Abstract
Background
Hematoma is the most common postoperative complication of rhytidoplasty, resulting in higher morbidity and longer recovery. Quilting suture for closure of the undermined area in abdominoplasty avoids the occurrence of seroma. Based on this principle and with the objective of reducing the number of patients with hematomas in rhytidoplasty, a similar surgical tactic was developed in which a hemostatic net is created with a running transfixing suture of 5-0 nylon encompassing the skin and the superficial musculoaponeurotic system-platysma.
Methods
The study enrolled 525 consecutive patients who underwent rhytidoplasty between July 2009 and February 2013. The first 120 patients (group A) were evaluated retrospectively and considered control subjects. The remaining 405 patients (group B) had application of the described tactic, with data collected prospectively. The occurrence of hematoma, ischemia, and necrosis was observed during the first 72 h after surgery.
Results
Control group A included 17 patients with hematoma (14.2 %) during the first 72 h, whereas no patient in group B experienced such a complication (p < 0.001). The surgical tactic did not significantly increase the occurrence of ischemia. This complication was experienced by 11 patients in group A (9.2 %) and 26 patients in group B (6.4 %) (p = 0.408). The tactic also did not change the incidence of necrosis, which was present in three group A patients (2.5 %) and six group B patients (1.5 %) (p = 0.723).
Conclusion
The hemostatic net is an efficient and safe method for preventing early hematomas in rhytidoplasties.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the greatest challenges in rhytidoplasty is effective avoidance of hematoma, the most frequent postoperative complication with this type of surgery [1–3]. Observed in up to 13.4 % of patients [4, 5], hematoma affects postoperative recovery because it is associated with an increased incidence of edema, ecchymosis, ischemia, infection, and necrosis in the area treated by rhytidoplasty [1].
Many methods have been described in attempts to eliminate this complication including drains [6, 7], tissue glues [8–11], tumescent infiltration [12], and dissection with ultrasonic instruments [13]. However, none of these methods have been completely effective in preventing hematoma. One study that observed no incidence of this complication was based on control of clinical variables (e.g., blood pressure), which often is difficult to reproduce [14].
A dramatically low incidence of seroma has occurred in abdominoplasty since the introduction of quilting suture to close the detached space, as originated by Baroudi and Ferreira [15]. The idea of placing similar internal sutures in rhytidoplasty to avoid hematoma is curbed by the difficulty in covering the entire flap area and also in achieving adequate skin traction.
Based on the principle of mechanical and compulsory closure of the areas treated by surgery, we have previously described a surgical tactic applied to rhytidoplasty in which a hemostatic net of continuous and transfixing sutures in the skin is created [16]. This method eliminated the occurrence of hematoma in a series of 246 consecutive patients. In the current study, we demonstrated the safety and efficacy of the procedure performed for a larger group of patients.
Patients and Methods
From July 2009 through February 2013, 525 consecutive patients underwent rhytidoplasty with the same surgical team in the same hospital facility. To be included in the study, a patient needed to have at least the middle third of the face approached. Other facial surgical rejuvenation procedures (blepharoplasty, endoscopic treatment of the frontal region, erbium/CO2 laser, fat injection, and/or platysmaplasty) were performed in association with the surgery when indicated.
The patients were divided into two groups. Group A, which served as the control, included the first 120 patients, who underwent surgery between July 2009 and March 2010. These patients were not treated using the proposed surgical tactic and had their data analyzed retrospectively.
Group B was composed of the remaining 405 patients, who underwent the described surgical tactic between April 2010 and February 2013. Data were collected prospectively for this group.
The descriptive data collected included age, gender, number of previous rhytidoplasties, association with submental platysmaplasty, smoking, and hypertension. All the patients were observed for the occurrence of hematoma, ischemia, and necrosis during the first 72 h after surgery.
Hematoma, as previously defined [4, 17], is a blood deposit larger than 30 mL that requires surgical drainage in the operating room. Ischemia, defined clinically, is an area 1 cm2 or larger, purple in color, that has a slower capillary refill, measured by digital compression and needle puncture, than the adjacent area with normal skin color. Necrosis is defined as a blackened area 1 cm2 or larger in which no perfusion has occurred, according to the criteria described earlier.
All the patients were photographed before and after surgery. All the patients agreed to sign an informed consent form. The group B patients (and their relatives whenever possible) received guidance about the proposed surgical procedure before surgery with the help of videos and photos.
This study was approved by the Research Ethics Committee of the Sociedade Evangélica Beneficente do Paraná on 21 July 2009.
