supported with reasonable confidence because measurements of change in both biological and water-quality factors were in good temporal agreement, with the presumed effects following the presumed causal agents. In other cases, causal relationships between water-quality conditions and biological condition could be established with reasonable confidence due to multiple measurements through an extended period of time, with a viable explanatory mechanism. The attribution of ambient toxicity in upper EFPC to chlorine (Stewart and others 1996), and the subsequent reduction in ambient toxicity in upper EFPC following effluent dechlorination and flow augmentation (Greeley and others 2011), may serve as examples of these cases. We suggest that frequent measurements of carefully selected water-quality factors at an appropriate selection of sites can establish a baseline on conditions suitable for detecting and quantifying operational upsets or episodic events. This, in turn, can guide the development of more focused experimental studies designed to ascertain causality.
The Significance of Spatial Scale
Several studies have shown strong longitudinal gradients in water-quality conditions in EFPC, with locations nearer to the Y-12 Complex being more severely affected than locations farther downstream. This situation was demonstrated at the whole-stream spatial scale by analyses of semi-conservative chemical properties of water (Stewart 2001). Further, a study of aquatic vegetation exported from New Hope Pond showed that a 1- to 3-km segment of upper EFPC was organically enriched by plant biomass for at least a portion of the year (Stewart and others 1992). Strong biological effects of chlorine (as the toxic oxidant, OCl−) were evident over about a 1.5-km reach of upper EFPC based on ambient toxicity tests (Stewart and others 1996), in situ tests with striped shiner and central stoneroller minnows (Lotts and Stewart 1995), and bank-side experiments with fathead minnows in aquaria (Stewart and others 1996). Since 1985, fish kills in EFPC typically have been restricted to (or at least were much more severe in) reaches upstream from EFK23 (Etnier and others 1996), and pollution-intolerant species of fish and macroinvertebrates were absent or much less common at sites closer to the Y-12 Complex than they were farther downstream (Ryon 2011; Smith and others 2011). High concentrations of ammonia were discovered in upper EFPC a few hundred meters upstream of Lake Reality, from improperly stored urea fertilizer, but elevated concentrations were not detected downstream of Lake Reality at Station 17. A fish-kill attributed to thermal shock from the rapid inflow of melting ice, as discussed previously, was restricted to about a 1.5-km section of upper EFPC upstream of Lake Reality. In short, water-quality degradation was much more evident and more severe in upper EFPC than it was farther downstream, and as discussed in other papers in this series, biological changes attributed to improvements in EFPC water-quality conditions were especially pronounced in upper EFPC (Hill and others 2010b; Ryon 2011; Smith and others 2011). The broad similarity in spatial pattern of water-quality perturbation and degradation of biological communities in EFPC (Ryon 2011; Smith and others 2011) is thus presumed to be causal, not merely coincidental.
Management Implications
From an environmental management perspective, flux is important because it is the most direct way to express the amount of a substance lost downstream over some defined period of time. For example, a U flux rate of 0.006 g/s (Fig. 7) translates to 189.2 kg of U per year. With this type of information, a straightforward management goal could be to reduce U flux to <150 kg/year. From a different but equally valid environmental management perspective, contaminant concentration is important because it and exposure duration are two key aspects of potential biological effects. For example, a fish kill caused by an acute exposure to an accidental release of a high concentration of uranium in soluble form could result in negative publicity, even if the annual flux rate of uranium is low.
Our studies of EFPC demonstrate the advantages of long-term, high-quality, water-quality monitoring. Having high-quality results on variables selected for their relevance to identified impacts and measured consistently through time offer significant advantages, as shown from this study. Annual trends in mean concentrations of constituents, or seasonal changes in concentrations of key nutrients or metals, can be revealed with confidence only if sampling frequency and duration are sufficient. Resources to accommodate these needs should be agreed upon early in a monitoring program’s conception, or the possibility of establishing causality or demonstrating trends can be compromised. Within the framework of acquiring a coherent and consistent set of water-quality measurements through time, it is also important to establish opportunity for obtaining measurements of selected water-quality factors at a higher sampling frequency to allow focused experiments or studies that can help identify cause-and-effect relationships. In such studies, the water-quality factors to be measured, and their measurement frequency, will vary depending upon the type(s) of impact(s) identified, the question(s) being addressed, and the experimental design selected to answer the question.
With respect to spatial scale, a key management implication of the examples we provide is that it may be easier to establish cause-and-effect relationships between changes in water-quality conditions and the conditions of stream biological communities when sampling can be accomplished close to the source of the water-quality changes. If a biological monitoring program is being considered, we suggest that there is value in effort invested early in the selection of a suite of monitoring sites that can increase the likelihood of establishing cause-and-effect relations.
