Abstract
Background
This study aimed to determine the efficacy and safety of a new facial rejuvenation procedure that combines a fractional carbon dioxide (CO2) laser, an ultrasound emitter, and a cosmeceutical preparation to be applied intraoperatively.
Methods
A split-face, double-blind randomized prospective study of 14 patients was designed, in which one half of the face was treated with a fractional CO2 laser, with the other half receiving the same laser and acoustic pressure ultrasound for transepidermal delivery of cosmeceuticals. Two semiquantitative scales and two visual analog scales were completed to evaluate the efficacy of each treatment. The results were assessed on the basis of photographs taken before treatment and then after 1, 2, and 6 months afterward. Potential adverse effects and complications were recorded.
Results
Both treatments achieved significant improvements in all parameters evaluated (p < 0.001). The combined ultrasound and cosmeceutical treatment had better scores for reduced fine lines and wrinkles as well as for overall facial aging at 6 months (p < 0.01), with nearly 80 % overall improvement in facial aging. The treatment was well tolerated, and no unexpected adverse effects were observed. The majority of the patients (86 %) stated that they were satisfied or very satisfied with their results.
Conclusion
One session of fractional ablative CO2 laser and acoustic pressure ultrasound technology for transepidermal delivery of cosmeceuticals is an effective method for treating facial rejuvenation.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Facial rejuvenation is one of the most demanded aesthetic procedures. The most common treatments involve topical retinoids, cosmeceuticals, botulinum toxin, soft tissue fillers, light/laser procedures, and surgery (face-lifting). The laser industry has developed many different treatment possibilities that have proved to be efficient. This is of particular interest to plastic surgeons because these can be an important complement to face-lifting procedures. In practice, the success of laser techniques in facial rejuvenation is directly related to the procedure used, the therapist’s experience in its application, and the patient’s interests and expectations.
The use of fractional ablative resurfacing is on the increase. It thermally ablates microscopic columns of epidermal and dermal tissue in regularly spaced arrays over a fraction of the skin surface. The popularity of fractional resurfacing in recent years is due to its highly satisfactory results achieved with a brief and well-tolerated postprocedure phase. The industry is therefore seeking to manufacture new equipment to provide improvements in addition to those achieved to date [1].
The prescription of bioactive cosmetic products before and after application of a laser treatment (LT) is deemed to be essential by most experts. Lupo and Jacob [2] state that incorporating cosmeceuticals into the perioperative skin care regimen can promote a better overall patient experience by hastening postoperative healing, reducing common side effects, and enhancing overall rejuvenation. However, almost no studies have investigated the latter. Such bioactive cosmetics often include peptides, which have proved to favor the in vivo synthesis of collagen [3], antioxidants that seek to prevent photoaging by reducing the synthesis of reactive oxygen species (ROS) [4], products obtained from plants with pharmacologic activity, and vitamins and growth factors with well-known effects on cutaneous physiology. The problem is that such substances, when applied topically, fail to reach the cellular targets or receptors in the dermis [2].
Ultrasound has an ever-increasing role in the delivery of therapeutic agents including proteins and chemotherapeutic drugs. Cavitating gaseous bodies such as microbubbles are the mediators through which the energy of relatively noninteractive pressure waves is concentrated to produce forces that permeate cell membranes and disrupt the vesicles that carry drugs. The presence of microbubbles and the acoustic pressure notably enhances delivery of proteins and smaller chemical agents [5].
This study aimed to examine the efficacy and safety of a new fractional ablative carbon dioxide (CO2) laser and acoustic pressure ultrasound technology for transepidermal delivery of cosmeceuticals in the treatment of facial rejuvenation. A split-face comparison is used, whereby one side of the face is treated only with a laser while the other side is treated with the same laser plus cosmeceuticals applied via ultrasound.
