Abstract
Background
Breast implants may be responsible for secondary deformities produced by parenchymal atrophy. However, few studies in the literature have reported changes in breast tissue after augmentation surgery. In this study, the breast thickness of patients undergoing breast augmentation was monitored by ultrasound, and correlations with surface, volume and projection measurements were examined.
Methods
We studied the parenchymal thickness at the lower pole of the breast with ultrasound in 36 women (72 breasts). In another group of 33 patients (66 breasts), we studied the thickness at the upper and lower poles along the meridian of each breast by ultrasound and measured the anthropometric metrics, volume and projection of the breast with a 3D camera.
Results
Midline measurements close to the areola showed reduced thickness at the lower pole, with 31.8% at the midpoint of the lower pole and 42% at the infra-areolar level (p < 0.0001). At the upper pole, there was a decrease of 14.6% (p < 0.001), but the thickness was increased by 6% and 38% at more cranial levels. No correlations with volume were found. Anatomical implants produced more atrophy at the lower pole, and round implants at the upper pole. More atrophy was found with subfascial than submuscular augmentation. Compared with the expected values, the final volume was very similar, but the projection was 29% less. Surface measurements changed significantly up to 4 months postoperatively and remained stable thereafter.
Conclusions
Implants affect significatively the thickness of the glandular tissue. All changes occur very soon postoperatively but stabilize after 4 months.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In our experience, it is not unusual to encounter a patient who has lost volume after breast augmentation. However, in our daily practice, we also observe changes in the lower pole of the breast during the months following the operation, and it is believed that breast implants can cause tissue atrophy [1]. Tebbets [2] established that the optimal use of any implant requires an understanding of the characteristics of the device, the dynamics of the tissues and the surgical techniques that maximize the advantages and reduce the disadvantages of each implant.
Vegas and Del Yerro [3] theorized that breast deformation in the context o f implants is related to the viscoelastic properties of the tissues and their responses to compression and loading, but no objective measurements were provided in the study. Recently, Roxo et al. [4,5,6] demonstrated with magnetic resonance imaging (MRI) that submuscular augmentation with high-profile, round implants reduced the volume of the major pectoral muscle, while subglandular implantation caused a decrease in the glandular tissue.
Ultrasound is an extraordinarily useful tool in breast implantation [7, 8]. Additionally, ultrasound is an inexpensive and accessible technique. However, although volume cannot be measured by ultrasound, the tissue thickness can be evaluated.
This study of patients undergoing breast augmentation consisted of two phases. In our practice, we have observed that during the postoperative period, a relaxation of the lower pole occurs, resulting in increased convexity in most patients undergoing breast augmentation. Additionally, some sagging of the implant seems to occur, especially with anatomical implants. We aimed to determine whether this phenomenon could be due to atrophy of the gland and whether a relationship exists with the volume of the implant. Therefore, we examined the thickness of the glandular tissue at the lower pole. After obtaining the preliminary results of this study, we measured the thickness of the glandular tissue at both the upper and lower poles in a second group of patients, and the surface measurements, volume and projection of the breast were also examined and analysed for correlations with changes in the parenchymal thickness, the implant type and anatomical position.
Materials and Methods
For the echographic study, a Mindray ultrasound device (Mindray, China) with a 4-cm-long, 10-MHz transducer was used. For the volumetric study, an Artec Eva camera (Artec, Luxembourg) was used.
This work was carried out in two phases: the first included 36 consecutive patients (72 breasts) who underwent breast augmentation with implants; in these patients, the degree of atrophy of the subcutaneous tissue along the midline of the lower pole was studied with ultrasound. In the second phase, 33 patients (66 breasts) were included, and the thickness of the glandular tissue was measured along the midline of both the lower and upper poles with ultrasound; the recordings were captured using a 3D camera. The study conformed to the Declaration of Helsinki.
