Abstract
Background
Some authors have mentioned that the endoscopic harvesting of the latissimus dorsi muscle flap for breast reconstruction is an uncommon technique that has been abandoned due to its technical complexity. Therefore, its use for immediate breast reconstruction after skin-sparing total mastectomies is reported for only a few patients, without clinical images of the reconstructed breast or of the donor site. This report describes 14 breast reconstructions using the aforementioned approach, with the latissimus dorsi muscle flap harvested by endoscopy plus the insertion of a breast implant in a single surgical procedure. The objective is to show images of the long-range clinical aesthetic results, both in the reconstructed breast and at the donor site as well as the complications so the reader can evaluate the advantages and disadvantages of the technique.
Clinical Cases
From 2008 to 2011, 12 women who experienced skin-sparing total mastectomy and 2 women who underwent modified radical mastectomy were reconstructed using the aforementioned technique. The average age was 42 years (range 30–58 years), and the average body mass index was 29 kg/m2 (range 22–34 kg/m2). Three patients were heavy smokers: one had undergone a previous abdominoplasty; one had hepatitis C; and one had undergone massive weight loss. Immediate reconstructions were performed for 11 patients, and 3 reconstructions were delayed. The implant volume ranged from 355 to 640 ml. The average endoscopic harvesting time was 163.5 min (range 120–240 min), and the average bleeding was 300 ml. Four patients experienced seromas at the donor site. Acceptance of the reconstructed breast was good in six cases, moderate in seven cases, and poor in one case. Acceptance of the donor site was good in 13 cases and moderate for 1 case.
Conclusions
Endoscopic harvesting of the latissimus dorsi muscle has technical difficulties that have limited its acceptance. However, this technique offers the same quality of breast reconstruction as the open harvesting technique, with the advantage of a smaller scar at the donor site. Based on the results, the authors consider the reported technique to be useful and valid.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endoscopic harvesting of the latissimus dorsi muscle flap in reconstructive surgery was initially described in 1994 [1], but its use for breast reconstruction was not reported until the year 2000 [2]. Since then, only a few reported series have shown its use for oncologic breast reconstruction. The largest series reported its utility for immediate breast reconstruction after skin-sparing partial mastectomy [3, 4]. Only three series of respectively 52, 14, and 8 patients have reported immediate breast reconstruction after skin-sparing total mastectomy [5–7]. None of these three studies showed images of the clinical results.
Endoscopic harvesting of the latissimus dorsi muscle with an open harvesting technique has not achieved popularity because of difficulties maintaining the optic cavity and thorax anatomic curvature [5]. Consequently, endoscopic harvesting with this method requires prolonged surgical time. Menke et al. [8] reported only two endoscopically harvested latissimus dorsi muscle flaps among 121 cases of breast reconstruction using the latissimus dorsi muscle flap. Aly et al. [9] pointed it out as an uncommon technique, and Vasconez [10] stated that he has abandoned this harvesting technique.
However, some patients who undergo skin-sparing total mastectomy desire the fewest possible complications, effective postoperative care, and diminished surgical stages for breast reconstruction. These patients generally are young and thin with insufficient abdominal tissue for use as a transverse rectus abdominis muscle (TRAM) flap. Other patients do not want a TRAM flap because of its morbidity, and still others desire a smaller scar at the donor site or have other personal reasons. Hence, these patients can benefit from breast reconstruction using the latissimus dorsi muscle flap harvested by endoscopy plus breast implant insertion in a single surgical procedure.
For these reasons, we report 14 patients who underwent reconstruction using the aforementioned technique with the aim to show images of the long-term aesthetic clinical results regarding the reconstructed breast, donor site, and complications. In this way, the reader can evaluate the advantages and disadvantages of this technique. An additional objective is to increase the number of reported cases, thereby maintaining endoscopic harvesting of the latissimus dorsi muscle as a valid option for breast reconstruction.
Technique
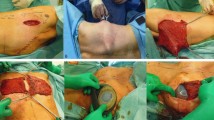
The flap was harvested after the mastectomy. For this, the patient was placed in the ventral decubitus position. Initially, 500–1,000 ml of Klein’s formula was infiltrated into the subcutaneous tissue of the latissimus dorsi muscle area. We continued with an initial 6- to 8-cm-long incision in the posterior axillary line anterior to the lateral border of the latissimus dorsi muscle. Through this incision, the free border of the latissimus dorsi muscle was identified as we continued the macroscopic subcutaneous and submuscular dissection to create the optic cavity.
A blunt dissector was carefully introduced, first through the subcutaneous tissue and then under the muscle to amplify the optic cavity. To continue with the endoscopic dissection, a 10-mm trocar was introduced through the initial incision, and the wound was sutured with 2-0 nylon. To avoid skin distension and to prevent carbon dioxide (CO2) gas leakage, a plastic protector was adhered over the skin and around the trocar. This was followed by optic cavity insufflation with CO2 gas at a pressure of 8 mmHg and a flow of 3 l/min.