Materials and Methods
Patients
This study recruited 14 patients via systematic sampling among scheduled visits at the Instituto Médico Vilafortuny (Cambrils, Tarragona, Spain) who were interested in undergoing facial rejuvenation treatment. Those who met the inclusion criteria were offered the possibility of taking part in a split-face prospective study, with a clear explanation of the treatment aims and expected results. They were informed that a clear improvement was expected 1 month after treatment but that definitive results would not be assessed until 6 months after the therapy. At this point, the patients would be asked to evaluate their degree of satisfaction using the following terms: very dissatisfied, dissatisfied, somewhat satisfied, satisfied and very satisfied.
All the subjects agreed to the terms and conditions for inclusion in the study and undertook to appear at the subsequent control and follow-up visits on the set dates. The assay was conducted according to the Declaration of Helsinki and approved by the Antoni de Gimbernat Foundation Ethics Committee. All the patients signed a written informed consent before inclusion in the study, thus authorizing the diffusion of their photographs in the scientific media.
The inclusion criteria specified an age of 40–79 years, moderate or severe signs of facial aging relative to chronological age, acceptance of the terms and conditions for active participation, and commitment to the follow-up phases thereof. The patients with important organic or psychological conditions, those receiving oral or topical treatments that could interfere with results, those who had undergone previous facial procedures of any kind (e.g., light or LTs, Botox, fillers, surgery), and pregnant and lactating women were excluded from the study.
A randomized double-blind, split-face prospective study was conducted, in which one side of the face was treated with a fractional CO2 laser (LT), with the other side treated using the same laser and ultrasound injected cosmeceuticals [combined treatment (CT)]. The application of LT or CT treatments to the right or left side of the face was randomized via sealed envelope so that LT was allocated to seven half-faces, and CT was allocated to another seven half-faces. Only the therapist (main researcher), not the patient or blind evaluators (double-blind), had knowledge of the treatment applied to each half-face.
All the included subjects were evaluated before treatment and then 1, 2, and 6 months after treatment. The instructions provided to the patients with regard to interpretation and completion of the questionnaires, application of the treatment, and follow-up evaluation and gathering of photographic images were handled by a single physician specifically trained to perform the procedure.
During each evaluation, standardized frontal and bilateral 45° side photographs were taken using the same camera (Nikon CoolpixP50, 12.1 Megapixels; Tokyo, Japan) settings, lighting, and patient positioning.
The Laser Device
A CO2 laser (Pixel CO2 laser; Alma Lasers, Caesarea, Israel) in fractional mode was used. This device is an ultrashort synchronized pulsing CO2 laser, and the console is fitted with three programs for fractional treatment with varying degrees of pulse power and density. The high program uses 60 W, whereas the medium and low options use 40 and 20 W, respectively. To achieve ablation channels, the treatment settings were high (60 W), with 50–70 mJ per pixel at a corresponding penetration depth of 120 μm. The laser settings and parameters used were exactly the same in both treatments (LT and CT).
The tip of the laser handpiece used as a spacer is fitted with a rolling device that emits laser beams when in contact with the skin and rolled over it. During treatment, the handpiece is rolled over the skin surface, and the speed, the number of passes, and the crisscross passing technique of applying laser beams produce a greater or lesser number of pixels per area. Therefore, pulse density is correlated with a greater or lesser area of treated skin, which is likewise significant for the effects and the recovery time.
Ultrasound
The console is fitted with an ultrasound generator with pulse modulation control that emits an output power of 40 W, with impacts ranging from 10 to 100 %. The output frequency is 27.5 Hz, with variables between 10 and 100 Hz. The (impact) ultrasound parameters on the control screen are expressed in percentages (%) of intensity between 0 and 100 %. The pulse rate is expressed in Hz (1/T). Regardless, the impact is a low-frequency ultrasound that operates in ~30 Hz.
The treatment is applied by moving the tip of the ultrasound handpiece, which has a trumpet-like design, in a circular motion. The time is set according to the size of the area to be treated.