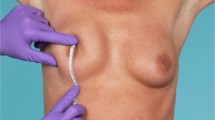
In the echographic study, the midpoint and both ends of the transducer were used as references. Thickness measurements (from the muscular or thoracic fascia to the dermis) were collected at four levels: beside the areola (point 3 at the lower pole and point 4 at the upper pole), at the midpoint (points 2 and 5 at the lower and upper poles, respectively), close to the inframammary fold (point 1) and the most cranial point at the upper pole (point 6) (Fig. 1).
Points of measurement. At the lower pole, 1, 2, and 3 correspond to caudal, medial, and cranial (areolar) measurements. At the upper pole, 4, 5 and 6 correspond to caudal (areolar), medial and cranial measurements. Points 3 and 4 correspond to the juxta-areolar level, points 2 and 5 correspond to the medial measurements, and points 1 and 6 correspond to further measurements of the areola (caudal at the lower pole, cranial at the upper pole)
In the 3D study, the volume and the geodesic distances on the surface from the sternal notch to the nipple (SN-Ni), the nipple to the inframammary fold (Ni-IMF), between the nipples (Ni-Ni) and straight from the nipple to the inframammary fold were measured (NI-IMF L) (Fig. 2).
The expected final volume (breast volume + implant volume) and the expected final projection (breast projection + implant projection) were calculated to determine the final variation between these values and the real values at 12 months.
In all cases, preoperative and postoperative measurements were collected at 1 month, 4 months and 12 months.
For statistical purposes, all the data corresponding to measurements of the lower pole were pooled from a total of 69 patients (138 breasts).
Statistical analysis was performed using SPSS 19.0 software (Armonk, NY, IBM Corporation), with a significance level of p < 0.05 (alpha, 5%). Student’s test was performed to compare the means and Pearson’s correlation was used to assess relationships between the variables.
Results
Lower Pole
The group consisted of 69 patients (138 breasts). The average patient age was 30.5 years (20–50). The average body mass index (BMI) was 20 (16.5–29).
The mean implant volume was 325 cc (210–480). Anatomical implants were used in 106 breasts (76.8%), and round implants were used in the remaining breasts (32 breasts, 23.2%). The mean volume and projection for the anatomical implants were 324.4 cc (210–480) and 5.18 cm (3.8–6.7), and the corresponding values were 323 cc (220–430) and 4.36 cm (3–5.4) for round implants.
For the anatomical implants, the following profiles were used: an extra-high profile in 22 breasts (20.8%), a high profile in 66 breasts (62.3%), a moderate profile in 17 breasts (16%) and a low profile in 1 case (0.9%). Polytech was used in 44 breasts (41.5%), Allergan was used in 24 breasts (22.6%), Mentor was used in 18 breasts (17%), Eurosilicone was used in 10 breasts (9.4%), Sebbin was used in 8 breasts (7.5%), and B-lite was used in 2 breasts (1.9%).
For round implants, the following profiles were used: an extra-high profile in 2 breasts (6.7%), a high profile in 10 breasts (33.3%), a moderate profile in 17 breasts (56.7%) and a low profile in 1 breast (3.3%). Polytech was used in 12 breasts (37.5%), Mentor was used in 6 breasts (18.8%), Allergan was used in 6 breasts (18.8%), Sebbin was used in 6 breasts (18.8%) and Nagor was used in 2 breasts (6.3%).
The areolar, axillary and inframammary approach was used in 18 (13.05%), 102 (73.9%) and 18 (13.05%) breasts, respectively. The augmentation was subfascial in 40 breasts (29%), submuscular in 80 breasts (58%) and dual-plane type II or III in 18 breasts (13%).
Thickness at Point 1: Caudal
The mean preoperative thickness was 0.58 cm (0.12–1.6). At 1, 4 and 12 months postoperatively, the mean thickness was 0.63 cm (0.17–1.71), 0.55 cm (0.19–1.55) and 0.53 cm (0.17–1.40), respectively (Fig. 3).
The difference in thickness between the preoperative and 12-month postoperative values was − 0.045 cm ± 0.26 cm (− 7.8%), which was not significant (p = 0.15).
