Abstract
Objectives
The retroauricular fascia flap (RFF) is one of the most commonly used vascularized linings for auriculocephalic sulcus reconstruction in staged total auricular reconstruction. This study aims to investigate the histomorphometric features regarding the retroauricular fascia.
Methods
Histological evaluation included qualitative observation and quantitative analysis of sections of RFF stained with hematoxylin and eosin, Masson’s trichrome, Elastica van Gieson, CD31, and Lyve-1. Ultrasonographic evaluation included measurement of the thickness of the superficial layer of the retroauricular fascia (RFF origin) at three different positions in microtia patients. P < 0.05 was considered statistically significant.
Results
RFF was a thin, highly organized layer with mainly collagen fibers. From its superior to inferior portions, the percentage of collagen fibers differed significantly (superior 87.57 ± 10.85%, middle 68.29 ± 29.02%, inferior 53.31 ± 33.33%, p < 0.05). The percentages of elastic fibers in the superior (4.86 ± 5.17%) and middle (5.05 ± 5.37%) areas were higher than that in the inferior (2.14 ± 2.42%, p < 0.05). RFF blood vessel density (20× magnification) decreased significantly from the superior to inferior portions (superior 6.39 ± 1.18, middle 5.17 ± 1.15, inferior 2.67 ± 0.78, p < 0.05). Lymphatic vessel density (20× magnification) also decreased significantly from the superior to inferior regions (superior 6.80 ± 0.62, middle 5.26 ± 1.17, inferior 2.11 ± 0.46, p < 0.05). Thickness of the superficial layer of retroauricular fascia increased significantly from the superior to inferior regions (superior 0.29 ± 0.06 mm, middle 0.36 ± 0.09 mm, inferior 0.53 ± 0.14 mm, p < 0.001).
Conclusions
From cranial to caudal, the RFF became thicker, less elastic, and less vascularized, and contained fewer lymphatic vessels. Therefore, when the retroauricular fascia is large enough, the superior portion would be preferred for RFF in auriculocephalic sulcus reconstruction.
No Level Assigned
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The retroauricular fascia, also known as the “postauricular” or “superficial mastoid” fascia, represents the fascial structure posterior to the auriculocephalic sulcus. It has been used extensively in several kinds of facial repairs and congenital defect reconstructions, such as augmentation rhinoplasty [1], blepharoplasty [2], and lip advancement [3]. The most common use of the retroauricular fascia flap (RFF) has been for auricular reconstruction [4,5,6]. During ear elevation in two-staged total auricular reconstruction, retroauricular fascia has become the preferred vascularized lining for many surgeons [7].
Park et al. [8] were the first authors to describe the subcutaneous fascial layers and blood supply of the retroauricular area. Thereafter, Datta et al. [9] performed detailed dissection in the retroauricular region and clearly elucidated the relationship between the temporal fascial and mastoid fascial layers. Other related anatomical studies provided further details regarding the vascularity of this area, including the posterior auricular artery (PAA), superficial temporal artery (STA), and occipital artery (OCA), and their patterns of anastomosis [10,11,12]. Nevertheless, to the best of our knowledge there is a paucity of literature regarding the histomorphometric features of the retroauricular fascia.
This study aimed to investigate the anatomical and histological characteristics of the RFF in patients with congenital microtia. We analyzed histological sections of redundant fresh RFF tissue obtained at surgery and preoperatively measured the thickness of the superficial layer of retroauricular fascia, in these patients, by ultrasonography. By improving our understanding of this area, we can explore more reliable modifications of the RFF and availability of other possible flaps for total auricular reconstruction, or even for other types of deformity reconstruction.
Materials and Methods
Histological Study
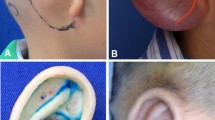
This study was approved by the Ethics Committee of Shanghai 9th People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine, China. RFF specimens were collected during the second stage of two-stage auricular reconstruction surgery. In the selected cases, the outer edge of the RFF (over 3 mm in width) was found to be redundant after this fascial flap was harvested. We excised three fragments (3 × 10 mm2) from the superior, middle, and inferior point of the redundant RFF edge in each case. The superior fragment corresponded to the level of the otobasion superius, the inferior fragment corresponded to the level of the otobasion inferius, and the middle fragment was located at the midpoint between the otobasion superius and otobasion inferius (Fig. 1). Operations were performed by a single surgeon (Zhang R).