Cosmeceuticals
The manufacturer (Alma Lasers, Caesarea, Israel) recommends a gel and cream treatment as a complement to the laser (Profound Pixel Pixelprep, Profound Pixel Pixelcleanse, Profound Pixel PixelRelief, and Profound Pixel FinalTouch), to be applied before and after each session. These cosmeceuticals contain hydrating components, soaps, moisturizers, oligopeptides, vitamins, lipids (oils), and natural extracts.
After laser resurfacing, the PixelTreatSR serum (Alma Lasers, Caesarea, Israel) is applied on the treated area, followed immediately by ultrasound passes to help the transepidermal absorption. The serum contains 18 ingredients for transepidermal delivery including keratolytics (allantoin), lipids (bisabolol), vitamins (C and E), and bioactive peptides (palmitoyl oligopeptide, palmitoyl tetrapeptide-7, SH-polypeptide-15, tripeptide-1, trifluoroacetyl tripeptide-2, acetyl tetrapeptide-2, palmitoyl hexapeptide-19, and tripeptide-8).
In this study, both half-faces received the same cream program regimen, except for the PixelTreatSR serum, which was applied only to the half-faces receiving ultrasound treatment (CT). Apart from this exception, both the pre- and posttreatment creams were the same for LT and CT.
Procedure
Before the LT, a lidocaine cream (Lambdalina; Lab. Isdin, Barcelona, Spain) was used on the whole face. The cream was applied 2 h before resurfacing, and the patients were advised that if they experienced too much pain during treatment, local anesthesia would be injected.
Half of the face was randomly selected for LT or CT resurfacing, and the selected laser settings were the same for all the patients. The high program option was chosen. The laser was set at 60 W, and pulse density (pixels per area) was set at the maximum, which means that the laser beam hits on the skin were 1 mm apart from each other.
The whole face was treated with two passes. The first pass was performed rolling the tip of the handpiece in one direction, with the second pass crisscrossing the first. Extra passes were given to those areas with wrinkles and flaccidity. Fractional treatment was adapted to tissue need (i.e., with more or fewer passes, 4–6 extra passes) to achieve the best possible outcome in one single treatment session.
During pixel fractional resurfacing, cold air was used (Cryo V; Zimmer, Ulm, Germany) set on program 5, which corresponded to a flux speed of 600 l/min. The nozzle was pointed directly over the tip of the laser handpiece, following its movement. Cold air flow at this speed decreases the sensation of heat from the laser and helps to ease pain.
Once resurfacing was completed, the half of the face selected was treated with the ultrasound device. Before the ultrasound, the PixelTreatSR serum supplied by the manufacturer was applied homogeneously to the treated area, and the ultrasound then was passed gently over the skin. For this purpose, the console display was programmed to operate a total of 6 min over half of the face, although treatment was terminated early if onset of pinpoint bleeding occurred at passing of the device.
The medication prescribed before treatment was 10 mg of diazepam together with 1 g of paracetamol. The posttreatment medication comprised 1 g of paracetamol every 6 h during 2 days and 4 mg of methylprednisolone three times per day for 3 days.
Assessment of Results
All pre- and posttreatment evaluations were carried out on the basis of photographic images viewed on the computer screen. A blinded physician investigator rated the degree of pigmentation (P), fine lines/wrinkles (W), and overall aging (OA) using a 5-point grading scale (0–4GS) as follows: 0 (absent), 1 (slight), 2 (mild), 3 (marked), 4 (severe). Ratings were allocated by viewing the photographs in pairs (before and after) without knowing whether LT or CT had been applied.
Clinical efficacy was assessed by the same blinded physician investigator using a 6-point Global Aesthetic Improvement Scale (0–5GAIS) to evaluate the overall aesthetic change as follows: 0 (no improvement), 1 (20 % improvement), 2 (40 % improvement), 3 (60 % improvement), 4 (80 % improvement), 5 (100 % improvement). Scores were allocated to each half-face independently and at each follow-up visit.