Surgical Technique
All the patients were anesthetized by the same group of anesthesiologists. Control group A and the first 281 patients of study group B had local anesthesia under sedation. The remaining 124 patients of the study group underwent general anesthesia. This change in method was prompted by the fact that the subplatysmal structures were more frequently explored in the last 2 years of the authors’ practice. Because these deep structures are not locally anesthetized well, general anesthesia was chosen.
Local Anesthesia and Sedation
A combination of midazolam, ketamine, propofol, and fentanyl was used for sedation [18]. After induction of anesthesia, the patients were infiltrated with two tumescent local anesthetic solutions [17]. The first solution, for use in the incisions, was more concentrated and comprised 20 mL of lidocaine 2 %, 10 mL of ropivacaine 1 %, 220 mL of saline solution 0.9 %, and epinephrine 1:400,000. The second anesthetic solution, for use in the areas of dissection, was less concentrated and prepared with 20 mL of lidocaine 2 %, 10 mL of ropivacaine 1 %, 470 mL of saline solution 0.9 %, and epinephrine 1:500,000. Depending on the area of the face, part of these solutions was not used. The two sides of the face were infiltrated before the surgery was begun.
General Anesthesia
After induction of anesthesia with propofol (0.2–0.4 mg/min), fentanyl (5 μg/kg), and pancuronium bromide (0.08 mg/kg), the patients were maintained with a mixture of isofluorane 0.25 %, oxygen 40 %, nitrous oxide 60 %, and propofol 0.25–0.30 mg/min. To improve comfort and to diminish the use of general anesthesia drugs during surgery, the same local anesthesia infiltration described earlier was used. With both local anesthesia plus sedation and general anesthesia, cephalexin (1 g) and dexamethasone (10 mg) were infused intravenously during induction, as was clonidine, for better blood pressure control [19].
Intermittent pneumatic compression was used for prophylaxis of deep venous thrombosis during and after surgery until the patient began ambulation, usually 12 h after surgery. Elastic stockings were used during surgery and until postoperative day 15. Subcutaneous enoxaparin was used in the preoperative, operative, and postoperative periods according to strict indications based on personal risk factors and family history of deep venous thrombosis [20, 21].
The surgical procedure was performed as previously described [22, 23]. Rhytidoplasty to the middle third of the face was performed with an incision that extended from the preauricular area to just above the line of hair implantation anteriorly and to the retroauricular region posteriorly. The superficial musculoaponeurotic system (SMAS) in the middle third of the face was treated with plication or resection. No patient was treated with an SMAS flap. A lateral plication of the SMAS-platysma flap was performed.
The dissection in the neck and middle third of the face, which was always wide, was performed with Metzenbaum scissors (Marina Medical, Sunrise, FL, USA). In the neck, we tried to release the last inferior skin fold. The middle right third of the face was managed before the left third. The limit of dissection in this region followed a longitudinal line passing through the outer corner of the eye, with attention paid to ensuring that dermal ligaments had an adequate release. Hemostasis with electrocautery (Electrosurgical Generator SS-200A; WEM, Brazil, or Force FX; Valleylab, Boulder, CO, USA) was predominantly performed on larger vessels, avoiding cauterization of the flap.
Proposed Surgical Tactic
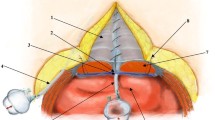
After traction and fixation of the skin-fat flap on the right side, and taking advantage of the head’s left lateral position, the hemostatic net is begun. Performed with continuous sutures, this procedure is focused on closure of the virtual space generated during dissection of the skin in the middle third of the face and the neck. Mononylon 5-0 suture is used to cover most of the dissected area (Ethilon 5-0, triangular 26-mm needle; Ethicon, São José dos Campos, SP, Brazil). Mononylon 4-0 suture (Ethilon 4-0, triangular 30-mm needle; Ethicon) may be used in areas of thicker skin.
The first line of the net begins in the most inferior and medial dissected area of the neck (Fig. 1). The needle passage follows a uniform pattern, transfixing perpendicular to the skin, plunging into the SMAS-platysma at 45°, and emerging at the same angle at a distance of 0.8–1 cm from the point of entry (Figs. 1, 2). This spacing and a mild traction on the thread by the assistant ensures that the suture will not be loose and not too tense, thus preventing impairment in blood circulation. The SMAS-platysma is encompassed at each passage of the needle so as to bring it into contact with the skin, thereby closing the space (Fig. 2).
The first line of suture ends in the most posterior portion of the retroauricular incision. The second line begins about 1 cm above, following parallel to the first and so on until all the dissected areas are covered by the hemostatic net (Figs. 3, 4, 5). After the right side is completed, the left side is dissected and treated in the same manner.