There was an increase in thickness at 1 month postoperatively, which decreased significantly at 4 months postoperatively (p < 0.05) and stabilized thereafter.
Thickness at Point 2: Midpoint of the Lower Pole
The mean preoperative thickness was 0.97 cm (0.31–1.95). At 1, 4 and 12 months postoperatively, the mean thickness was 0.83 cm (0.22–1.81), 0.69 cm (0.23–1.96) and 0.66 cm (0.26–1.73), respectively (Fig. 4).
The difference in thickness between the preoperative and 12-month postoperative values was − 0.3 ± 0.3 cm (− 31.85%), which was significant at p < 0.0001.
The reduction in thickness at 1 month postoperatively was significant (− 0.14 cm, p < 0.0001), and this atrophy continued until 4 months (− 0.14 cm, p < 0.0001); no significant differences in thickness were found between 4 and 12 months postoperatively (p = 0.45).
Thickness at Point 3: Areolar
The mean preoperative thickness was 1.3 cm (0.22–2.40). At 1, 4 and 12 months postoperatively, the mean thickness was 0.97 cm (0.29–2.15), 0.77 cm (0.24–2.17) and 0.77 cm (0.24–2.01), respectively (Fig. 5).
The difference in thickness between the preoperative and 12-month values was − 0.56 ± 0.37 cm (a reduction of 42.4%), which was significant at p < 0.0001.
At this level, the gland thickness was reduced by − 0.36 cm (p < 0.0001) at 1 month postoperatively. At 4 months, the thickness was further reduced by 0.19 cm (p < 0.0001), and no significant changes occurred thereafter (p = 0.8).
Relationship Between Tissue Thickness and Implant Volume, Projection or Implant Shape
No apparent relationships were observed between atrophy of the glandular tissue and implant volume or projection (profile category: extra-high, high and moderate).
Anatomical implants caused significantly more atrophy than round implants at the lower pole (Fig. 6) at all levels: p = 0.02 at the caudal level (point 1, p < 0.05); p = 0.001 at the medial level (point 2, p < 0.05); and p = 0.007 (p < 0.05) at the areolar level (point 3). Atrophy at point 3 was greater for anatomical implants than for round implants for the high profile (p < 0.001) and moderate profile (p < 0.05).
No correlation was found between the brand of the implant and the differences in thickness, and no significant differences were observed between the brands for the same profile.
Relationship of Tissue Thickness with Implant Volume and BMI
The BMI showed no significant correlation with the implant volume or the thickness at the caudal (point 1) and medial (point 2) levels. However, the BMI was significantly correlated with the areolar thickness (point 3) (R2 = 0.07, p = 0.007, p < 0.05); a higher BMI resulted in a lower reduction in thickness.
Relationship of Tissue Thickness with Implant Shape and Position
Generally, subfascial placement produced more atrophy than submuscular placement at all three levels and was significant at the areolar (p < 0.05) and medial levels (p < 0.0001) (Table 1). With submuscular placement, round implants caused significantly less of a reduction in the glandular thickness than anatomical implants at all levels (all p < 0.05).
There were no significant differences between the different planes of placement for round implants. For anatomical implants, we found significant more atrophy for subfascial placement only the areolar level (p < 0.05) (Table 2).
The incision was not related to changes in thickness.
Upper Pole and 3D Measurements
Thirty-three patients were included in this group (66 breasts). The mean age was 29.3 years (20–48). The mean BMI was 19.8 (17.6–24.5). The mean implant volume and projection were 325 cc (220–480) and 5 cm (3.1–6.7), respectively.
Anatomical implants were used in 52 breasts (78.8%), while round implants were used in 14 breasts (21.2%). The periareolar, transaxillary and inframammary approach was used in 12 (18%), 44 (66%) and 10 (15%) breasts. Dual-plane, subfascial and submuscular placement was used in two (3%), 14 (21.2%) and 50 (75.8%) breasts.