A visual analog scale (VAS) with the degree of facial aging severity marked on a 100-mm horizontal line was established, in which 0 mm indicated no signs of aging and 100 mm indicated very severe signs of aging relative to the patient’s chronological age. This VAS scale was completed separately by two blinded dermatologists (VAS-1 and VAS-2) on the basis of the lateral photographs taken before treatment and 6 months after treatment. A total of 56 lateral photographs viewed online and out of sequence were scored, without the raters knowing whether the photographed patients had been treated or not. In each photograph, only the overall aging was assessed.
Side Effects and Complications
Any possible complications or side effects were recorded during the procedure, in the immediate posttreatment stage, and at subsequent follow-up visits. Enquiries were made as to pain felt during the procedure for each of the half-faces treated. The pain was classified as light, moderate, severe, or very severe.
The degree of pain was evaluated through a questionnaire immediately after the procedure. The next day and on days 4 and 7, visual signs at inspection and any discomfort reported by each patient were investigated and recorded in detail in the patient’s medical history.
Statistical Analysis
Data were entered and processed using SPSS v.13.0 for the Windows program (Chicago, IL, USA). The descriptive statistical data included the average value or arithmetic mean (m), the standard deviation (SD), and the percentage (%). Baseline and posttreatment evaluations were compared by the Wilcoxon signed rank test, and the results of the two methods were compared by the Mann–Whitney U test (nonpaired results). A p value lower than 0.05 was considered statistically significant.
Results
Patients
The study included 14 patients (11 women and 3 men) ranging in age from 42 to 76 years (mean, 59.4 years) with Fitzpatrick skin types 2–4 exhibiting moderate to severe facial aging relative to chronological age. All the patients completed the study.
Improvement in Pigmentation, Fine Lines/Wrinkles, and Overall Facial Aging
Table 1 shows the observed improvements in pigmented lesions (P), fine lines and wrinkles (W), and overall facial aging (OA) separately by means of a 0–4GS. The 1-month follow-up assessment showed the achievement of significant improvements in the three parameters studied with both treatments. Lessening or disappearance of pigmented lesions was observed early in the first month with both the LT and CT treatments (p < 0.001), with no significant differences between them. Fine lines and wrinkles were reduced after the first month with both treatments (p < 0.001), but better results were not achieved with the LT after 2 and 6 months. On the other hand, significant reductions were observed after 6 months with CT, over and above those obtained with the LT (p < 0.01). Overall aging was gradually reduced with both treatments, but the scores at months 1, 2, and 6 were lower with CT, proving it to be more effective (p < 0.05).
At 30 days after control, no side effects were noticed. Interestingly, with fractional treatment, despite its high intensity and with various passes in some areas, tissue healed rapidly, and erythema was practically nonexistent at this control point. Increased risk of adverse effects was not observed even in those patients with darker skin phototypes. At the 30-day control assessment, the patients did not complain, and the results were acceptable. No hyperpigmentation was seen in any patient.
Clinical Efficacy According to the Blinded Physician Investigator
The average 0–5GAIS ratings on the sides of faces that had received the LT were 3.14 ± 0.93 at 1 month, 3.36 ± 0.64 at 2 months, and 3.44 ± 0.76 at 6 months. This is equivalent to respective overall improvement averages of 63, 67, and 69 % (Fig. 1). The ratings increased in months 2 and 6 compared with month 1, without reaching statistically significant differences.
The average scores for the sides of faces that had undergone CT were of 3.22 ± 0.84 at 1 month, 3.58 ± 0.73 at 2 months, and 3.96 ± 0.65 at 6 months, denoting respective overall improvement averages of 64, 72, and 79 %. When the two treatments were compared, the results at month 6 for the half-faces treated with CT proved to be better (p < 0.05).
VAS-1 and VAS-2
After the out-of-sequence viewing of the photographs, the first dermatologist (VAS-1) determined an average reduction in facial aging of 66–41 mm for LT (p < 0.001) and of 69–35 mm for CT (p < 0.001). The reduction was significantly greater for the sides of the faces treated with the CT (p < 0.01).
