Abstract
Feeding marine wildlife as a tourism experience has become a popular means by which to attract both people and wildlife, although management efforts are still in their infancy. “Stingray City Sandbar” in the Cayman Islands, where visitors can hand feed free-ranging Southern Stingrays (Dasyatis americana), is a world-famous attraction currently undergoing visitor and wildlife management. One plan is to decrease the amount of nonnatural food provided by tourists with the intention of decreasing stingray habituation to the artificial food source and promoting stingray health. However, the effectiveness of this action is uncertain given that neither the extent of squid composition in the stingray diet nor the degree of nutrient similarity between the fed and natural diets is unknown. We used fatty acid (FA) profile analysis to address these questions by assessing the serum nonesterified FA composition of fed and unfed stingrays around the island and compared them with FA profiles of (1) the provisioned food source (squid) and (2) other warm- and cold-water elasmobranchs (sharks and rays). Our results indicated that fed stingrays were distinct. The FA profiles of the fed stingray population were expressly different from those of the unfed populations and showed a remarkable similarity to the FA composition of squid, suggesting that squid is the main food source. The tropical fed stingrays also exhibited essential FA ratios, specific to both species and habitat, comparable with those of elasmobranchs and squid from cold-water environs, implying that the provisioned food does not provide a similar nutritional lipid composition to that eaten in the wild. Our results suggest that FA profiles are a valuable indicator for the management and monitoring of fed Southern Stingrays because they can be used to assess differences in diet composition and provide an index of nutritional similarity. Our findings are currently being used by Caymanian stakeholders in designing practical management actions for their wildlife attraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, a growing worldwide demand to interact with wildlife has given increase to a wide range of wildlife tourism products (Reynolds and Braithwaite 2001). Marine wildlife tourism (MWT), in particular, has become one of the leading foreign exchange earners for countries with coastlines (Green and Higginbottom 2000, Garrod and Wilson 2004). The ultimate benefit of MWT is its potential to create a positive feedback between resource persistence and tourism demand that results in a common incentive to protect the natural environment. Despite benefits, MWT is not a panacea for conservation that can unfailingly both protect the environment and support economic activity. Instead, MWT can be prone to unmitigated development that is progressed at the cost of ecologic integrity (Miller 1993, Orams 1999). The ironic negative outcome of people’s increased value of and desire for wildlife interaction opportunities stems from the conflict amongst conservation values, visitor satisfaction, and profitability of the stakeholders involved (Reynolds and Braithwaite 2001).
Deliberate feeding of marine wildlife as a tourism attraction is one such opportunity that has potential for conflict. Marine tourists are now sanctioned to hand-feed wild dolphins, coral reef fish, sharks, and stingrays (and manatees and sea turtles without permission). Although this activity permits close observation of the focal species, allows the tourist to experience unusual or exciting animals, and returns a large economic benefit, feeding can also affect the wildlife. Food provisioning has been demonstrated to alter an animal’s natural behavior patterns and population levels beyond carrying capacity and to increase aggression levels and habituation to human contact (see Orams 2002 for a review). Other suspected disturbances include dependence, overfeeding, malnourishment, and disease. The pathologies of these latter outcomes are often subtle and delayed (especially in long-lived species) and are therefore difficult to assess without explicit physiologic examinations. Conversely, feeding can also produce positive impacts in the form of increased reproduction and enhanced survival during nutritionally stressed periods (Orams 2002, Dunkley and Cattet 2003). The issue of wildlife feeding as a tourism attraction is consequently complex because the social and economic benefits are vast and the negative ecologic outcomes uncertain at best, particularly in a marine setting. Addressing the issue of feeding wildlife therefore involves a considerable amount of empirical research, management, and understanding (Newsome and others 2005).
The Cayman Islands in the Caribbean are internationally known for their stingray-feeding tourism attraction. Stingray City Sandbar (SCS) is a warm, shallow water (1.6 m maximum depth) sandbar in the North Sound of Grand Cayman, approximately 7740 m2 in area and located roughly 300 meters inland from the fringing reef. Here, a congregation of Southern Stingrays (Dasyatis americana) gather to be fed frozen squid (Illex and Loligo spp.) by tourists and tour-boat operators. SCS is an enormous attraction draw to the Cayman Islands, with its off season during the spring and summer months and its on season in fall and winter. Today, the site supports at least 56 local dive and snorkel tourism operations (increased from 36 in 1998; Shackley 1998) and hosts >1 million tourists a year (almost half of the entire tourism visitor market). Visitor numbers have more than doubled from 3 years ago (Cayman Islands Ministry of Tourism 2002), and ≤2500 tourists (from approximately 40 boats) can be simultaneously present at the sandbar, feeding, touching, and holding stingrays as part of their marine tourism experience.
With no concerted management effort of the site since its inception in 1984, Caymanian stakeholders (The Cayman Islands Department of Environment, Cayman Islands Marine Conservation Board, local residents, Cayman Islands Tourism Association, and tour operator representatives) are concerned that this wildlife-based recreational activity has negative impacts on the fed stingray population. The Department of Environment has distributed a press release regarding its misgivings with stingray feeding, fearing ray fitness consequences (http://www.divecayman.ky/divemaster/press 01.asp). In 2003, the Caymanian stakeholders formed the North Sound Committee―Status Overview panel to discuss possible management options for SCS. The resultant ecologic plan (in addition to social and economic ones) suggested the control of the quantity of food (squid) provided to the stingrays with the intent to decrease their habituation to the constant supply of squid. This action would also serve to encourage stingrays to increase foraging in their own natural habitat, thus ensuring a more balanced diet in terms of nutritional composition. However, the panel was hesitant regarding the appropriateness or adequacy of the feeding restrictions, owing to the uncertainty of the extent to which the provisioned squid makes up the fed stingrays’ diet and whether it has a nutritional composition dissimilar to one obtained naturally in the wild. The panel could not, therefore, predict whether management, including food restrictions, would prove effective in terms of their concern about stingray well being.