The mean preoperative volume was 135.74 cc (48–243), and the mean preoperative projection was 3.6 cm (2.2–5). Anatomical implants showed a mean volume of 319 cc (220–480) and a mean projection of 5.16 cm (3.8–6.7). Round implants showed a mean volume of 347 cc (300–400) and a mean projection of 4.5 cm (3.1–5.4). The expected final volume (preoperative breast volume + implant volume) was 474.40 cc (303–730). The expected final projection (preoperative breast projection + implant projection) was 8.6 cm (7–10.9).
For anatomical implants, an extra-high profile was used in 13 breasts (25%), a high profile was used in 31 breasts (59.6%) and a moderate profile was used in 8 breasts (15.4%). Polytech was used in 26 breasts (50%), Allergan was used in 10 breasts (19.2%), Mentor was used in 6 breasts (11.5%), Sebbin and Eurosilicone were used in 4 breasts each (7.7% each) and B-lite was used in 2 breasts (3.8%).
For round implants, a high profile was used in 7 breasts (50%), a moderate profile was used in 6 breasts (42.9%) and a low profile was used in 1 breast (7.1%). Polytech, Allergan and Sebbin were used in 4 breasts (28.6%) each, and Nagor was used in 2 breasts (14.3%).
Thickness at Point 4: Areolar
The mean preoperative thickness was 1.43 cm (0.52–2.09). At 1, 4 and 12 months postoperatively, the mean thickness was 1.29 cm (0.43–1.76) (p < 0.05 vs preoperative), 1.23 cm (0.52–1.79) (p = 0.17 vs 1 month) and 1.22 cm (0.44–2.17) (p = 0.25, vs 4 months), respectively (Fig. 7).
The difference in thickness between the preoperative and 12-month postoperative values was − 0.21 ± 0.34 cm (− 14.6%), which was significant at p < 0.001.
Thickness at Point 5: Midpoint of the Upper Pole
The mean preoperative thickness was 1.1 cm (0.32–1.74). At 1, 4 and 12 months postoperatively, the mean thickness was 1.2 cm (0.35–1.91) (p = 0.06 vs preoperative), 1.17 cm (0.49–1.77) (p = 0.57 vs 1 month) and 1.17 cm (0.13–1.94) (p = 0.83 vs 4 months), respectively (Fig. 8).
The difference in thickness between the preoperative and 12-month postoperative values was 0.07 ± 0.34 mm (+ 6.2%), which was not significant (p = 0.25).
Thickness at Point 6: Cranial Upper Pole
The mean preoperative thickness was 0.79 cm (0.18–1.41). At 1, 4 and 12 months postoperatively, the mean thickness was 1.07 cm (0.27–1.92) (p < 0.0001 vs preoperative), 1.07 cm (0.41–1.96) (p = 0.96 vs 1 month) and 1.09 cm (0.49–1.80) (p = 0.7 vs 4 months), respectively (Fig. 9).
The difference in thickness between the preoperative and 12-month postoperative values was 0.30 ± 0.31 cm (+ 38%), which was significant at p < 0.0001.
Relationship of Tissue Thickness with Implant Volume and Projection
Regarding the volume of the implant, at the areolar level (point 4), a reduction in thickness occurred, but at the medial level (point 5), an increase in thickness occurred (p < 0.05 at both levels). No relationships were found at the upper (point 6) level.
Regarding the implant projection, we found a correlation (p < 0.05) with reduction in the thickness at the areolar (point 4) and medial (point 5) levels for round implants and at only the areolar level (point 4) for extra-high anatomical implants.
No differences were observed between brands and profile of the implants.
Relationship Between Thickness and Implant Shape
The glandular tissue was thinner for round implants than for anatomical implants at all levels (Fig. 6). The mean change at the areolar level was − 0.19 cm (− 0.9 to 0.65) for anatomical implants and − 0.25 cm (− 0.47 to 0.25) for round implants (p < 0.0001). At the medial level (point 5), the mean change was 0.09 cm (− 1.25 to 1.01) for anatomical implants and − 0.036 cm (− 0.42 to 0.5) for round implants (p < 0.0001). At the cranial level (point 6), the mean change was 0.31 cm (− 0.28 to 1.1) for anatomical implants and 0.26 cm (− 0.28 to 0.83) for round implants (p < 0.0001).