Conclusions
This paper broadly summarizes changes in water quality in EFPC over a period of nearly 25 years resulting from several pollution abatement measures and remedial actions taken by the Oak Ridge Y-12 Complex to help meet imposed water-quality standards. Clear and significant improvements in water quality in EFPC were documented. However, as has been known for many years (Karr 1995), and as demonstrated by the changes in biological communities in EFPC (Ryon 2011; Smith and others 2011), simply meeting water-quality criteria does not guarantee protection of a water body’s biological integrity. Understanding the changes in water quality as environmental conditions improve provides an important piece of the puzzle in understanding the benefits to biological integrity and ecological recovery of stream ecosystems—which is the objective of this series of papers. With a better knowledge of the ecological consequences of their decisions, environmental managers can better evaluate alternative actions and more accurately predict their effects.
References
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Beck AJ, Janssen F, Polerecky L, Herlory O, de Beer D (2009) Phototrophic biofilm activity and dynamics of diurnal Cd cycling in a freshwater stream. Environmental Science and Technology 43:7245–7251
Cicerone DS, Stewart AJ, Roh Y (1999) Diel calcite production in a eutrophic spill-control basin. Environmental Toxicology and Chemistry 18:2169–2177
Etnier EL, Opresko DM, Talmage SS (1996) Second report on the Oak Ridge Y-12 Plant fish kill of upper East Fork Poplar Creek. ORNL/TM-12636. Oak Ridge National Laboratory, Oak Ridge, TN
Fox DR (2006) Statistical issues in Ecological Risk Assessment. Human and Ecological Risk Assessment 12:120–129
Graf MH, Arnwine DH (2009) 2007-8 Probabilistic monitoring of wadeable streams in Tennessee. vol 4—Water chemistry. Tennessee Department of Environment and Conservation. http://www.state.tn.us/environment/wpc/publications/pdf/wsa08volume4.pdf. Accessed 20 Nov 2009
Greeley MS Jr, Kszos LA, Morris GW, Smith JG, Stewart AJ (2011) Role of a comprehensive toxicity assessment and monitoring program in the management and ecological recovery of a wastewater receiving stream. Environmental Management (this series)
Griffith MB, Lazorchak JM, Herlihy AT (2004) Relationships among exceedences of metals criteria, the results of ambient bioassays, and community metrics in mining-impacted streams. Environmental Toxicology and Chemistry 23:786–1795
Hill WR, Mulholland PJ, Marzolf ER (2001) Stream ecosystem responses to forest leaf emergence in spring. Ecology 82:2306–2319
Hill WR, Smith JG, Stewart AJ (2010a) Light, nutrients, and herbivore growth in oligotrophic streams. Ecology 91:518–527
Hill WR, Ryon MG, Smith JG, Adams SM, Boston HL, Stewart AJ (2010b) The role of periphyton in mediating the effects of pollution in a stream ecosystem. Environmental Management 45:563–576
Hinzman RL (ed) (1998) Third report of the Oak Ridge Y-12 Plant Biological Monitoring, Abatement Program for East Fork Poplar Creek. Environmental Sciences Division Publication No. 4260. Y/TS-889. Oak Ridge National Laboratory, Oak Ridge
Karr JR (1995) Protecting aquatic ecosystems: clean water is not enough. In: Davis WS, Simon TP (eds) Biological assessment and criteria: tools for water resource planning and decision making. Lewis Publishers, Boca Raton, pp 7–13
Kszos LA, Stewart AJ, Taylor PA (1992) An evaluation of nickel toxicity to Ceriodaphnia dubia and Daphnia magna in a contaminated stream and in laboratory tests. Environmental Toxicology and Chemistry 11:1001–1012
Kszos LA, Morris GW, Konetsky BK (2004) Source of toxicity in storm water: zinc from commonly used paint. Environmental Toxicology and Chemistry 23:12–16
Levandowsky M (1972) Ecological niches of sympatric phytoplankton species. The American Naturalist 106:71–78
Loar JM, Stewart AJ, Smith JG (2011) Twenty-five years of ecological recovery of East Fork Poplar Creek: review of environmental problems and remedial actions. Environmental Management. doi:10.1007/s00267-011-9625-4
Lotts JW, Stewart AJ (1995) Minnows can acclimate to total residual chlorine. Environmental Toxicology and Chemistry 14(8):1365–1374
Luo J, Cirpka OA, Wu WM, Fienen MN, Jardine PM, Mehlhorn TL, Watson DB, Criddle CS, Kitanidis PK (2005) Mass-transfer limitations for nitrate removal in a uranium-contaminated aquifer. Environmental Science and Technology 39:8353–8459
McCune B, Grace JB (2002) Analysis of ecological communities. MJM Software Design, Gleneden Beach
McMaster WM (1967) Hydrologic data for the Oak Ridge area, Tennessee. U.S. Geological Survey Water Supply Paper No. 1825-N. U.S. Government Printing Office, Washington, DC