The second dermatologist (VAS-2) established a reduction of 73–48 mm with LT (p < 0.001) and of 67 to 32 mm with CT (p < 0.001). The reduction also proved to be significant for the sides treated with the CT compared with LT (p < 0.01).
Intra- and Postoperative Phases, Side Effects, and Complications
Pain was moderate for three patients (22 %), severe for nine patients (64 %), and very severe for two patients (14 %), who required anesthetic infiltration for completion of the procedure. Pain was felt during the LT but not during the application of ultrasound and cosmeceuticals. All the patients reported a burning and stinging sensation, which subsided within the first 24 h, except in three patients for whom the discomfort continued as long as 72 h.
Among the signs observed by the therapist were erythema, edema, slight bleeding, and formation of thin scabs, which were more evident on the side treated with CT. In most cases, such signs disappeared completely during the first week after treatment. At the 1-, 2-, and 6-month follow-up assessments, no type of local or systemic side effect attributable to LT or CT was observed; nor was there any hypersensitivity or intolerance reaction to the cosmeceuticals applied.
Patient Satisfaction at Completion of the Study
At month 6 of treatment, the patients rated their satisfaction with treatment as follows: two patients were somewhat satisfied (14 %); seven patients were satisfied (57 %); and five patients were very satisfied (29 %). When asked about the possible minimum differences they observed between the sides of their faces when looking at themselves in the mirror, ten patients (71 %) reported a relative improvement on the side of the face treated with CT, whereas four patients (29 %) reported improvement in the half-face treated with LT.
Images
Figures 2, 3, 4 and 5, show the results with illustrative photographs and the scores obtained on the 0–4GS, 0–5GAIS, VAS-1, and VAS-2 scales.
Postprocedural evolution shown for a 47-year-old woman a before, b 1 week after, and c 1 month after treatment. A delayed shedding of scabs, which are deeper and more attached on the left half-face (subjected to CT) is apparent. After 1 month, fine lines and wrinkles were notably less visible (forehead, orbital area, and lower third of the face), without significant differences between the two half-faces (0–5GAIS: LT = 3, CT = 3). 0–5GAIS, 6-point Global Aesthetic Improvement Scale; LT laser treatment, CT complete treatment
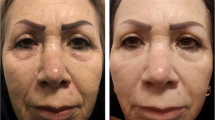
A 76-year-old woman a before and b 6 months after right half-face complete treatment (CT) and left half-face laser treatment (LT). An improvement in the clearing of hyperpigmentation, fine lines, wrinkles, and overall aging on both half-faces can be observed, particularly on the right half-face. This is a clear case of improved results with CT (0–5GAIS: LT = 3, CT = 5). 0–5GAIS, 6-point Global Aesthetic Improvement Scale
A 62-year-old woman a before and b 6 months after complete treatment (CT) of the left half-face. Significant improvement is shown by the following pattern of results in terms of the 5-point grading scale (0–4GS): a pigmentation (P) = 3, fine lines/wrinkles (W) = 4, overall aging (OA) = 3; b P = 1, W = 1, OA = 1; 0–5GAIS = 4, VAS1A = 75, VAS1B = 38, VAS2A = 70, VAS2B = 49. 0–5GAIS, 6-point Global Aesthetic Improvement Scale; VAS visual analog scale
A 51-year-old woman a before and b 6 months after complete treatment (CT) of the left half-face. Significant improvement is shown by the following pattern of results in terms of the 5-point grading scale (0–4GS): a pigmentation (P) = 4, fine lines/wrinkles (W) = 3, overall aging (OA) = 3; b P = 0, W = 0, OA = 0; 0–5GAIS = 5, VAS1A = 68, VAS1B = 29, VAS2A = 64, VAS2B = 37. 0–5GAIS, 6-point Global Aesthetic Improvement Scale; VAS visual analog scale
Discussion
The first studies on fractional ablative CO2 facial rejuvenation suggested that improvements of 70 % could be obtained on ratings of overall facial rejuvenation scales by viewing photographs [6]. An excellent efficacy and safety profile is recognized, but there have been many attempts to improve on these results [7–9].