The composition and nutritional requisiteness of an animal’s diet can be partly determined through an analysis of its fatty-acid (FA) profile (Cartland-Shaw and others 1998, Ishigame and others 2006). Because prey lipids and the constituent FAs are integrated during a significantly longer period than the “snap-shot” provided by direct observation of dietary intake, stomach content, and scat analysis (Schaufler and others 2005), FA compositions can provide a more complete picture of animals’ diets with time. The use of FAs as indicators of diet composition is particularly applicable to the marine environment because marine profiles have been quantitatively established, are qualitatively diverse, and possess unique FAs. FA structures can also be transferred largely unaltered across trophic levels and thus can have distinctive groupings traceable to a specific origin (Smith and others 1997). FA profiles have been used to characterize the foraging ecology and diet of a wide variety of marine species (Ackman and Eaton 1966, Iverson and Oftedal 1992, Virtue and others 2000), to assess among- and within-species population differences (Guitart and others 1999, Seaborn and others 2005) and to determine the nutritional adequacy of artificial diets fed to captive aquaculture fish (Seaborn and others 2000, Rodriguez and others 2004).
In addition to diet characterization, FA levels can be used as physiological biomarkers because they directly reflect the physiologic status of an animal and are therefore useful indices of marine animal population health (Ballantyne and others 1996, McKinley and others 1993). Essential FAs (EFAs) are preformed long-chain FAs important for normal growth, development, and reproduction. Because EFAs cannot be biosynthesized in sufficient quantities to ensure optimal physiologic performance (Sargent and others 1999, Arts and others 2001), marine fishes (including elasmobranchs, i.e., sharks and rays) require dietary sources of EFAs (Ballantyne 1997, Sargent and others 1995). The EFA requirements of marine fish can be met by supplying, by way of the diet, three long-chain polyunsaturated FAs (PUFAs) of the n−6 and n−3 varieties: 20:4n−6 (arachidonic acid [AA]), 20:5n−3 (eicosapentaenoic acid [EPA]), and 22:6n−3 (docosahexaenoic acid [DHA]). The relative and absolute amounts of these EFAs can be linked to the metabolic demands of disease resistance and immune response (Lall 2000) and are species and habitat specific (Sargent and others 1999). A comparison of EFA compositions of tissues of artificially fed marine fish with those of naturally foraging fish can provide an estimation of the suitability of the provisioned diet, assuming that the natural diet presumably contains the desirable composition for the lipid nutrition of the fish species in question (Rodrigez and others 2004).
We examined the capacity of FA profile analysis to serve as an ecologic indicator for the management of Southern Stingrays fed by Cayman Island tourists. This is the first study using FAs to investigate human-induced physiologic changes in a wildlife-tourism context. Our objectives were to (1) compare FA composition in the serum, measured as nonesterified FAs (NEFAs), between unfed Cayman Island rays from nontourist sites and rays fed by tourists; (2) compare the stingray FA profiles with the FA signature of squid, the provisioned food source, to establish any similarities in FA composition; and (3) explore the degree of correspondence in FA composition between the provisioned and natural diets through a comparison of EFA profiles of fed and unfed Cayman Island rays with those of warm-water elasmobranchs (sharks and rays), cold-water elasmobranchs, and squid. Our results provided information for Caymanian stakeholders to determine regulations to minimize any potential impacts of food provisioning as well as the means by which to monitor their effectiveness.
Materials and Methods
Study Species and Study Site
The Southern Stingray is a long-lived, common inshore ray frequenting tropical and subtropical shallow bays of the Southern Atlantic Ocean, the Caribbean, and the Gulf of Mexico. The Southern Stingray is a continuous forager, feeding opportunistically on a varied diet of crustaceans and teleosts, and to a lesser extent, on molluscs and annelids (Gilliam and Sullivan 1993). Stingrays are typically solitary animals, forming groups only for mating purposes annually or for antipredator protection when protective cover is lacking (Semeniuk and Dill 2005). Although Southern Stingrays inhabit all shallow bays around the Cayman Islands, it is only in the vicinity of SCS that these rays can be found year-round in a dense aggregation of individuals of both sexes. This amassment results from the unregulated quantity of provisioned squid (Illex and Loligo spp.), a nonnatural diet item shipped in from the North Atlantic and North Pacific (C. A. D. S., personal observation; Gina Ebanks-Petrie, Director, Cayman Islands Department of Environment, personal communication). The feeding routine (daily, except for the off season when weekends are excluded) lasts from early morning until midafternoon as tour boats continuously deliver tourists (mainly cruise line passengers) for an average 45–minute visit to SCS. As a result of this regime, the fed rays have become diurnal (compared with their nonfed counterparts around the island) and have confined their activity space to the shallow waters immediately surrounding the feeding area (Corcoran 2006). Nearly 170 individuals from the fed group have been tagged since 2002. The rays have a mean yearly recapture rate of 92.5% (0.03% SD; CADS unpublished data, Corcoran 2006), indicating a very strong temporal and spatial fidelity to the feeding site.
Sample Collection
During a 3-month period from May until July 2004, blood samples were collected from a total of 168 stingrays (90 from SCS) as part of a larger study on the physiological effects of stingray feeding. For this study, a subsample of serum aliquots was selected from 25 immature and adult stingrays at SCS and from two control nontourist sites on the southern (opposite) side of Grand Cayman (under a Cayman Islands research permit and Simon Fraser University animal care protocol 708R-04). Stingrays with similar disc widths were selected from the fed and unfed sites so that diets would be comparable. Because stingrays from the two southern nontourist sites do not interact with the tourists in SCS (radiotelemetry tracking data; Corcoran 2006), all of their food is presumed to come from natural sources. Sampled rays included both male and female animals in a range of disc sizes:
-
1.
Fed sitefemales: n = 7, size (disc width) = 87 to 95 cm, weight = 21 to 31 kg
-
2.
Fed sitemales: n =3, size = 56 cm, weight = 5 to 5.4 kg
-
3.
Unfed site 1females: n = 5, size = 87 to 95 cm, weight = 19.5 to 26 kg
-
4.
Unfed site 1males: n =3, size = 48 to 54 cm, weight = 4 to 5.4 kg
-
5.
Unfed site 2females: n = 4, size = 88 to 93 cm, weight = 22 to 26 kg
-
6.
Unfed site 2males: n =3, size = 38 to 46 cm, weight =1.4 to 2.7 kg.