The specimens were placed immediately on a plastic board with 4% paraformaldehyde to avoid deformation artifacts and then processed according to routine histological procedures. Cross-histological sections were obtained and stained with hematoxylin and eosin (H and E) for cellular components and general tissue, Masson’s trichrome for collagen fibers, Elastica van Gieson (EVG) for elastic fibers, CD31 for vascular tissue, and Lyve-1 for lymphatic vessels.
The specimens were visualized with a Nikon Eclipse E600 microscope, and documentation of microscopy findings was collected with a Nikon Sight ds-L1 digital camera. Quantitative analysis of collagen and elastic fibers was performed using a Pannoramic MIDI (3DHISTECH, Ltd., Budapest, Hungary) scanner, along with its image analysis system, including the Pannoramic viewer and Quant center. Blood vessel and lymphatic vessel densities were calculated in five randomly selected microscope visual fields per subject (objective 20× magnification).
Ultrasonography
Patients with microtia were assessed by ultrasonography before the first stage of their two-stage auricular reconstruction surgery. Ultrasonography was performed with a SL3116 probe by a single registered senior medical sonographer (Ding A) using the MyLab™ Class C system (ESAOTE, Italy) at a central frequency of 22 MHz.
A layer of gel was applied to minimize compression of the skin and subcutaneous tissue by the transducer, and the probe was placed perpendicular to the skin. The thickness of the superficial layer of retroauricular fascia (the layer from which the RFF was obtained) was measured at three points in the retroauricular region on the operative side, which generally corresponds to the three assessment positions in our histological study. The three points were 4 cm posterior to the helix of the reconstructed ear, at the level of the otobasion superius, the otobasion inferius, and the midpoint between these two points. The three points were recorded as the superior, middle, and inferior point. Each point was measured three times, and we recorded the mean value as the thickness at that point of each patient.
Statistical Analysis
Statistical analyses were performed with SPSS version 22.0 (SPSS, Inc., Chicago, IL). Wilcoxon and Kendall tests were used to compare histological characteristics (percentage of collagen fibers, percentage of elastic fibers, blood vessel density, and lymphatic vessel density) among the three retroauricular fascia points. Repetitive measures analysis of variance (ANOVA) was used to compare thickness among these three points. P < 0.05 was considered statistically significant.
Results
Histological Study
From January 2015 to May 2017, ten patients with microtia met our criteria for collection of RFF specimens. The patients included eight males and two females, aged 9 to 21 years [mean ± standard deviation (SD) 14 ± 4 years] (Table 1). Except for congenital aural atresia, none of the patients exhibited other related symptoms, such as hemifacial microsomia or facial diplegia.
According to the sections stained with H&E, Masson’s trichrome, and EVG, samples from different RFF positions (the superior point, middle point, and inferior point) shared similar morphologic characteristics (Fig. 2). The superficial layer of retroauricular fascia was a thin and highly organized layer of connective tissue predominantly consisting of collagen fibers. The collagen fibers were grouped into thick and wavy bundles in a regular longitudinal way, and the bundles composed parallel sheets, similar to a layer cake. In the EVG-stained sections, bundled or dispersed thin elastic fibers were recognized; the elastic fibers covered a small proportion of the tissue and were not arranged in any characteristic pattern. Blood vessels and lymphatic vessels of various diameters could be observed among the collagen bundles, and some fibroblast nuclei with basophilic staining were aligned dispersedly among the collagen bundles (Fig. 3). Adipose tissue was observed between the collagen fiber sheets or within the outer and inner surfaces of the fascia layer samples. In addition, muscle bundles were observed deep to the fascia layer in the inferior sample of three patients.