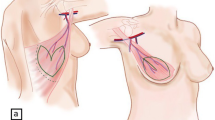
Two trocars were additionally introduced into the optic cavity under endoscopic vision according to the principles of endoscopic surgery triangulation (Fig. 1). The endoscopic dissection was initially subcutaneous, followed by a submuscular dissection. Posteriorly, the paravertebral and inferior insertions of the latissimus dorsi muscle were sectioned with an ultrasonic scalpel, and the perforator vessels were occluded with hemoclips. Thus, the muscle was extracted through the initial incision and rotated to the recipient site. The thoracodorsal nerve was conserved in all patients. A 19-Fr Blake drain was placed in the donor site. Staggered points of the subcutaneous tissue to the deep muscular plane were placed in the macroscopically accessible areas. Finally, the wounds were sutured.
To proceed with the surgery, the patient was turned over to the supine position. In the recipient site, the medial and inferior insertions of the pectoralis major muscle were sectioned. The latissimus dorsi muscle was rotated and sutured along the inframammary fold. The breast implant was placed under the pectoralis major and latissimus dorsi muscles, and both muscles were sutured (Fig. 2). A 19-Fr Blake drain was introduced under both muscles. At the recipient site, the skin was sutured according to the breast reconstruction surgical plan. A shoulder immobilizer was placed and used for 10 days.
Clinical Cases
From 2008 to 2011, 12 women who had undergone skin-sparing total mastectomy and 2 women who had undergone modified radical mastectomy benefited from breast reconstruction with an endoscopically harvested latissimus dorsi muscle flap and breast implant placement in a single surgical procedure. The demographic and clinical data are presented in Table 1. In the bilateral reconstruction cases (patients 2, 4, and 12), the skin-sparing total mastectomy indication was breast cancer and prophylaxis in the contralateral breast.
The volume of the implants used, the surgical procedure time for the endoscopic harvesting of each latissimus dorsi muscle, the total surgical procedure time, the average hospitalization duration and surgical bleeding, the number of patients with transfusions, the seroma rate and complications in the donor and receptor sites, and the follow-up time are shown in Table 2. Patient 7 required two units of packed red cells and had a prolonged hospital stay due to hematomas in the reconstructed breast and flap donor site.
Patient 12 experienced an accidental sectioning of the thoracodorsal artery of one flap, requiring microsurgical repair and prolongation of the total surgical time. Four patients experienced donor-site seromas (patients 6, 8, 10, and 14), which were treated with serial percutaneous punctures on an outpatient basis. Patient 2, who had a heavy smoking history, experienced thoracic cutaneous flap necrosis and underwent wound care on an outpatient basis until secondary healing was achieved.
The patients evaluated their results. One of the authors directly interviewed them at least 6 months after their surgery. They were questioned about several concepts regarding their approval of the reconstructed breast (Table 3) and acceptance of the donor site (Table 4). Six patients described good acceptance of their reconstructed breast (Figs. 3, 4), and seven patients described moderate acceptance (Fig. 5). Their most frequent complaint was asymmetry compared with the contralateral breast when the reconstruction was unilateral, and one patient with bilateral reconstruction experienced Baker’s grade 2 capsular contracture (patient 2). Patient 7 reported poor acceptance. This patient had a history of hepatitis C and experienced volume, position, and shape asymmetry as well as Baker’s grade 3 capsular contracture (Table 5).
A 30-year-old woman (patient 11) who had a thoracic wall sarcoma and underwent resection of the third, fourth, and fifth costal arches; pectoralis major and minor resection; skin-sparing total mastectomy; and pleura resection. a, c Preoperative view. b, d Delayed breast reconstruction with the latissimus dorsi muscle endoscopic flap and breast implant of 315 ml and a contralateral mastopexy with breast implant of 195 ml. View 6 months after surgery. e The latissimus dorsi muscle has been rotated at the recipient site. f Resulting donor-site scar
A 42-year-old woman (patient 12) who had in situ breast cancer and a positive family history for breast cancer. a, c Preoperative view. b, d Postoperative outcome after bilateral skin-sparing total mastectomy with immediate reconstruction using endoscopic latissimus dorsi muscle and 355-ml implants. View 18 months after surgery. e Skin-sparing total mastectomy. f Resulting donor-site scar 18 months after surgery
A 46-year-old woman (patient 10) who had a modified radical mastectomy and massive weight loss. a, c Preoperative view. b, d View 2 years after breast reconstruction with endoscopic harvesting of the latissimus dorsi muscle flap and a 350-ml breast implant. Breast augmentation with a 140-ml breast implant in the right breast. e Breast implant, pectoralis major, and latissimus dorsi muscles. f Resulting donor-site scar 24 months after surgery
Satisfaction regarding the donor site was good for 13 patients. Patient 2 had moderate satisfaction with the donor site because she desired smaller scars.
Discussion
The main difference between open and endoscopic harvesting of the latissimus dorsi muscle is the resulting scar at the donor site. With the open technique, this scar generally is wide with irregularities and folds [11]. Although Hammond [12] reported improvement of the resultant scar and Vasconez [10] stated that the resulting scar will be of no importance to the patient if the breast reconstruction is appropriate, the scar still is the main disadvantage of this method.