Relationship Between Thickness and BMI
There were no significant correlations between the BMI and tissue thickness. However, when considering the implant volume as well, we found smaller reductions in the tissue thickness with increasing BMI and implant volume (p < 0.05) at the areolar and midpoint levels.
Relationship Between Thickness and Implant Position
Subfascial placement resulted in greater reductions in thickness at all points (4, 5 and 6) than submuscular placement (p < 0.05). The mean thickness at the areolar level was − 0.16 cm (− 0.9 to 0.65) and − 0.44 cm (− 0.36 to 0.07) for submuscular and subfascial placement, respectively. The mean thickness at the medial level (point 5) was 0.12 cm (− 1.25 to 1) and − 0.16 cm (− 0.32 to 0.46) for submuscular and subfascial placement, respectively. At the cranial level (point 6), the mean thickness was 0.36 cm (− 0.28 to 1.1) and 0.07 cm (− 0.07 to 0.51) for submuscular and subfascial placement, respectively.
Considering the implant shape, we did not find a significant difference between round and anatomical implants. However, subfascial placement produced more atrophy than submuscular placement for anatomical implants (p < 0.05) (Table 3).
Anthropometric Measurements
All surface measurements are compiled in Table 4. For all measurements, a significant difference was found between the baseline and the first month after the operation (p < 0.0001). No major changes occurred afterwards.
The increase in the SN-Ni distance at 12 months postoperatively was greater for round implants (1.3 cm, 0.08–2.5) than anatomical implants (1.1 cm, − 0.34 to 2.8) (p < 0.001).
The difference between the theoretical (474.42 cc; 303–750) and final breast volume at 12 months postoperatively (473.78 cc; 288–660) was not significant (p = 0.97) (Fig. 10). There were no significant differences between round and anatomical implants.
The final projection (6.7 cm; 5–8) was 29% less than expected (8.63 cm; 7–10.9) (p < 0.0001) (Fig. 11). The difference between the final projection at 12 months postoperatively and the expected projection was − 1.2 cm for round implants (− 13.8%) and − 2.1 cm for anatomical implants (− 24.4%).
There was a positive correlation between the BMI and SN-Ni distance (R2 = 0.56, p < 0.0001) and a negative correlation between the BMI and Ni-IMF distance (R2 = − 0.24, p < 0.05).
Influence of Implant Shape
The implant shape was not correlated with any of the anthropometric measurements except for the final breast volume (p = 0.044, p < 0.05), as there was a greater gain in breast volume at 12 months postoperatively with round implants than with anatomical implants (398 cc vs 338.7 cc) because the volume of the implant was higher for round than anatomical implants (mean, 347 cc vs 310 cc).
Influence of Implant Position
There were no significant differences in any of the measurements according to the position or shape of the implant (Tables 5, 6), with the exception of a significant difference (p < 0.05) in the NI-IMF distance for anatomical implants between dual-plane and subfascial placement.
Expected Versus Final Volume
The difference between the final volume and the expected volume was 0.6 cc (− 176 to 122). The final volume can be estimated as follows (R2 = 0.67, good correlation):
Expected Versus Final Projection
The final projection was always less than expected (29%). The correlation was weak (R2 = 0.1). The final projection can be estimated according to the following formula:
Correlation Between Tissue Thickness and Distance Measurements
The greater the reduction in the tissue thickness near the areola (cranial measurement at the lower pole), the greater the increase in the Ni-IMF distance (p < 0.05).
We did not find any correlations between the implant volume and the SN-Ni, Ni-IMF or Ni-IMF L distances. Additionally, there were no correlations between the implant type and position. However, although the differences were not significant, the anatomical implants produced a greater increase in the Ni-IMF distance than round implants (3.97 ± 1.47 cm vs 3.65 ± 0.5 cm, p = 0.385), as did submuscular implants compared with subfascial implants (4 ± 1.3 cm vs 3.5 ± 1.5 cm, p = 0.21).