The morphologic characteristics of the RFF: a H and E stain (× 5). The RFF sample predominantly consisted of collagen fibers. The collagen fibers were arranged in wavy and parallel bundles (red arrow). Vessels (black arrows) and adipose tissue (black pentacle) were observed. b Masson’s trichrome stain (× 10). Adipose tissue (black pentacle) is located within the outer and inner surfaces of the fascia layer, and vessels (black arrows) were found between the fiber sheets. Muscle bundles (red arrows) were laid deep beneath the fascia layer. c EVG stain (× 40). The elastic fibers (blue arrows) were undulant and arranged either dispersed or in bundles. Several fibroblast nuclei (green triangles) stained in brown aligned dispersedly. Vessels (black arrows) were located between fiber sheets
The mean ± SD collagen fiber percentage of RFF specimens was 87.57 ± 10.85% at the superior point, 68.29 ± 29.02% at the middle point, and 53.31 ± 33.33% at the inferior point (Psuperior vs. middle = 0.037, Psuperior vs. inferior = 0.005, Pmiddle vs. inferior = 0.007). The mean ± SD elastic fiber percentage of RFF specimens was 4.86 ± 5.17% at the superior point, 5.05 ± 5.37% at the middle point, and 2.14 ± 2.42% at the inferior point (Psuperior vs. middle = 0.333, Psuperior vs. inferior = 0.005, Pmiddle vs. inferior = 0.017) (Fig. 4). The mean ± SD blood vessel density of RFF samples (under 20× magnification) was 6.39 ± 1.18 vessels per field at the superior point, 5.17 ± 1.15 vessels per field at the middle point, and 2.67 ± 0.78 vessels per field at the inferior point (Psuperior vs. middle = 0.017, Psuperior vs. inferior = 0.005, Pmiddle vs. inferior = 0.005). The mean ± SD lymphatic vessel density of RFF samples (under 20× magnification) was 6.80 ± 0.62 vessels per field at the superior point, 5.26 ± 1.17 vessels per field at the middle point, and 2.11 ± 0.46 vessels per field at the inferior point (Psuperior vs. middle = 0.007, Psuperior vs. inferior = 0.005, Pmiddle vs. inferior = 0.005) (Fig. 5).
The collagen fiber percentage and elastic fiber percentage of the RFF in three different regions. The collagen fiber percentage differed significantly from superior to inferior portions. The elastic fiber percentages in the superior and middle portions were higher than that in the inferior. P < 0.05 was considered statistically significant. The horizontal lines inside each column represent the median values
The blood vessel density and lymphatic vessel density of the RFF in three different regions. The blood vessel density and lymphatic vessel density (per 20× microscope vision) both decreased significantly from the superior to inferior regions. P < 0.05 was considered statistically significant. The horizontal lines inside each column represent the median values
Ultrasonography
From August 2016 to May 2017, 46 patients with microtia were assessed by ultrasonography. We excluded 15 from this study because we were unable to distinguish the low-density zone between fascial layers when examining the inferior point. The remaining 31 patients included 21 males and 10 females, aged 7–21 years (mean ± SD 13 ± 4 years) (Table 2). The high-density fascial layers ran in parallel arrays between the skin and muscularis (Fig. 6). The thickness of the superficial layer of retroauricular fascia was significantly different at the superior (mean value ± SD 0.29 ± 0.06 mm), middle (mean ± SD 0.36 ± 0.09 mm), and inferior (mean ± SD 0.53 ± 0.14 mm) points (p < 0.001) (Fig. 7).
Discussion
RFF has been widely used in facial repairs and reconstructions, especially during ear elevation in two-staged total auricular reconstruction [6, 7, 13]. This flap was first described as a “retroauricular fascia flap” by Ou et al. [14] in 2001 and has also been referred to as a “superficial mastoid fascia flap,” “postauricular fascia flap,” or “book cover flap” over the years. Undoubtedly, when comparing the intrinsic quality of a fascial flap for ear elevation, the temporoparietal fascia flap (TPFF) is superior. TPFF is thinner and has a more reliable vascularity [15, 16], leading to a deeper reconstructed auriculocephalic sulcus and fewer flap complications. Nevertheless, many authors [5, 6, 8, 11, 14] regard RFF as an appropriate alternative for ear elevation, reserving TPFF for secondary salvage. Besides, the RFF technique is simpler and leaves a less conspicuous scar than TPFF.