The fed rays at SCS are habituated to human presence and easily captured by simply holding them against one’s chest when they approach for food. Once caught, a ray was placed in a landing net (1-m diameter) and transferred into a seawater-filled canvas pool (4 m2) aboard a 24-foot long, 225-hp dusky boat. Control rays from nontourist sites were located visually from a 14-foot long, 45-hp double-hull boat and then encircled in a hand-drawn seine net (30 feet long), guided into a landing net, and transferred aboard into the holding pool. Once transferred, binder clips were placed over the barb on the ray’s tail for protection, and in an average of 15 minutes, the ray was identified or tagged, weighed, and its disc width measured, injuries recorded, dermal parasites counted, and venous blood collected from the underside of the tail. All but one ray from the tourist site had been previously captured and tagged within the last year. On completion, stingrays were placed back into the landing net, had the tail clip removed, and were gently returned to the water. When released, fed stingrays resumed feeding at once, and nonfed rays swam away from the immediate area. For all rays, blood was drawn from the caudal vein using 21G x 1.5–inch needles into 3-mL serum vacutainers, and samples were kept chilled until their return to the wet laboratory at Georgetown, Cayman Islands, where they were immediately centrifuged. The separated serum was then stored at –70°C. Samples chosen for analysis came from rays that were caught on 22 separate occasions, with no more than 2 samples originating from the same day. Five capture events occurred in May, 8 in June and 9 in July, all between 9:00 AM and 4:30 PM.
FA Analysis
Serum NEFAs were methylated as described by Singer and others (1990) and then extracted from sera by way of the addition and subsequent centrifugation of hexane. The methyl esters were next redissolved in 25 μl carbon disulfide. Next, 1 μl solution was injected into a gas chromatograph (6890N; Agilent Technologies, Palo Alto, CA) fitted with a flame ionization detector and an automatic injector. Methyl esters were separated on a DB-23 column (J&W Scientific, Folsom, CA). The column temperature was initially set at 50°C, increased to 180°C during 10 minutes, held at 180°C for 5 minutes, and then increased during 5 minutes to 240°C, where it was held for 5 minutes. FAs were identified by comparing their retention times with those of known standards (GLC 463 augmented with 22:5(n−6) and 23:0 [Nu-Check Prep, Elysian, MN]). Absolute FA amounts were calculated by adding a known amount (15 μg) of internal standard heptadecanoic acid (17:0) to the serum samples before methylation. Preliminary analyses showed only trace amounts of endogenous 17:0 in the samples.
Data Analysis
Comparisons of total FA concentrations per site were made using analysis of variance (ANOVA) in JMP 6.0 (version 6; SAS, Cary, NC) to determine differences in the sum total concentration of 23 FAs between tourist and nontourist sites. Four FAs were not included in the analysis because their detection was negligible (see Appendix 1). Data were tested first for univariate normality and homogeneity of variance before proceeding. To compare FA composition (i.e., different types of FAs) between sites, we minimized the number of FA variables, and using nonparametric multivariate ANOVA (MANOVA) with an unbalanced design and with location as a fixed factor (DISTLIM; Anderson 2004a), carried out a compositional analysis of FA concentrations with FAs grouped as either saturates, monoenes (monounsaturates), or PUFAs n−3 and n−6. Because of the small sample size and our inability to confirm multivariate normality and homogeneity of covariances, we selected a nonparametric technique. The nonparametric MANOVA analysis uses a test statistic analogous to Fisher’s F ratio calculated from a distance matrix generated from the original data. P values were obtained using permutations. Because nonparametric MANOVA analysis is sensitive to differences in multivariate dispersion between groups (which can inflate type-1 error even when centroids have identical locations), the same model was tested for differences in dispersion using the program PERMDISP (Anderson 2004b). In this test, an F statistic is generated to compare the average distance of observation units to their group centroid (or spatial median), which is defined in the space identified by the chosen dissimilarity measure. A p value is then obtained by permuting appropriate residuals. Significant results would indicate that the significant effect observed in DISTLIM was an artefact of variable dispersions. The effects of location on the individual FA groups (in nmol/mL) were further explored within each population using posthoc nonparametric Kruskal-Wallace tests in SPSS (v.14, SPSS, Chicago, IL), with α = 0.0125 (α = 0.05/4; Bonferroni method to decrease the risk of a type-1 error). Mann-Whitney U tests were then used to examine the nature of the differences for each FA group as indicated by the significant nonparametric ANOVAs. Effect sizes were also calculated with confidence intervals (CIs) based on the Mann-Whitney U statistic after Newcombe (2006). θ, the test statistic, can be regarded as a measure of separation, or equally, a measure of discriminatory ability. Its value ranges from 0 to 1, with values of 0 and 1 indicating no overlap, and a value of 0.5 indicating that the two variables are identically distributed.
FA concentrations (nmol/mL) were analyzed using classification and regression tree (CART) analysis with SEE 5.0 (Rulequest Research 1997) to provide a quantitative estimate of diet similarity and to determine which set of FAs were most important in discriminating diets between tourist and nontourist sites. CART analysis is a nonparametric multivariate classification technique that allows the statistical interpretation of FA patterns containing a high number of variables (FAs) per observation (Smith and others 1997). This analysis sequentially selects the “best” variable as well as the best splitting point of that variable to separate the data into two groups (or nodes) that are as divergent as possible (Kirsch and others 1998). The splitting continues until one of two stopping criteria (based on deviance and number of observations) is met. A classification as well as a misclassification rate were made. CART analysis is particularly appropriate for data in which the number of variables exceeds the number of samples. A second CART analysis was done on selected FAs that contributed ≥80% of the total NEFA concentration to ascertain whether diets differed with respect to the most abundant FA. A final CART analysis was performed using mean percent of the most dominant FAs to compare the FA signature of both fed and unfed stingray groups to the FA signature of squid (Illex spp.; obtained from Kirsch and others 1998 and renormalized using the same subset of 23 FAs).