Discussion
Understanding the relationship between an implant and the breast tissue is crucial to the final outcome of breast augmentation. Implants can cause various types of deformities, such as bottoming out, inframammary fold descent, tissue atrophy and distortion due to implant visibility [9]. Vegas and Del Yerro [3] theorized that the breast undergoes an instantaneous deformation (creep deformation) that is intimately related to the viscoelasticity of the tissue. The implant, especially if it is large, would exert stress on the lower pole of the breast proportional to the weight of the implant and to the projection, leading to atrophy of the parenchyma, thinning of the tissue and distension of the lower pole [2, 3, 9, 10]. However, most of these opinions from reputed surgeons have not been supported with scientific data.
In our study, using ultrasonography, we selected the midline because this is where the implant is expected to exert the greatest pressure, in terms of both its weight and projection. We performed two consecutive studies, the first in the lower pole and the second in both the upper and lower poles of the breast, and obtained 3D measurements. The pooled study of the lower pole showed that most caudal measurements (point 1) exhibited few differences, with a thickness reduction of 7.8%. However, the atrophy was more significant at the medial (point 2) and subareolar (point 3) levels, with a thickness reduction of 31.8% and 42.4%, respectively, compared to baseline values. Atrophy occurs immediately and is very significant at 1 month postoperatively (p < 0.0001); it worsens somewhat until 4 months postoperatively and then stabilizes, with no significant progression occurring between 4 and 12 months postoperatively. The atrophy was not clinically significant, but it was likely linked to the feeling of naturalness and softness expressed by the patient.
At the upper pole, we found significant parenchymal atrophy (p < 0.05) in the juxta-areolar zone (point 4) at 1 month postoperatively, with no further progression thereafter. Paradoxically, we found an increase in the parenchymal thickness in points 5 and 6, i.e. the medial (+ 6%) and cranial (+ 38%) levels. The cause of this phenomenon is unclear. A potential explanation could be a compensation for the atrophy in other areas of the gland, although recruitment of the glandular tissue is more likely, resulting in pushing of the gland upward, similar to the effect of a bra. The shape of the anatomical implant promotes some upward rotation of the tissue compared to round implants, which push the tissue forward. This change could potentially explain why in the long run, round implants can become indistinguishable from anatomical implants at the upper pole, as they produce more thinning of the parenchyma, as our results show, and of the muscle, as Roxo et al. [5, 6] demonstrated.
Contrary to what might be expected, we did not find a correlation between the implant volume and the decrease in thickness at the lower pole; however, at the upper pole, the implant volume was related to the degree of atrophy at only the juxta-areolar level. The implant projection only had an influence on breast atrophy at the upper pole near the areola. A higher BMI reduces the impact on the tissue at the areolar level. We found that the implant shape was the most significant factor. Anatomical implants caused more atrophy than round implants.
Submuscular placement provides some protection from parenchymal atrophy, which explains why we found more glandular atrophy with subfascial placement (especially in the case of anatomical prostheses). Possibly, as demonstrated by Roxo et al. [4, 5], the muscle absorbs the impact of the increase (with reduction of volume of the muscle by 46.38% at 6 months and 49.80% at 12 months with respect to baseline preoperative levels). They also found less of a reduction in the parenchymal volume in patients with submuscular implants than in those with subglandular implants (27% at 6 months and 22% at 12 months with respect to the baseline) [6].