According to existing anatomical studies, there are two groups of subcutaneous fascial layers in the retroauricular area: the temporal fascial layers superficial to the temporalis and the mastoid fascial layers superficial to the sternocleidomastoid muscle. The transition area between these two fascial groups is superficial to the inferior margin of the temporalis, where the sternocleidomastoid muscle inserts on the mastoid process [17]. Therefore, in our study, the superior point of the RFF came from the superficial temporal fascia, whereas the inferior point likely originated from the superficial mastoid fascia; the histological origin of the middle point may differ between patients.
In sections stained with H and E, Masson’s trichrome, and EVG, the RFF was a highly organized, well-defined fibrous connective tissue layer, which followed the general pattern of superficial fascia [18]. In addition, muscle bundles were observed in the inferior section in some cases. This may be attributed to difficulty in dissecting the inferior portion when harvesting the RFF, resulting in some fibers of the sternocleidomastoid muscle being raised as well. In our greater than 10 years of experience with auricular reconstruction [19], the RFF showed gradual differences from its cranial to caudal portions; one of the differences was that the cranial portion was easier to harvest than the caudal area. As described by Park et al. [8] and Datta et al. [9], in the retroauricular area, the temporal fascial layers in the cranial portion were elastic and discrete, whereas the mastoid fascial layers in the caudal portion were not distinctly separated and presented as “a pad of heavy fibrous, fascial, and muscular tissue.” Park also mentioned that because the caudal portion was “thick, heavy, fibrous,” sharp dissection was necessary when harvesting the fascial flap.
Quantitative analysis indicated that the RFF was mainly formed by collagen fibers; the estimated collagen fiber percentage decreased from the superior to inferior points. Although we did not quantitatively determine the adipose tissue and muscle fiber content, the decrease in collagen may suggest that the percentage of these tissues increased from the superior to inferior portions of the RFF. Collagen fibers increase the strength of connective tissue, and collagen plays an important role in tissue regeneration [20]. Elastic fibers composed a small proportion of RFF. Although the elastic fiber percentage did not differ significantly between the superior and middle points, it was significantly lower at the inferior point. Elastic fibers are responsible for elasticity and play an important role in the healing of dermal lesions [20]. These results suggest that the inferior RFF portion is less favorable for use during ear elevation.
Blood vessel and lymphatic vessel density both decreased significantly from the superior to the inferior RFF regions. This suggests that the superficial temporal fascia (cranial portion of RFF) is more vascularized and may exhibit better lymphatic drainage. These findings correspond with our clinical findings that the RFF, derived from the superior area, produces less hypertrophic scars and partial skin necrosis [21]. Flap complications are mainly related to arterial or venous issues, but the influence of lymphatic dysfunction is also important. The lymphatic system functions in tissue fluid regulation, fat and vitamin absorption, macromolecular homeostasis, immune responses, and cancer metastases. Accordingly, lymphatic disruption can result in tissue edema, reduced blood perfusion, impaired immune function, and fibrosis [22].
Our observation of decreasing blood vessel density from the superior to inferior RFF is consistent with the findings of published anatomical studies. The retroauricular region receives its arterial supply via the PAA, STA, and OCA [10,11,12], which are mostly distributed in the superficial layer [23]. In 97% of cases, this area is perfused predominantly by the PAA [11], which ascends in the auriculocephalic sulcus and sends branches posteriorly to supply the retroauricular surface. In addition, the PAA supplied the major part of the occipital area of the scalp [24]. This indicates that an anterior-pedicled RFF could be supplied by the PAA in an axial pattern, which is the most commonly recommended flap pattern in the retroauricular region [23]. Furthermore, the PAA, STA, and OCA anastomose with each other in the region posterior and superior to the auricle. Conversely, only the PAA and OCA anastomose with each in the inferior portion of retroauricular area because the STA ascends in front of the ear.
Our ultrasonography study showed that from the cranial to caudal areas, the superficial layer of retroauricular fascia became significantly thicker. This indicates that in the retroauricular region, the superficial temporal fascia is thinner than the superficial mastoid fascia, which is consistent with the results of previous anatomical studies. Our finding is also consistent with the results of a histologic study by Hong et al. [25], which measured the thickness of the superficial retroauricular fascial layer in H and E-stained sections from cadavers. In that study, the thickness of the cephalic vertical section was less than the thickness of the caudal vertical section.