Finally, we conducted a discriminant analysis in JMP 6.0 comparing the nutritional balance of percent dietary EFA between fed and unfed Cayman Island rays, squid, and cold- and warm-water elasmobranchs to determine the extent to which fed stingrays could be distinguished from the other elasmobranch and squid groups using their EFA profiles as discriminating variables. Group classification was computed, and differences in mean canonical scores between groups were examined using ANOVAs with α = 0.01 (α = 0.05/5). Posthoc comparisons were made using Scheffé’s test (α = 0.05) when results were significant. We were less concerned about violating the key assumption of equality of variance–covariance matrices in this case because our purpose was to use discriminant analysis as an exploratory means to describe the gradients of variation in the data set; moreover, in wildlife research, there is little evidence that moderate violations significantly change classification success (McGarigal and others 2000). A scatterplot of mean canonical scores for the significant discriminant functions was constructed to show the positions and orientations of the integrated profiles of individuals in their species groups relative to each other. The relative percentage values of AA (20:4n6), EPA (20:5n3), and DHA (22:6n3) of squid and other elasmobranchs were obtained from various published and unpublished sources. Data for cold-water elasmobranchs Amblyraja radiata and Raja rhina (homogenized tissue total FA) were used from Budge and others (2002), and Squalus acanthias, Leucoraja erinacea, Raja rhina, and Bathyraja spp. (plasma NEFA) data were from Speers-Roesch (2005). Warm-water elasmobranch Chiloscyllium punctatum and Taeniura lymma (plasma NEFA) data were obtained from Speers-Roesch (2005); Dasyatis zugei (muscle total FA) data came from Gibson and others (1984); and Dasyatis kuhlii (muscle total FA) data came from Hansel and others (1993). Squid (Illex illecebrosus [homogenized tissue total FA]) data were used from Jangaard and Ackman (1965) and Kirsch and others (1998), and Moroteuthis ingens (mantle total FA) data were from Phillips and others (2001).
Results
The sum total serum NEFA concentrations of the 10 fed and 15 unfed stingrays were similar (mean ± SEM total NEFA concentration [nmol/mL]): fed = 455.4 ± 18.9; unfed 1 = 465.9 ± 21.2; unfed 2 = 463.2 ± 22.6; ANOVA: F 2,22 = 0.075; p = 0.93). However, the NEFA profiles, i.e., total saturates, monoenes, n−3 FAs, and n−6 FAs, of the fed rays exhibited marked group compositional differences (nonparametric MANOVA F = 11.9; p <0.01; Table 1). The overall test for differences in dispersion among groups was nonsignificant (F = 2.02, p = 0.16), indicating that the effect of location caused a shift in the FA composition. All FAs but total saturated FAs were found to differ significantly between the fed and unfed sites (Kruskal-Wallace H = 13.7, p < 0.001). The two nontourist sites did not differ significantly for any FA subgroup (see Appendix 1). Fed rays had significantly higher concentrations of n−3 PUFAs but significantly lower amounts of monoenes and n−6 PUFA (Table 1). Effect sizes between fed and unfed stingrays for each significant FA group were large (θ = 0.01 to 0.11; 95% CI 0.0 to 0.39).
We initially investigated variation in FA profiles using concentrations (nmol/mL) of all 23 FAs. CART analysis resulted in the algorithm selecting a minor component (20:3n6) that maximized the change in deviance between groups at the root node (90.9%). Classification required only 4 FAs (Table 2 [all 23 FAs]) and resulted in a simple tree of 5 terminal nodes. Fed stingrays were immediately resolved into a terminal node, with the 2 nontourist sites needing further classification to enable differentiation. Only 2 of the 25 stingrays were misclassified; 1 error occurred between rays from the nontourist sites, and 1 fed ray was classified nearer to the root node as an unfed ray from the second nontourist site.
In a second CART analysis, we used six of the 23 FAs that contributed to ≥80% of total FAs in all 3 subpopulations (16:0, 18:0. 18:1, 20:4n−6, 20:5n−3, and 22:6n−3). The same trend was observed when using the full set of FAs with similar classification accuracy. A tree using 20:5n3 at the root node generated 5 terminal nodes using only 3 FAs, with an overall classification success rate of 92% (Table 2 [6 most common FAs]). All fed rays were correctly classified and terminated in a single node according to 1 FA (20:5n3). Again, only 2 individuals were incorrectly classified, this time as a result of difficulty differentiating rays between the 2 nontourist sites.
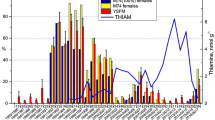
In the CART analysis comparing the FA signature of Cayman Island rays with the FA signature of squid (the tourist-provided diet), we again used the six dominant FAs as in the previous test but added the additional sample of the percent FA composition of squid (I. illecebrosus, using the same six FAs). A similar classification accuracy resulted, with two FAs needed to fully resolve all Cayman Island ray groups, and one FA to distinguish fed from nonfed rays. One misclassification error occurred, with CART analysis classifying squid as a fed ray (Fig. 1).
Regression tree showing generalized relationships between fed and unfed stingrays of the Cayman Islands. Tree shows mean percent common FAs of Southern Stingrays from subpopulations that are either fed squid by tourists (fed rays; n = 10) or not (nontourist site 1: unfed 1, n = 8; and nontourist site 2: unfed 2, n = 7), and of squid (Illex spp.; Kirsch and others 1998), n = 1), i.e., the nonnatural food source. The FA and the cutpoint are given for each node in the tree, with the “>” sign referring to the right-hand decision. The fractions under each terminal node refer to the total number of observations for that node (numerator) over the number and source of misclassifications (denominator)
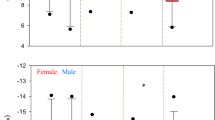
Discriminant analysis resulted in the five species groups being classified correctly 82% of the time (Table 3). As expected, unfed rays were misclassified more frequently than the other groups, owing to their similarity in EFAs to other warm-water elasmobranch species. Two significant discriminant functions were produced (Wilk’s Lambda = 0.04; F 12,82 = 15.1; p <0.01; Fig. 2), with fed Southern Stingrays having a statistically similar first mean canonical score to cold-water elasmobranchs and a statistically similar second mean canonical score to squid (ANOVA, both p < 0.001; Scheffé’s test with α < 0.05; Table 3). The scatter plot of mean canonical scores showed that the two discriminant functions could be used to differentiate between the five species groups (although there was a great deal of overlap between warm-water and unfed elasmobranchs). The first canonical axis was also more effective than the second at separating the five groups. Although the assumption of homogeneity of group dispersions was not met (Box’s M-test p < 0.05), the canonical functions that resulted from the discriminant analysis are of ecologic significance because they (1) have an ecologically meaningful and consistent interpretation; (2) contribute more to group separation than any other canonical functions that fail to satisfy; and (3) result in significant separation of at least two groups consistent with the ecologic interpretation of the functions (McGarigal and others 2000).