This tissue atrophy is almost immediate and presumably due to the acute distension and stretching by the implant. Anatomical implants have more caudal convexity and projection than round implants, causing greater pressure and distension of the lower pole. Unlike the lower pole, the upper pole showed significantly greater tissue atrophy with round implants than with anatomical implants (p < 0.0001). This difference is supported by the fact that the juxta-areolar atrophy was greater caudally (infra-areolar) for anatomical implants and cranially (supra-areolar) for round implants. Tebbets and Teitelbaum [9] warned of the risk of using high or extra-high profile implants because they would produce greater tissue pressure and consequently more atrophy, but our results have not supported this hypothesis. We did not find a correlation or differences between implant profiles (extra-high, high, moderate) and atrophy. No differences were observed between high and extra-high projections, although extra-high implants produced more atrophy than the moderate profile at point 4 (supra-areolar). Regardless of the profile, anatomical implants caused more atrophy at the infra-areolar point and midpoint of the lower pole.
We used implants from different manufacturers (hence with different gel properties and shells), but this did not make any difference in the thickness of the gland or surface measurements.
Regarding the surface distances (SN-Ni, Ni-IMF, Ni-Ni, and Ni-IMF L), there was a significant difference between the values at 1 month postoperatively and at baseline; however, practically no differences were observed after that point. With 3D imaging, Eder et al. [11] also showed similar changes in patients with subpectoral augmentation, and these changes stabilized after 3 months.
There was a relationship between the caudal areolar thickness and the Ni-IMF distance; the greater the atrophy, the greater the distance. This relationship is why the anatomical implants showed a greater Ni-IMF distance than did the round implants, although this difference was not significant.
We estimated the volume and projection of the breast before and after augmentation. The volume at 12 months postoperatively was similar to the calculated volume. However, the postoperative projection was less (29%) than the theoretical projection, by 1 cm for round prostheses and approximately 2 cm for anatomical prostheses. These results are similar to those reported by Tepper et al. [12] (21% lower projection).
The implant volume has not been shown to be a determining factor of the degree of parenchymal atrophy, at least within the first postoperative year. Based on these findings, it is not clear that a lighter implant can cause less tissue atrophy at the lower pole than a heavier implant, as has been proposed [13]. Perhaps over the long term, once the tissue has become thinner and weaker, the implant volume could be a determining factor in the deformity of the lower pole.
Our results may be useful during implant selection. The selection of one or another type of implant or placement plane has been the subject of numerous publications and debates [14,15,16,17]. An anatomical implant, because of its effect at the lower pole, would be indicated in cases with a tight skin envelope, restriction of the lower pole or tuberous breasts. Although the difference was not significant, the Ni-IMF distance is also greater for anatomical implants than for round implants. Tebbets [2] pioneered the 2-cm pinch for plane selection, with lower values indicating that submuscular placement would be better. Considering the degree of atrophy at the lower pole, especially with anatomical prostheses, and that subglandular/subfascial placement has a greater impact on the mammary parenchyma, this recommendation is absolutely valid. Another strategy would be to supplement the augmentation with fat grafting for subfascial/subglandular placement [18, 19].
A potential limitation of this study is the bias in implant selection. Our preferences are to use moderate profile, round implants for submuscular augmentation and high-projection anatomical implants in patients with pseudoptosis or pole restriction. Other potential limitation could be the number of cases in phase II in terms of comparison between groups.
Our main conclusions are as follows:
-
Implants have an impact on the glandular tissue and reduce the tissue thickness, especially at the centre of the breast.
-
Anatomical implants result in more atrophy at the lower pole, while round implants result in more atrophy at the upper pole; the implant shape is the main variable influencing atrophy.
-
The implant volume has no effect on changes in the parenchyma in the first postoperative year, but the implant projection plays a role, especially in the periareolar area.
-
While the final breast volume is similar to the expected value, the final projection is approximately 29% less than expected.
-
Significant changes occur very early, as soon as 1 month postoperatively. However, no major changes in thickness or surface measurements occur after 4 months postoperatively.