Díaz and Sánchez [12] suggested that the safest area for the RFF, based on the PAA, was approximately a fan shape with average dimensions of 10.7 cm long and 7.07 cm wide. Accurate localization of the flap depended on three points: insertion of the helix, external auditory canal, and mastoid process. Lohasammakul et al. [23] characterized RFF vascularity by investigating the anastomoses among the PAA, STA, and OCA. They concluded that the area 5 cm above and 3 cm below the external acoustic meatus, and approximately 6 cm posterior to the external acoustic meatus (where the OCA posteriorly limits the fascial flap border), was the maximal size of the RFF receiving axial blood supply from the PAA. In addition, the RFF may be enlarged superiorly by combining the blood supply from the parietal branch of the STA. Although such a large area is available for the RFF, the optimal region for the flap should be based on the variations observed between the superior and inferior portions.
This study elucidated the anatomical and histological characteristics of the RFF, including their cranial to caudal differences. We have previously proved that the modified RFF (mainly consisting of the cranial portion of the retroauricular fascia) has more clinical advantages [21]. In this research, the histomorphometric findings offered an anatomical basis to our former work. On the other hand, the histological study was limited by its small sample size. For the surgeon skilled with auricular reconstruction, there were not many patients with an oversized RFF. Besides, this study was also limited by not comparing the RFF with the TPFF, because we were unable to obtain TPFF specimens in our regular surgery. A multicenter study would be useful in the future to compare the RFF and TPFF, to provide a more comprehensive evaluation of the fascia in the auriculotemporal region.
Conclusion
The RFF has been widely used in facial repair, especially auricular reconstruction, although it has some disadvantages when compared with TPFF. Our results showed that when proceeding from the cranial to caudal portions of the RFF, the flap became thicker, less elastic, and less vascularized, and contained fewer lymphatic vessels. These findings infer that when the available retroauricular fascia is sufficiently large, the superior portion would be preferred as a vascularized lining for auricular reconstruction, which would make the quality of the RFF histologically more similar to the superficial temporal fascia.
References
Guerra AB (2014) Postauricular fascia in augmentation rhinoplasty. Ear Nose Throat J 93(6):212–218
Cho JM, Jeong JH, Woo KV, Lee YH (2013) Versatility of retroauricular mastoid donor site: a convenient valuable warehouse of various free graft tissues in cosmetic and reconstructive surgery. J Craniofac Surg 24(5):e486–e490
Recupero WD, McCollough EG (2010) Comparison of lip enhancement using autologous superficial musculoaponeurotic system tissue and postauricular fascia in conjunction with lip advancement. Arch Facial Plast Surg 12(5):342–348
Brent B (1999) Technical advances in ear reconstruction with autogenous rib cartilage grafts: personal experience with 1200 cases. Plast Reconstr Surg 104(2):319–334
Zhang Q, Quan Y, Su Y, Shi L, Xie Y, Liu X (2010) Expanded retroauricular skin and fascial flap in congenital microtia reconstruction. Ann Plast Surg 64(4):428–434
Zhang Q, Zhang R, Xu F, Jin P, Wu J, Li D, Chin W (2010) Firm elevation of the reconstructed auricle with a retroauricular fascial flap wrapping an EH (a mixture of epoxide acrylate malelic and hydroxyapatite) composite wedge. J Plast Reconstr Aesthet Surg 63(9):1452–1458
Breugem CC, Stewart KJ, Kon M (2011) International trends in the treatment of microtia. J Craniofac Surg 22(4):1367–1369
Park C, Lee TJ, Shin KS, Kim YW (1991) A single-stage two-flap method of total ear reconstruction. Plast Reconstr Surg 88(3):404–412