The patients in group A received a Jackson-Pratt 15-Fr tubulolaminar drain (Jac-Cell Medic, Lachine, Quebec, Canada) in each side of the face. The patients in group B, for whom the hemostatic net was used, were not drained.
The skin suture is performed with surgical staples (Proximate; Ethicon Endo-Surgery, Cincinnati, OH, USA) in the scalp, with 5-0 nylon stitches (Ethilon 5-0; Ethicon, São José dos Campos, SP, Brazil) in the retroauricular region and ear lobes, and with 4-0 poliglecaprone (Monocryl 4-0; Ethicon, São José dos Campos, SP, Brazil) in the preauricular areas.
Patients normally are hospitalized for 48 h. During this period, an occlusive dressing is maintained and changed after 24 h. On the second postoperative day, 2–3 h before discharge of the patient, the bandage and the hemostatic net are removed. As with simple skin sutures, each loop of the net is cut and removed individually.
With the described surgical procedure, it is natural to have some concerns about the quality of the scarring at the needle entry sites in the hemostatic net. To assess this, the patients in group B were evaluated at the end of postoperative months 1 and 3 for the presence or absence of skin hyperpigmentation and hypopigmentation.
Statistical Analysis
The results were analyzed using Fisher’s exact test. A p value of 0.05 or lower or 5 % was established for rejection of the null hypothesis.
Results
The descriptive analysis of both groups is shown in Table 1. The average age was 55.4 years for the patients in group A and 55.39 years for the patients in group B. Both groups had a higher percentage of women. Most of the patients were nonsmokers, normotensive, and undergoing surgery for the first time.
Figures 6, 7, 8, 9, 10, 11 illustrate the postoperative evolution of a 62-year-old patient submitted to rhytidoplasty, frontal videoendoscopy, upper and lower blepharoplasty, and submental platysmaplasty. The hemostatic net was used in this patient.
In group A, 102 patients (85 %) also were submitted to submental platysmaplasty, compared with 388 patients (96 %) in group B (Table 2). Hematomas developed in 17 group A patients (14.2 %) during the first 72 h. In group B, the hemostatic net was used, and no patient experienced this complication (p < 0.001) (Table 3).
Ischemia was observed in 11 group A patients (9.2 %), an incidence higher than in group B (6.5 %, 26 patients). This difference was not statistically significant (p = 0.408) (Table 4).
Necrosis was observed in three group A patients (2.5 %) and six group B patients (1.5 %). This difference was not statistically significant (p = 0,723) (Table 5).
Hyperpigmentation at the points of needle puncture in the hemostatic net was observed in 42 patients (17.1 %), mostly those with Fitzpatrick skin types 3 or 4. This symptom resolved in an average of 3 months. For these patients, topical hydroquinone 2 % was used after the first postoperative month to accelerate recovery. Figure 12 presents a patient who received this treatment. A month after surgery, hyperpigmentation developed in the region of the neck where the sutures had been applied. This hyperchromia disappeared after 3 months.
Persistent hypopigmentation was found in three patients (1.2 %). The patient in Fig. 13 illustrates this occurrence.
Discussion
The occurrence of hematoma complicates the postoperative evolution of rhytidoplasty by leading to more edema and fibrosis [1–4]. In large hematomas, often found when a delay in the diagnosis is longer than desirable, ischemia and necrosis of the skin-fat flap may occur, seriously compromising the outcome of surgery.
The rate of hematoma in the control group (14.2 %), although high compared with the incidences described by other authors (up to 8 %) [4], should be interpreted in light of the amplitude of the dissection performed. As stated earlier in the “Methods” section, the patients had systematically ample skin flaps in the middle third of the face. Furthermore, whereas most published works do not indicate the rate at which the neck is opened, in this analysis, 85 % of patients in the control group and 96 % of the patients in the group with the hemostatic net were submitted to submental platysmaplasty.
As observed in a study that evaluated 1,078 patients, approaching the neck via a submental incision is the factor most associated with hematoma, carrying a relative risk of 4.3, higher than the risk for arterial hypertension, gender, use of aspirin, and smoking [5]. In that study [5], 26 (13.4 %) of 194 patients who had platysmaplasty experienced hematomas, an incidence similar to that of our control group. In our experience, this incidence represents an average of 17 patients with hematoma per year. Besides the number, this rate meant stress for the surgical team and for the patients in a way that forced the development of a different solution to this problem.
Such a solution was developed for seroma in abdominoplasty with the use of internal sutures [15, 24]. The paradigm of using drains in this surgery was transformed, and many surgeons currently do not use them because of the confidence the quilting suture has generated.