Segregation of integrated EFA profiles for warm (n = 4) and cold-water (n = 6) elasmobranchs; fed (n=10) and unfed (n=15) D. Americana; and squid (n = 3). Circles represent the profile means and their 95% CIs. Contributions of individual EFAs to the separation of locality centroids are indicated by the magnitude and direction of the EFA vectors, which are scaled relative to the pooled within-group SDs
Discussion
This study examined the value of using FA profiles as an indicator in describing the diet composition of provisioned stingrays and as an indicator of diet similarity between naturally feeding elasmobranchs and provisioned stingrays in terms of lipid nutrition, specifically EFAs. Our results support the utility of FA analysis in the assessment of human-induced physiological change in a wildlife tourism context. Although we acknowledge that no “control” group is truly representative of the natural state of the environment because of global anthropologic impacts, we nonetheless believe the rays used as baseline correspond to the closest natural condition of the environment and are foraging on species still common to the area. Our analysis detected significant differences in serum FA composition between the unfed and fed Southern Stingrays in Grand Cayman as a result of their contrasting diets. With there being no difference in the sum concentration of FAs between rays from the tourist and nontourist sites, we nonetheless found substantial differences between the NEFA profiles. These results therefore indicate that fed rays had significantly higher relative and absolute concentrations of PUFA n−3 and lower PUFA n−6 and monoene concentrations than unfed rays. Although the differences between the fed and unfed Cayman Island rays were significant, the FA profiles within these groups were internally consistent. These findings suggest that the tree classifier model assigned rays according to their FA profile into fed or nonfed groups with high accuracy. Indeed, using CART analysis models, discrimination between the two unfed ray populations was possible with some error, but both groups were distinctly different from fed rays, which in turn had a minimal misclassification rate. The differences in NEFA profiles between fed and unfed Cayman Island rays were so pronounced that the two groups could be differentiated solely by the comparison of the proportion of one FA. Although these results are typical of evaluations made between farm-reared and wild sea turtles and teleosts (Joseph and others 1985, Seaborn and others 2000, Rodrigurez and others 2004), or between geographically and seasonally separated individuals (Bradshaw and others 2003), its usefulness in detecting human-induced changes from wildlife tourism is as yet underappreciated.
Our FA analysis also indicates that squid provided by tourists are the SCS stingrays’ major food source. Although the technique we used cannot quantify the diet per se (as discussed later), we can describe the FA composition with confidence. To begin with, CART analysis discriminated between fed and unfed rays but was unable to differentiate squid into its own terminal node, instead mistakenly classifying squid as a fed ray. Although this result is based on one sample for squid and may be an oversimplification, it nevertheless reveals that the contribution of squid to the diet is extremely high because the FA profile of fed stingrays more closely resembled that of squid rather than their unfed counterparts (whose profiles reflected the assimilation of natural-prey FAs into their lipid metabolism). Next, canonical discrimination analysis further demonstrated that fed Cayman Island rays’ essential FA profiles were significantly different from those of warm-water species groups, but again they most closely resembled EFA profiles of squid and other cold-water elasmobranchs. Finally, FAs can have distinctive groupings traceable to a specific origin (Smith and others 1997); therefore, it is not unreasonable to suggest that the rays’ food source was predominantly the provisioned one.
Although FAs from a storage tissue (e.g., liver) would clearly demonstrate the long-term dependency of Southern Stingrays on squid, we suggest that serum NEFAs are equally valuable. First, plasma NEFAs in fish show percentages of FAs that match very closely the levels seen in muscle and liver (Henderson and Tocher 1987, Greene and Selivonchick 1987). Second, plasma NEFAs partially represent mobilized FAs from the liver and not just recently acquired dietary lipids (Greene and Selivonchick 1987). Third, the stingray groups in our study (from tourist and nontourist sites) were sampled at various times during the day during a 3-month period, and the fed rays (all but one) had been previously caught and tagged within the previous year. This suggests that our sample of stingrays came from a resident population, and that our results reflect a long-term integration of dietary FAs. In sum, because we have tested what the rays were eating during an extended period of time, and in every instance the profile returned had resembled that of squid, it is reasonable once again to assume that the rays were feeding predominantly on squid. Our serum NEFA data, therefore, reflect the body composition of FAs in the Southern Stingray and provide conclusive evidence of a strong, enduring incorporation of tourist-fed squid in their diet.
Our results further reveal that fed, tropical Cayman Island rays do not exhibit the typical essential FA profile of their unfed counterparts but instead possess a profile that more closely resembles those of elasmobranchs and squid from cooler waters. Patterns resulting from the canonical discrimination analysis of EFAs revealed that marine elasmobranchs living at latitudes characterized by cold waters have a higher proportion of DHA and EPA than AA, whereas elasmobranchs in warm waters have relatively higher concentrations of AA and lower concentrations of DHA and EPA (Fig. 2 and Table 4). Not surprisingly, squid from temperate-zone and sub-Antarctic waters had EFA profiles similar to those of other cold-water species in this analysis. These trends are widespread in the marine environment: n−6 FAs (especially AA and its essential precursor 18:2n−6) are more common in inshore tropical versus cold-water marine food webs, whereas high concentrations of PUFA n−3, such as EPA and DHA, are generally found in marine organisms inhabiting cooler environs (including flagellates and diatoms that are a major trophic source of these FAs and their precursors; Sinclair and others 1984; Castell and others 1994). An outcome of the Southern Stingray’s diet of squid is a shifted essential FA profile that does not correspond to what occurs naturally in unfed Southern stingrays’ serum.
Management Implications
The goal of our study was to determine the efficacy of FA profile analysis as an indicator tool for the ecologic management of SCS in the Cayman Islands. Specifically, we set out to address concerns raised by Caymanian stakeholders as to the degree to which fed Southern Stingrays were habituated to the nonnatural provisioned food source and whether this source provided a diet comparable with that of the wild. Our results revealed that squid represents a major prey item in the fed-stingray diet and that this food source does not provide a diet comparable with one of the nonfed wild rays with respect to monoene, n−3 FA, and n−6 FA concentrations (in particular, EFAs). Health implications and management options that stem from these findings are discussed here.