A single LT or CT treatment session produces an improvement in the overall cosmetic outcome at 6 months of 69 % with LT and 79 % with CT. Both results seem clearly superior to those described by other authors after various resurfacing sessions using a fractional ablative CO2 laser [10, 11].
Tajirian and Goldberg [12], reviewing the literature, showed that fractional ablative laser skin resurfacing can have ablative efficacy similar to that of skin resurfacing using a nonfractional CO2 laser, with fewer side effects, but over a greater number of sessions. Findings also have shown that the clinical improvements of rhytids and pigmentations in facial and nonfacial skin are proportional to increasing energy and density settings [13]. The high treatment program was selected after discussion with patients on whether they preferred treatment in three sessions or one single session.
To eliminate wrinkles around the mouth, four to six passes at an energy of 60 W were performed, which corresponded to the high-intensity program of the laser system (pixels per area) set at the maximum (e.g., the laser beam hits on the skin are 1 mm apart from each other; see Procedure section). Four to six passes produce a residual thermal deposit in the dermis and a progressive increase in collagenosis. Mechanisms of thermal repair are activated with production of new collagen by fibroblasts [14].
The manufacturer of the laser actually recommends two to three sessions for any treatment, but we found that patients were more inclined to undergo only one session. A greater number of treatment sessions obviously has the advantage of a faster recovery after each session due to the lower power settings of the medium and low programs. However, although the use of the high program in one single session means a longer recovery period, patients have to go through it only once, and the total number of days for recovery is shorter.
We assumed that with one treatment session using the high program, notable effects could be achieved relative to various sessions with lower energy programs. This observation was based on the authors’ experience and backed by the residual heat deposit in tissue that produces neocollagen formation during wound repair [15, 16].
In our study, LT and CT procedures proved to be very effective in treating facial aging. Although most of the effect was achieved with the LT, the combination of ultrasound and cosmeceuticals yielded better statistical results at month 6 in terms of reduced fine lines and wrinkles and overall facial rejuvenation ratings.
The results show that the overall effect on facial rejuvenation assessed using the 0–4GS was better with CT during the first month of treatment. However, when the 0–5GAIS was used, no significant differences were obtained until month 6. It was striking to observe, however, that, at the end of the study, when the patients were asked to decide which side of the face satisfied them best, 71 % preferred the side treated with CT, which is consistent with all the observations made by blinded expert evaluators at month 6. The VAS scales, which merely compared photographs before the treatment and 6 months afterward, proved that CT yields better results in the medium to long term.
On a theoretical level, transepidermal application of cosmeceuticals by ultrasonic emulsification might improve the results of fractional ablative resurfacing due to different action mechanisms. What the medical histories prove is that the acoustic trauma caused to the dermis significantly increases pinpoint bleeding and the formation of larger and more numerous thin scabs during the postoperative stage. This greater skin reaction may lead to a greater increase in the formation of new collagen in the medium term, although chemical reactions favoring neocollagenogenesis or oxidative metabolic inhibitors may intervene due to the action of the cosmeceuticals. On the other hand, this possible delayed effect is consistent with our results, with statistical significance obtained on four different scales by three blinded investigators.
This study included only patients who had not previously undergone any other facial cosmetic procedures. Nevertheless, CT could be a highly interesting complementary treatment for patients who have previously undergone face-lifting. When prescribed, face-lifting is the best alternative for correction of skin flaccidity and sagging without affecting other signs of aging such as hyperpigmentation and small periocular and perioral wrinkles.
In our experience with three patients who received CT after previously undergoing face-lifting, the results were very satisfactory. Therefore, the new treatment also seems to be an ideal complement to this type of surgery (awaiting publication).