Encouraged by the outcomes obtained with these abdominoplasty sutures, the authors of this study began to develop the idea of using a hemostatic net to prevent the development of hematoma. The tactic also was based on the transfixing sutures for flap stabilization in rhytidoplasty, as shown by Pontes [25], and on the work of Rho et al. [26], who showed their use in preventing the occurrence of hematoma after liposuction of the axilla in the treatment of hyperhidrosis in this region.
Previously, our routine for treating small hematomas was to drain them at the bedside, according to established procedures [27, 28]. Large hematomas were eliminated in the operating room, with drainage achieved by opening the skin sutures and cauterizing the bleeding vessels. This approach was modified about 8 years ago.
Initially, recurrent and minor hematomas (localized areas up to 5 cm in diameter) were treated by aspiration of the blood and placement of transfixing sutures to stabilize the skin and close the virtual space. It was feared that these stitches in the skin flap could lead to ischemia or necrosis, but this was not seen. Good quality of skin healing was obtained when the suture was applied and held for up to 48 h. The authors also considered that the passage of the needle in the SMAS-platysma was safe because it was not deeper than that commonly used in the SMAS plication.
The authors expanded the therapeutic use of these sutures, already in the form of a hemostatic net, to patients with large hematomas involving, for example, an entire side of the face. In fact, 15 of the 17 patients in the control group of this study (group A) who experienced hematomas were treated according to this procedure. This experience led the authors to a more daring step: to use the hemostatic net preventively and not only as a way to treat hematomas.
A routine for the surgical tactic described in this report was established. The results indicate that the hemostatic net prevents the occurrence of hematoma in the first 72 postoperative hours. The mechanisms that led to this observation may have been a combination of several factors such as total obliteration of spaces, pressure of the skin on the SMAS-platysma, and stability of the flap.
Furthermore, it was noted that the net did not lead to a higher incidence of ischemia or necrosis, two complications naturally feared with application of the suture to the flap. In this regard, some technical details should be considered. First, in the group with the net, extensive cauterization of the detached area was not necessary. This cauterization, especially for patients who bleed more, can compromise blood flow.
Second, and very important, operations with extensive dissections, as performed in this study, should ensure regular flaps thick enough to maintain good perfusion. Normally, thinner flaps tend to have more ischemia and necrosis. Such complications may be attributed to the hemostatic net when in fact the quality of the flap is itself a key determinant of its survival.
Once the surgeon is confident enough with his or her dissections, the hemostatic net may be used. It should be noted that this procedure is reversible, and if after the net is applied the quality of vascularization is in doubt, the stitches can always be removed.
Another aspect of this surgical tactic, which has been debated, is the possibility of overtreatment because, statistically, the majority of patients would not experience a hematoma. The main problem is that a hematoma is not a predictable event although risk factors are identifiable.
Considering that hematoma is followed by a more difficult recovery with delayed results and that the presented tactic eliminated this complication, the authors currently view the tactic as a necessary step during surgery. However, for those surgeons who might want to introduce the tactic in their practice, high-risk patients for hematoma could be considered first.
An additional advantage of the net is worth mentioning: skin accommodation in patients with deep wrinkles in the lower aspect of the neck. The net allows better positioning of the neck flap, leading to a more effective redraping of this region. The patient in Figs. 14 and 15 illustrates this application of the hemostatic net.
The question most often asked when this tactic is presented to patients relates to the unusual visual aspect of the hemostatic net. The use of an occlusive dressing during the hospital stay (48 h) helps to improve patient acceptance of the procedure.
Skin healing in the area of hemostatic net performance is a natural concern when this surgical tactic is considered. The first cases of hematoma management using the net showed that hyperpigmentation, when it occurred, was temporary in most cases.
It is important to note that due to the ethnic background of the city and region in which the authors have their practice, only patients with Fitzpatrick skin types 1–4 were treated. Therefore, conclusions cannot be drawn from this study about the healing quality of the hemostatic net for patients with skin types 5 or 6.
On the other hand, the few patients with hypopigmentation did not experience major repercussions because the retroauricular region was the most frequently affected, often in a barely noticeable manner. It was observed that all the affected patients had solar leukodermia. It is therefore advisable to discuss this possibility with all patients, particularly those presenting with this preoperative condition.
Conclusions
The hemostatic net is an efficient method for preventing early hematomas in rhytidoplasty. This surgical procedure did not lead to a significant increase in the incidence of ischemia or necrosis.