The longevity of stingrays (many decades), in combination with their protracted gestational time and small litter sizes, suggests that diet-related impacts on growth, reproduction, and survival rates would take many years to detect. For these reasons, our discussion here of the possible consequences of differences in FA composition, while compelling, are speculative and based on what is known for piscivorous fishes. Potential areas for further research on fed Southern Stingrays are identified.
Recent developments in research exploring fish nutrition suggest that differences in EFA requirements for different fish species reflect dietary and metabolic adaptations to distinctive habitats and ecosystems (Sargent and others 1999, Harel and others 2002, Bell and Sargent 2003). Moreover, the optimal tissue requirement for any individual long-chain PUFA cannot be considered meaningfully in isolation (Sargent and others 1999). Instead, the relative levels (i.e., ratios of all three EFA) must be considered because conjointly they are crucial requirements of lipid nutrition that influence immune health, disease resistance, and membrane fluidity in an optimal manner (Sargent and others 1999, Tocher 2003, Rodriguez and others 2004). As such, care should be exercised in deviating too far from “natural” ratios of n−3 o n−6 PUFA (for example, in commercial fish diets; Sargent and others 1995). With regard to the fed Cayman Island Southern Stingrays, they are acquiring a diet that is disproportionate in FA and EFA composition compared with the diet of naturally feeding Cayman Island Southern Stingrays (which presumably reflects a dietary and metabolic adaptation to the environment). Fed stingrays are not only receiving less absolute amounts of AA and higher amounts of EPA and DHA from their diet (note the similar sum total NEFA concentrations between the stingray groups; see also Appendix 1), but their essential FA ratios (AA:EPA and AA:DHA) are also relatively skewed in the opposite direction from related warm-water species (Table 4). Furthermore, these FA profiles are present as plasma-free FAs, a metabolically dynamic fraction of serum lipids, and thus represent mobilized FAs that are actively important in metabolic processes (Henderson and Tocher 1987, Ballantyne and others 1996). Based on the premise that a diet should match the environment and its requirements, there may be long-term effects on the fed stingrays’ growth, immune function, resistance to parasites and disease, and eventual survival. The implications of these findings, and the possibility of other physiological differences between the fed and unfed Southern Stingrays, should be further studied (e.g., an assessment of macrophage and lysozyme activities to determine circulating levels of leukocytes, and quantifying serum cytokines; Rice and Arkoosh 2002), and considered in future management plans.
Potential management options stemming from these findings include decreasing the amount of artificial food so that stingrays become less habituated and forage in their natural habitat (as originally suggested by the North Sound Committee – Status Overview panel) and/or changing the composition of the provisioned food to a higher quality, varied diet more characteristic of a tropical environment as a precautionary approach. Because serum FA profiles provide evidence of tourist effects and are labile enough to reflect changes in diet (Kirsch and others 1998), they also can be used as a monitoring tool to gauge whether there is continued evidence of stingrays feeding disproportionately on squid and whether any management actions concerning food quality are having an effect. If properly managed, tourist-provisioned food could be used in a positive manner (i.e., the appropriate ratio of EFAs), and FA markers would serve as a bioindicator of such use.
Conclusion
In this article, we chose to focus on FA analysis as an ecologic indicator of (physiologic) change in tourism settings where feeding wildlife is the main attraction. However, we must stress that this is only one indicator and that the behavioural effects of fed animals and resulting ecologic effects (e.g., effects on habitat, incidental effects on nontarget species, effects of surpassing carrying capacity, and predator–prey dynamics, etc.) are equally important. We nonetheless wished to demonstrate the physiologic changes that can be brought about by artificial feeding (and diet), to caution about the possible negative impacts of skewed (essential) FA ratios of an unbalanced diet, and to discuss the use of FA analysis as an indicator and monitoring tool. FA analysis can be used in a qualitative manner (such as in this study) in a wide range of animals to describe temporal and spatial patterns in the diet and is therefore especially relevant to other studies in which artificial feeding of wildlife is an issue. This technique is particularly appropriate for when direct observation of feeding is rarely possible and when indirect methods of scat or regurgitation analysis are prone to differential rates of digestion among prey species that can seriously bias estimates in favor of species with large and robust hard parts (Iverson and others 2004). Although tissue analysis of FAs is preferred, when biopsy samples are not an option, plasma or serum FAs can be used provided that samples are collected during a range of days and at various times during the day to minimize reflecting a point-source feeding bout. Because the techniques required are instrument intensive, we recommend collaborating with other laboratories that are adequately equipped. Another type of FA analysis involves quantifying the composition of prey species in the diet. However, this task is more challenging and necessitates multiple sources of information, such as an understanding and correction for the effects of predator lipid metabolism on FA deposition, consideration of variability in FA composition within and among prey species, appropriate sampling and chemical analysis of predator and prey lipid tissue, and a statistical estimation model (quantitative FA signature analysis; Thiemann and others 2004). Nevertheless, we believe that FA analysis is an important first step in determining whether changes are present in lipid nutrition and in pinpointing possible downstream effects that require further investigation.