References
FDA (2018) Risk of breast implants. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/breastimplants/ucm064106.htm. Accessed 14 July 2018
Tebbetts JB (1998) Use of anatomic breast implants: ten essentials. Aesthet Surg J 18:377–384
Vegas MR, Del Yerro JLM (2013) Stiffness, compliance, resilience, and creep deformation: understanding implant-soft tissue dynamics in the augmented breast: fundamentals based on materials science. Aesthet Plast Surg 37:922–930
Roxo AC, Nahas FX, Bazi F, de Castro CC, Aboudib JH, Marques RG (2015) Evaluation of the effects of silicone implants on the breast parenchyma. Aesthet Surg J 35:929–935
Roxo AC, Nahas FX, Salin R, de Castro CC, Aboudib JH, Marques RG (2016) Volumetric evaluation of the mammary gland and pectoralis major muscle following subglandular and submuscular breast augmentation. Plast Reconstr Surg 137:62–69
Roxo AC, Nahas FX, Rodrigues NCP, Salles JI, Cossich VRA, de Castro CC et al (2017) Functional and volumetric analysis of the pectoralis major muscle after submuscular breast augmentation. Aesthet Surg J. 37:654–661
Benito-Ruiz J, de Cabo F (2014) Ultrasonography: a useful tool for plastic surgeons. Aesthet Plast Surg 38:561–571
Sieber DA, Stark RY, Chase S, Schafer M, Adams WP Jr (2017) Clinical evaluation of shaped gel breast implant rotation using high-resolution ultrasound. Aesthet Surg J 37:290–296
Tebbetts JB, Teitelbaum S (2010) High- and extra-high-projection breast implants: potential consequences for patients. Plast Reconstr Surg 126:2150–2159
Handel N (2006) Secondary mastopexy in the augmented patient: a recipe for disaster. Plast Reconstr Surg 118:152S–163S (discussion 164S-165S, 166S-167S)
Eder M, Waldenfels F, Sichtermann M, Schuster T, Papadopulos NA, Machens HG et al (2011) Three-dimensional evaluation of breast contour and volume changes following subpectoral augmentation mammaplasty over 6 months. J Plast Reconstr Aesthet Surg 64:1152–1160
Tepper OM, Small KH, Unger JG, Feldman DL, Kumar N, Choi M et al (2009) 3D analysis of breast augmentation defines operative changes and their relationship to implant dimensions. Ann Plast Surg 62:570–575
Govrin-Yehudain J, Dvir H, Preise D, Govrin-Yehudain O, Govreen-Segal D (2015) Lightweight breast implants: a novel solution for breast augmentation and reconstruction mammaplasty. Aesthet Surg J 35:965–971
Montemurro P, Cheema M, Heden P, Ferri M, Li AQ, Avvedimento S (2017) Role of macrotextured shaped extra full projection cohesive gel implants in primary aesthetic breast augmentation. Aesthet Surg J. 37:408–418
Caplin DA (2014) Indications for the use of memory shape breast implants in aesthetic and reconstructive breast surgery: long-term clinical outcomes of shaped versus round silicone breast implants. Plast Reconstr Surg 134:27S–37S
Heden P, Montemurro P, Adams WP Jr, Germann G, Scheflan M, Maxwell GP (2015) Anatomical and round breast implants: how to select and indications for use. Plast Reconstr Surg 136:263–272
Montemurro P, Cheema M, Heden P, Agko M, Li AQ, Avvedimento S (2018) Do not fear an implant’s shape: a single surgeon’s experience of over 1200 round and shaped textured implants in primary breast augmentation. Aesthet Surg J 38:254–261
Auclair E, Blondeel P, Del Vecchio DA (2013) Composite breast augmentation: soft-tissue planning using implants and fat. Plast Reconstr Surg 132:558–568
Ozalp B, Aydinol M (2017) Breast augmentation combining fat injection and breast implants in patients with atrophied breasts. Ann Plast Surg 78:623–628
Acknowledgements
The authors declare that they have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benito-Ruiz, J., de Cabo, F., Manzano, M. et al. Effects of Silicone Implants on the Mammary Gland: Ultrasonographic and 3D Study. Aesth Plast Surg 43, 354–365 (2019). https://doi.org/10.1007/s00266-018-1253-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-018-1253-4