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Runck C (2007) Macroinvertebrate production and food web energetic in an industrially contaminated stream. Ecological Applications 17:740–753
Ryon MG (2011) Recovery of fish communities in a warm water stream following pollution abatement. Environmental Management. doi:10.1007/s00267-010-9596-x
Ryon MG, Kszos LA, Seagraves JL, Stewart AJ (1995) Investigation of a fish kill in East Fork Poplar Creek at the Y-12 Plant, January 6–8, 1995. Unpublished Report to the Environmental Surveillance Section, Health, Safety, Environment, and Accountability Division. Oak Ridge Y-12 Plant, Oak Ridge
Ryon MG, Stewart AJ, Kzsos LA, Phipps T (2002) Impacts on streams from the use of sulfur-based compounds for dechlorinating industrial effluents. Water, Air and Soil Pollution 136:255–268
Smith JG, Brandt CC, Christensen SW (2011) Long-term benthic macroinvertebrate community monitoring to assess pollution abatement effectiveness. Environmental Management. doi:10.1007/s00267-010-9610-3
Southworth GR, Peterson MJ, Roy WK, Mathews TJ (2011) Monitoring fish contaminant responses to abatement actions: factors that affect recovery. Environmental Management. doi:10.1007/s00267-011-9637-0
Stewart AJ (2001) A simple stream monitoring technique based on measurements of semiconservative properties of water. Environmental Management 27:37–46
Stewart AJ, Konetsky BK (1998) Longevity and reproduction of Ceriodaphnia dubia in receiving waters. Environmental Toxicology and Chemistry 17:1165–1171
Stewart AJ, Haynes GJ, Martinez MI (1992) Fate and biological effects of contaminated vegetation in a Tennessee stream. Environmental Toxicology and Chemistry 11:653–664
Stewart AJ, Hill WR, Boston HL (1993) Grazers, periphyton and toxicant movement in streams. Environmental Toxicology and Chemistry 12:955–957
Stewart AJ, Hill WR, Ham KD, Christensen SW, Beauchamp JJ (1996) Chlorine dynamics and toxicity in receiving streams. Ecological Applications 6:458–471
Suter GW II (1996) Toxicological benchmarks for screening contaminants of potential concern for effects on freshwater biota. Environmental Toxicology and Chemistry 15:1232–1241
ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and CanoDraw for Windows User’s guide: Software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca
Van den Brink PJ, Van den Brink NW, ter Braak CJF (2003) Multivariate analysis of ecotoxicological data using ordination: demonstrations of utility on the basis of various examples. Australasian Journal of Ecotoxicology 9:141–156
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP II (2005) The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24:706–723
Wetzel RG, Likens GE (1991) Limnological analyses, 2nd edn. Springer, New York
Acknowledgments
Many individuals made significant contributions to this project, including visiting scientists, dozens of intern students and teachers, subcontractors, and numerous scientific and technical staff from the Environmental Sciences Division at Oak Ridge National Laboratory (ORNL). The authors are particularly grateful to the staff of the Y-12 Complex’s Environment, Safety, and Health Organization for their long-term support and contributions to this program, including M. C. Wiest, L. Vaughn, and C. C. Hill. We also thank K. G. Hanzelka of the same organization for providing most of the raw water-quality data for upper EFPC. K. R. Birdwell of the National Oceanic and Atmospheric Administration’s Atmospheric Turbulence and Diffusion Division in Oak Ridge, TN, kindly provided precipitation data. Dale Robertson (journal editor) and two anonymous reviewers provided many helpful suggestions. This work was funded by the Environment, Safety, and Health organization of the Y-12 National Security Complex, which is managed by BWXT Y-12, LLC for the U.S. Department of Energy under contract number DE-AC05-00OR22800. ORNL is managed by the University of Tennessee-Battelle LLC for the U.S. Department of Energy under contract DE-AC05-00OR22725.
Author information
Authors and Affiliations
Corresponding author
Additional information
The submitted manuscript has been authored by a contractor of the U.S. Government under contract DE-AC05-00OR22725. Accordingly, the U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce the published form of this contribution, or allow others to do so, for U.S. Government purposes.
Rights and permissions
About this article
Cite this article
Stewart, A.J., Smith, J.G. & Loar, J.M. Long-Term Water-Quality Changes in East Fork Poplar Creek, Tennessee: Background, Trends, and Potential Biological Consequences. Environmental Management 47, 1021–1032 (2011). https://doi.org/10.1007/s00267-011-9630-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-011-9630-7