References
Ackman RG, Eaton CA (1966) Lipids in the fin whale (Balaenoptera physalus) from North Atlantic waters. III. Occurrence of eicosenoic and docosenoic fatty acids in the zooplankton Meganyctiphanes norvegica (M. Sars) and their effect on whale oil composition. Canadian Journal of Biochemistry 44:1561–1566
Anderson MJ (2004a) DISTLIM v.5: a FORTRAN computer program to calculate a distance-based multivariate analysis for a linear model. Department of Statistics, University of Auckland, New Zealand
Anderson MJ (2004b) PERMDISP: a FORTRAN computer program for permutatinoal analysis of multivariate dispersions (for any two-factor ANOVA design) using permutation tests. Department of Statistics, University of Auckland, New Zealand
Ballantyne JS (1997) Jaws, the inside story. The metabolism of elasmobranch fishes. Comparative Biochemistry and Physiology B, Comparative Biochemistry 118:703–742
Ballantyne JS, Mercure F, Gerrits MF Van Der Kraak G McKinley S Martens DW, et al. (1996) Plasma nonesterified fatty acid profiles in male and female sockeye salmon, Oncorhynchus nerka, during the spawning migration. Canadian Journal of Fisheries and Aquatic Sciences 53:1418–1426
Basiron MN (1997) Marine tourism industry–trends and prospects. Paper presented at the National Seminar on the Development of Marine Tourism Industry in South East Asia at Langkawi. September 25 to 28
Bell JG, Sargent JR (2003) Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture 218:491–499
Bradshaw CJA, Hindell MA, Best NJ, Phillips KL, Wilson G, Nichols PD (2003) You are what you eat: describing the foraging ecology of southern elephant seals (Mirounga leonina) using blubber fatty acids. Proceedings of the Royal Society of London B 270:1283–1292
Budge SM, Iverson SJ, Bowen WD, Ackman RG (2002) Among- and within-species variability in fatty acid signatures of marine fish and invertebrates on the Scotian Shelf, Georges Bank, and southern Gulf of St. Lawrence. Canadian Journal of Fisheries and Aquatic Sciences 59:886–898
Cartland-Shaw LK, Cree A, Skeaff CM, Grimmond NM (1998) Differences in dietary and plasma fatty acids between wild and captive populations of a rare reptile, the tuatara (Sphenodon punctatus). Journal of Comparative Physiology B, Biochemical, Systemic, and Environmental Physiology 168:569–580
Castell JD, Bell JG, Tocher DR, Sargent JR (1994) Effects of purified diets containing different combinations of arachidonic and docosahexaenoic acid on survival, growth and fatty acid composition of juvenile turbot (Scophthalmus maximus). Aquaculture 128:315–333
Cayman Islands Ministry of Tourism (2002) Focus for the future–a tourism policy framework for the Cayman Islands. The Tourism Company, London, UK
Corcoran M (2006) The effects of supplemental feeding on the activity space and movement patterns of the Southern stingray, Dasyatis americana, at Grand Cayman, Cayman Islands. Master’s thesis, Nova Southeastern University, Fort Lauderdale, FL
Dunkley L, Cattet MRL (2003) A comprehensive review of the ecological and human social effects of artificial feeding and baiting of wildlife. Canadian Cooperative Wildlife Health Center, Saskatoon, Saskatchewan, Canada
Fillion FL, Foley JP, Jaquemot AJ (1992) The economics of global tourism. Paper presented at the fourth World Congress on National Parks and Protected Areas, Caracas, Venezuela, February 10 to 21
Garrod B, Wilson JC (2004) Nature on the edge? Marine ecotourism in peripheral coastal areas. Journal of Sustainable Tourism 12:95–120
Gibson RA, Kneebone R, Kneebone GM (1984) Comparative levels of arachidonic acid and eicosapentaenoic acid in Malaysian fish. Comparative Biochemistry and Physiology C, Comparative Pharmacology 78:325–328
Gilliam D, Sullivan KM (1993) Diet and feeding habits of the Southern stingray Dasyatis americana in the Central Bahamas. Bulletin of Marine Science 52:1007–1013
Green RJ, Higgenbottom K (2000) The effects of non-consumptive wildlife tourism on free-ranging wildlife: a review. Pacific Conservation Biology 6:183–197
Greene D, Selivonchick D (1987) Lipids metabolism in fish. Progress in Lipid Research 26:53–85
Guitart R, Silvestre AM, Guerrero X, Mateo R (1999) Comparative study on the fatty acid composition of two marine vertebrates: striped dolphins and loggerhead turtles. Comparative Biochemistry and Physiology B, Comparative Biochemistry 124:439–443
Hansel M, Rao KS, Matsuoka T, Rali T, Burrows I, Huber ME (1993) The distribution of fatty acids in flesh and liver of Papua New Guinean fish. Comparative Biochemistry and Physiology B, Comparative Biochemistry 106:655–658
Harel M, Koven W, Lein I, Bar Y, Behrens P, Stubblefield J, et al. (2002) Advanced DHA, EPA and ArA enrichment materials for marine aquaculture using single cell heterotrophs. Aquaculture 213:347–362
Henderson RJ, Tocher DR (1987) The lipid composition and biochemistry of freshwater fish. Progress in Lipid Research 28:281–347
Ishigame G, Baxter GS, Lisle AT (2006) Effects of artificial foods on the blood chemistry of the Australian magpie. Austral Ecology 31:199–207
Iverson SJ, Field C, Bowen WD, Blanchard W (2004) Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecological Monographs 74:211–235
Iverson SJ, Oftedal OT (1992) Fatty acid composition of black bear (Ursus americanus) milk during and after the period of winter dormancy. Lipid 27:940–943
Jangaard PM, Ackman RG (1965) Lipids and component fatty acids of the Newfoundland squid, Illex illecebrosus (Le Sueur). Journal of the Fisheries Resource Board of Canada 22:131–137
Joseph JD, Ackman RG, Seaborn GT (1985) Effect of diet on depot fatty acid composition in the green turtle, Chelonia mydas. Comparative Biochemistry and Physiology B, Comparative Biochemistry 80:15–22
Kirsch PE, Iverson SJ, Bowen WD, Kerr SR, Ackman RG (1998) Dietary effects on fatty acid signature of whole Atlantic cod (Gadus morhua). Canadian Journal of Fisheries and Aquatic Sciences 55:1378–(1386)
Lall SP (2000) Nutrition and health of fish. Paper presented in Avances en Nutrición Acuicola Memorial V del V Simposium Internacional de Nutrición Acuicola. Cruz-Suarez, LE, Ricque-Marie D, Tapia-Salazar M, Olvera-Novoa MA, Civera-Cerecedo R (eds.) Mérida, Yucatán, Mexico, November19–22 to (2000). Available at: http://www.w3.dsi.uanl.mx/publicaciones/maricultura/acuiculturaV/lall.pdf. Accessed: August 2006