References
Baker TJ, Gordon HL (1967) Complications of rhytidectomy. Plast Reconstr Surg 40:31–39
Rees TD, Lee YC, Coburn RJ (1973) Expanding hematoma after rhytidectomy: a retrospective study. Plast Reconstr Surg 51:149–153
Stuzin JM (2008) Face lifting. Plast Reconstr Surg 121(1 Suppl):1–19
Baker DC, Stefani WA, Chiu ES (2005) Reducing the incidence of hematoma requiring surgical evacuation following male rhytidectomy: A 30-year review of 985 cases. Plast Reconstr Surg 116:1973–1985
Grover R, Jones BM, Waterhouse N (2001) The prevention of haematoma following rhytidectomy: a review of 1,078 consecutive facelifts. Br J Plast Surg 54:481–486
Huang TT, Blackwell SJ, Lewis SR (1987) Routine use of a suction drain in facial rhytidoplasty. Ann Plast Surg 18:30–33
Jones BM, Grover R, Hamilton S (2007) The efficacy of surgical drainage in cervicofacial rhytidectomy: a prospective, randomized, controlled trial. Plast Reconstr Surg 120:263–270
Marchac D, Sándor G (1994) Face lifts and sprayed fibrin glue: an outcome analysis of 200 patients. Br J Plast Surg 47:306–309
Fezza JP, Cartwright M, Mack W, Flaharty P (2002) The use of aerosolized fibrin glue in face-lift surgery. Plast Reconstr Surg 110:658–664
Kamer FM, Nguyen DB (2007) Experience with fibrin glue in rhytidectomy. Plast Reconstr Surg 120:1045–1051
Por YC, Shi L, Samuel M, Song C, Yeow VK (2009) Use of tissue sealants in face-lifts: a metaanalysis. Aesthet Plast Surg 33:336–339
Jones BM, Grover R (2004) Reducing complications in cervicofacial rhytidectomy by tumescent infiltration: a comparative trial evaluating 678 consecutive face lifts. Plast Reconstr Surg 113:398–403
Firmin FO, Marchac AC, Lotz NC (2008) Use of the harmonic blade in face lifting: a report based on 420 operations. Plast Reconstr Surg 124:245–255
Beer GM, Goldscheider E, Weber A, Lehmann K (2010) Prevention of acute hematoma after face-lifts. Aesthet Plast Surg 34:502–507
Baroudi R, Ferreira CA (1998) Seroma: how to avoid it and how to treat it. Aesthet Surg J 18:439–441
Auersvald A, Auersvald LA, Biondo-Simões MLP (2012) Hemostatic net: an alternative for the prevention of hematoma in rhytidoplasty. Rev Bras Cir Plást 27:22–30
Jones BM, Grover R (2004) Avoiding hematoma in cervicofacial rhytidectomy: a personal 8-year quest—reviewing 910 patients. Plast Reconstr Surg 113:381–387
Gruber RP, Morley B (1999) Ketamine-assisted intravenous sedation with midazolam: benefits and potential problems. Plast Reconstr Surg 104:1823–1825
Beninger FG, Pritchard SJ (1998) Clonidine in the management of blood pressure during rhytidectomy. Aesthet Surg J 18:89–94
Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL (2004) Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg 114:43E–51E
Durnig P, Jungwirth W (2006) Low-molecular-weight heparin and postoperative bleeding in rhytidectomy. Plast Reconstr Surg 118:502–507
Baker DC (1997) Lateral SMASectomy. Plast Reconstr Surg 100:509–513
McKinney P, Celetti S, Sweis I (1996) An accurate technique for fixation in endoscopic brow lift. Plast Reconstr Surg 97:824–829
Nahas FX, Ferreira LM, Ghelfond C (2007) Does quilting suture prevent seroma in abdominoplasty? Plast Reconstr Surg 119:1060–1064
Pontes R (2011) O Universo da Ritidoplastia. Revinter, Rio de Janeiro
Rho NK, Shin JH, Jung CW, Park BS, Lee YT, Nam JH, Kim WS (2008) Effect of quilting sutures on hematoma formation after liposuction with dermal curettage for treatment of axillary hyperhidrosis: a randomized clinical trial. Dermatol Surg 34:1010–1015
Pitanguy I, Ceravolo MP (1981) Hematoma post rhytidectomy: how we treat it. Plast Reconstr Surg 67:526–529
Baker DC, Chiu ES (2005) Bedside treatment of early acute rhytidectomy hematomas. Plast Reconstr Surg 115:2119–2122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Auersvald, A., Auersvald, L.A. Hemostatic Net in Rhytidoplasty: An Efficient and Safe Method for Preventing Hematoma in 405 Consecutive Patients. Aesth Plast Surg 38, 1–9 (2014). https://doi.org/10.1007/s00266-013-0202-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-013-0202-5