In conclusion, single-session fractional ablative CO2 laser and acoustic pressure ultrasound technology for transepidermal delivery of cosmeceuticals is a novel effective method in the treatment of facial rejuvenation. As shown in the photographs, clinical improvement is evident, and 86 % of the patients reported that they were either satisfied or very satisfied. The method that allows for these results to be achieved is very important for this reason, as fully described in the Materials and Methods section. Clinical trials with larger sample sizes are required to confirm the efficacy profile and the side effects described in this study.
References
Alexiades-Armenaka MR, Dover JS, Arndt KA (2008) The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol 58:719–737
Lupo M, Jacob L (2011) Cosmeceuticals used in conjunction with laser resurfacing. Semin Cutan Med Surg 30:156–162
Nusgens BV, Humbert P, Rougier A, Richard A, Lapière CM (2002) Stimulation of collagen biosynthesis by topically applied vitamin C. Eur J Dermatol 4:32–34
Humbert PG, Haftek M, Creidi P et al (2003) Topical ascorbic acid on photoaged skin: clinical, topographical, and ultrastructural evaluation: double-blind study vs placebo. Exp Dermatol 12:237–244
Pitt WG, Husseini GA, Staples BJ (2004) Ultrasonic drug delivery: a general review. Expert Opin Drug Deliv 1:37–56
Cassuto DA, Sadik NS, Scrimali L, Sirago P (2008) An innovative device for fractional CO2 laser resurfacing: a preliminary clinical study. Am J Cosmet Surg 25:97–101
Chan NP, Ho SG, Yeung CK, Shek SY, Chan HH (2010) Fractional ablative carbon dioxide laser resurfacing for skin rejuvenation and acne scars in Asians. Lasers Surg Med 42:615–623
Alexiades-Armenaka M, Sarnoff D, Gotkin R, Sadick N (2011) Multicenter clinical study and review of fractional ablative CO2 laser resurfacing for the treatment of rhytides, photoaging, scars, and striae. J Drugs Dermatol 104:352–362
Trelles MA, Shohat M, Urdiales F (2011) Safe and effective one-session fractional skin resurfacing using a carbon dioxide laser device in super-pulse mode: a clinical and histologic study. Aesthet Plast Surg 35:31–42
Tierney EP, Hanke CW (2011) Fractionated carbon dioxide laser treatment of photoaging: prospective study and review of the literature. Dermatol Surg 37:1279–1290
Stebbins WG, Hanke CW (2011) Ablative fractional resurfacing for photoaging of the hands: pilot study of 10 patients. Dermatol Ther 24:62–70
Tajirian AL, Goldberg DJ (2011) Fractional ablative laser skin resurfacing: a review. J Cosmet Laser Ther 13:262–264
Sasaki GH, Travis HM, Tucker B (2009) Fractional CO2 laser resurfacing of photoaged facial and nonfacial skin: histologic and clinical results and side effects. J Cosmet Laser Ther 11:190–201
Trelles MA, Garcia RJ, Mellor K (1998) Coherent versus sharplan dans le resurfacing cutane: modifications du collagene et de l’ elastine. J Med Esthet Chir Dermatol 97:15–20
Trelles MA (2003) Laser ablative resurfacing for photorejuvenation based on more than a decade’s experience and 1,200 patients: personal observations. J Cosmet Dermatol 2:2–13
Trelles MA, Rigau J, Mellor TK, Garcia L (1998) A clinical and histological comparison of flash scanning versus pulsed technology in carbon dioxide laser facial skin resurfacing. Dermatol Surg 24:43–49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trelles, M.A., Leclère, F.M. & Martínez-Carpio, P.A. Fractional Carbon Dioxide Laser and Acoustic-Pressure Ultrasound for Transepidermal Delivery of Cosmeceuticals: A Novel Method of Facial Rejuvenation. Aesth Plast Surg 37, 965–972 (2013). https://doi.org/10.1007/s00266-013-0176-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-013-0176-3