McGarigal K, Cushman S, Stafford S (2000) Multivariate statistics for wildlife and ecology research. Springer-Verlag, New York, NY
McKinley RS, Singer TD, Ballantyne JS, Power G (1993) Seasonal variation in plasma nonesterified fatty acids of Lake sturgeon (Acipenser fulvescens) in the vicinity of hydroelectric facilities. Canadian Journal of Fisheries and Aquatic Sciences 50:2440–2447
Miller ML (1993) The rise of coastal and marine tourism. Ocean and Coastal Management 20:181–199
Newcombe RG (2006) Confidence intervals for an effect size measure based on the Mann-Whitney statistic. Part 2. Asymptotic methods and evaluation. Statistics in Medicine 25:559–573
Newsome D, Dowling R, Moore S (2005) Wildlife tourism. Channel View Publications, Clevedon, USA
Orams M (1999) Marine tourism―development, impacts and management. Routledge, New York, NY
Orams MB (2002) Feeding wildlife as a tourism attraction: a review of issues and impacts. Tourism Management 23:281–293
Phillips KL, Jackson GD, Nichols PD (2001) Predation on myctophids by the squid Moroteuthis ingens around Macquarie and Heard islands: stomach contents and fatty acid analyses. Marine Ecology Progress Series 215:179–189
Reynolds PC, Braithwaite D (2001) Towards a conceptual framework for wildlife tourism. Tourism Management 22:31–42
Rice CD, Arkoosh MR (2002) Immunological indicators of environmental stress and disease susceptibility in fishes. In Adams SM (ed.), Biological indicators of aquatic ecosystem stress. American Fisheries Society, Symposium 8, Bethesda, MD. pp 187–220
Rodriguez C, Acosta C, Badia P, Cejas JR, Santamaria FJ, Lorenzo A (2004) Assessment of lipid and essential fatty acids requirements of black seabream (Spondyliosoma cantharus) by comparison of lipid composition in muscle and liver of wild and captive adult fish. Comparative Biochemistry and Physiology B, Comparative Biochemistry 139:619–629
RuleQuest Research. (1997) RuleQuest research data mining tools. RuleQuest Research, St. Ives, New South Wales, Australia
Sargent JR, Bell JG, Bell MV, Henderson RJ, Tocher DR (1995) Requirement criteria for essential fatty acids. Symposium of European Inland Fisheries Advisory Commission. Journal of Applied Ichthyology 11:183–198
Sargent JR, Bell G, McEvoy LA, Tocher DR, Estevez A (1999) Recent developments in the essential fatty acid nutrition of fish. Aquaculture 177:191–199
Schaufler L, Heintz R, Sigler M, Hulbert L (2005) Fatty acid composition of sleeper shark (Somniosus pacificus) liver and muscle reveals nutritional dependence on planktivores. International Council for the Exploration of the Sea, Conference Meeting, Session N:05, Elasmobranch Fisheries Science
Seaborn GT, Jahncke ML, Smith TIJ (2000) Differentiation between cultured hybrid striped bass and wild striped bass and hybrid bass using fatty acid profiles. North American Journal of Fisheries Management 20:618–626
Seaborn GT, Moore MK, Balazs GH (2005) Depot fatty acid composition in immature green turtles (Chelonia mydas) residing at two near-shore foraging areas in the Hawaiian Islands. Comparative Biochemistry and Physiology B, Comparative Biochemistry 140:183–195
Semeniuk CAD, Dill LM (2005) Cost/benefit analysis of group and solitary resting in the cowtail stingray (Pastinachus sephen). Behavioral Ecology 16:417–426
Shackley M (1998) “Stingray City”―Managing the impact of underwater tourism in the Cayman Islands. Journal of Sustainable Tourism 6:328–338
Sinclair AJ, O’Dea K, Naughton JM, Sutherland T, Wankowski J (1984) Polyunsaturated fatty acid types in some Australian and Antarctic fish. Proceedings of the Nutrition Society of Australia 9:188
Singer TD, Mahadevappa VG, Ballantyne JS (1990) Aspects of the energy metabolism of lake sturgeon, Acipenser fulvescens, with special emphasis on lipid and ketone body metabolism. Canadian Journal of Fisheries and Aquatic Sciences 47:873–881
Smith SJ, Iverson SJ, Bowen WD (1997) Fatty acid signatures and classification trees: new tools for investigating the foraging ecology of seals. Canadian Journal of Fisheries and Aquatic Sciences 54:1377–1386
Speers-Roesch B (2005) Metabolic organization of the chondrichthyan fishes: evolutionary implications. M.Sc. thesis, University of Guelph, Ontario, Canada
Thiemann GW, Budge SM, Bowen WD, Iverson SJ (2004) Comment on Grahl-Nielsen and others (2003) Fatty acid composition of the adipose tissue of polar bears and of their prey: ringed seals, bearded seals and harp seals. Marine Ecology Progress Series 281:297–301
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Reviews in Fisheries Science 11:107–184
Virtue P, Mayzaud P, Albessard E, Nichols P (2000) Use of fatty acids as dietary indicators in northern krill, Meganyctiphanes norvegica, from northeastern Atlantic, Kattegat, and Mediterranean waters. Canadian Journal of Fisheries and Aquatic Sciences 57(Suppl. 3):104–114
Acknowledgments
C. A. D. S and K. D. R. acknowledge financial support from Fonds Québécois de la Recherche sur la Nature et les Technologies and the National Sciences and Engineering Research Council of Canada (NSERC), respectively. B. S.-R. was supported by a NSERC postgraduate scholarship. This work was partially supported by a PADI AWARE research grant. We thank the following individuals for their assistance in the field: J. Verspoor, R. Wrangham, M. Potenski, A. Briggs, and C. Sherrit. We thank M. Murray of the Monterey Bay Aquarium, CA, for advice on stingray blood-sampling techniques, and the Cayman Island Department of Environment and the Guy Harvey Research Institute for the use of marine and laboratory equipment. We also thank J. S. Ballantyne for use of the gas chromatograph for FA analysis. Last, we thank M. Orams and two anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Semeniuk, C.A.D., Speers-Roesch, B. & Rothley, K.D. Using Fatty-Acid Profile Analysis as an Ecologic Indicator in the Management of Tourist Impacts on Marine Wildlife: A Case of Stingray-Feeding in the Caribbean. Environmental Management 40, 665–677 (2007). https://doi.org/10.1007/s00267-006-0321-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-006-0321-8