Datta G, Carlucci S (2008) Reconstruction of the retroauricular fold by ‘nonpedicled’superficial mastoid fascia: details of anatomy and surgical technique. J Plast Reconstr Aesthet Surg 61:S92–S97
Yang D, Morris SF (1998) Vascular basis of the retroauricular flap. Ann Plast Surg 40(1):28–33
Wang Y, Zhuang X, Jiang H, Yang Q, Zhao Y, Han J, Yu D, Zhang Z (2008) The anatomy and application of the postauricular fascia flap in auricular reconstruction for congenital microtia. J Plast Reconstr Aesthet Surg 61:S70–S76
Díaz OJG, Sánchez MDC (2016) Anatomical and clinical study of the posterior auricular artery angiosome: in search of a rescue tool for ear reconstruction. Plast Reconstr Surg Glob Open 4(12):e1165
Park C (1997) Modification of two-flap method and framework construction for reconstruction of atypical congenital auricular deformities. Plast Reconstr Surg 99(7):1846–1857
Ou LF, Yan RS, Tang YW (2001) Firm elevation of the auricle in reconstruction of microtia with a retroauricular fascial flap wrapping an autogenous cartilage wedge. Br J Plast Surg 54(7):573–580
Duvdevani SI, Magritz R, Siegert R (2013) Sulcus construction in microtia repair: a retrospective comparison of different techniques. JAMA Facial Plast Surg 15(1):17–20
Yoshimura K, Asato H, Nakatsuka T, Sugawara Y, Park S (1999) Elevation of a constructed auricle using the anteriorly based mastoid fascial flap. Br J Plast Surg 52(7):530–533
Talmi YP, Liokumovitch P, Wolf M, Horowitz Z, Kopolovitch J, Kronenberg J (1997) Anatomy of the postauricular island” revolving door” flap (“flip-flop” flap). Ann Plast Surg 39(6):603–607
Stecco C, Macchi V, Porzionato A, Duparc F, De Caro R (2011) The fascia: the forgotten structure. Ital J Anat Embryol 116(3):127–138
Zhang Q, Zhang R, Xu F, Jin P, Cao Y (2009) Auricular reconstruction for microtia: personal 6-year experience based on 350 microtia ear reconstructions in China. Plast Reconstr Surg 123(3):849–858
Morales-Avalos R, Soto-Domínguez A, García-Juárez J, Cardenas-Serna M, Esparza-Hernández CN, Carreño-Salcedo SA, Montes-de-Oca-Luna R, Loera-Arias MJ, Saucedo-Cárdenas O, Elizondo-Omaña RE, Guzmán-López S (2017) Morphological and histomorphometric evaluation of the ventral rectus sheath of the rectus abdominis muscle, fascia lata and pectoral fascia. The beginning of a morphological information bank of human fascias. Histol Histopathol 32(3):271–282
Li Y, Zhang R, Zhang Q, Xu Z, Xu F, Li D (2017) An alternative posterosuperior auricular fascia flap for ear elevation during microtia reconstruction. Aesthet Plast Surg 41(1):47–55
Li K, Min P, Sadigh P, Grassetti L, Lazzeri D, Torresetti M, Marsili R, Feng S, Liu N, Zhang YX (2017) Prefabricated cervical skin flaps for hemi-facial resurfacing: elucidating the natural history of postoperative edema using indocyanine green. Lymphat Res Biol. https://doi.org/10.1089/lrb.2015.0043 (ahead of print)
Lohasammakul S, Turbpaiboon C, Chompoopong S, Ratanalekha R, Aojanepong C (2017) Vascular nature and existence of anastomoses of extrinsic postauricular fascia: application for staged auricular reconstruction. Ann Plast Surg 78(6):723–727
Touré G, Méningaud JP, Vacher C (2010) Arterial vascularization of occipital scalp: mapping of vascular cutaneous territories and surgical applications. Surg Radiol Anat 32(8):739–743
Hong ST, Kim DW, Yoon ES, Kim HY, Dhong ES (2012) Superficial mastoid fascia as an accessible donor for various augmentations in Asian rhinoplasty. J Plast Reconstr Aesthet Surg 65(8):1035–1040
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Li, Y., Cui, C., Zhang, R. et al. Anatomical and Histological Evaluation of the Retroauricular Fascia Flap for Staged Auricular Reconstruction. Aesth Plast Surg 42, 625–632 (2018). https://doi.org/10.1007/s00266-018-1098-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-018-1098-x