Regardless of the scar’s appearance, it is almost always a concern, especially in young patients. With endoscopic harvesting, the resulting 6- to 8-cm scar extension contrasts with the 21.61-cm scar that resulted from the open harvesting technique of this muscle in one study [11]. Although our evaluation was subjective, the patients were satisfied.
Many patients have benefited from the addition of a skin island. However, in other patients, preoperative mastectomy planning can conserve much of the skin. These patients can benefit from endoscopic harvesting of the latissimus dorsi muscle flap. The resulting scar will be shorter.
Endoscopic harvesting of the latissimus dorsi muscle differs in various technical concepts (Table 6). All the current authors agree that patients who undergo reconstruction with this technique experience less pain, enjoy a faster recovery, and have a smaller scar at the donor site than those who undergo the open harvesting technique. In addition, the use of this muscle for breast reconstruction does not affect the patient functionally [13]. We understand that although all these benefits have been reported, it is necessary to perform comparative studies.
The dorsal decubitus position, the infiltration of Klein’s formula, and the maintenance of the optic cavity with CO2 allowed us to achieve total latissimus dorsi muscle extension more easily and comfortably. Pomel et al. [7], Missana and Pomel [5], and Güemes et al. [14] reported that endoscopic harvesting of the latissimus dorsi muscle is a mixed technique involving macroscopic and endoscopic dissection. The 6- to 8-cm initial incision facilitates identification of the anatomic planes and macroscopic dissection of a larger initial optic cavity. It also facilitates placement of the 10-mm trocar in the back in a position more medial to the posterior axillary line. All the aforementioned factors allow performance of endoscopic dissection in the paravertebral region despite the thoracic curvature.
The reported surgical time for harvesting of the latissimus dorsi muscle with the open technique ranges from 88 to 289 min [8, 11]. Therefore, the paradigm of prolonged surgical time required to harvest the latissimus dorsi muscle endoscopically remains in doubt compared with the times listed in Table 6. In 168 cases of endoscopic harvesting of the latissimus dorsi muscle flap for breast reconstruction, Nakajima et al. [4] managed to decrease the surgical harvesting time to 50 min. Missana and Pomel [5] decreased the surgical time to 64 min in 52 cases. The surgical time of 163.5 min for endoscopic harvesting in the current 14 cases also can be decreased once the learning curve is overcome by the constant use of this technique. With this method, a main disadvantage of endoscopic harvesting of the latissimus dorsi muscle flap can be eliminated.
We consider that despite our prolonged surgical time for endoscopic harvesting, the reported technique is useful for breast reconstruction because it results in an objectively smaller scar at the donor site while maintaining the same aesthetic results in the reconstructed breast according to the evaluation by our patients.
The use of acellular dermal matrixes has allowed the completion of breast reconstruction with breast implants in a single procedure. The percentage of capsular contractures reported with this technique is 0.5 %, and the percentage of total complications is 2–15 % [15]. Hence, this type of reconstruction has gained great popularity. However, when risk factors exist, such as cutaneous flaps with circulatory disturbances, smoking, obesity, radiotherapy, or previous scars, breast reconstruction in a single surgical procedure is preferentially performed with autologous tissues [16].
The cost of acellular dermal matrixes is another factor that can limit their use, resulting in the use of autologous tissues. Finally, the general condition of the patient, the local circumstances, and the surgeon’s experience all help to determine the best technique.
The total extension of the latissimus dorsi muscle together with the pectoralis major muscle provided a robust muscular cover over the breast implant and created a more favorable microenvironment. No implant loss occurred despite the thoracic cutaneous flap necrosis in patient 2. Thus, we agree with Ramakrishnan et al. [2], who reported that this technique is indicated for patients at a high risk of thoracic cutaneous flap necrosis such as patients with a smoking history.
The utility of harvesting the latissimus dorsi muscle endoscopically is well defined for breast reconstruction secondary to skin-sparing partial mastectomy because the provided volume is sufficient to achieve the breast reconstruction in a single surgical procedure [3, 4]. Some authors have added subfascial fat to the flap to maintain a permanent transferred volume [3]. However, application of this technique in skin-sparing total mastectomy has been reported for very few patients. Missana and Pomel [5] and Nakajima et al. [6] reported a main series but did not show clinical images of their results.
In our 14-case report, we showed the clinical images of three reconstruction cases. These will guide the readers in evaluating and comparing the usefulness of endoscopic harvesting of the latissimus dorsi muscle plus breast implant insertion in a single surgical procedure for breast reconstruction in patients who have undergone skin-sparing total mastectomy.
Breast reconstruction with a latissimus dorsi muscle flap harvested using the open technique plus breast implant insertion in a single surgical procedure is associated with a capsular contracture rate reaching 75 % [12]. For this reason, several authors first place a breast expander, and then during a second surgical procedure 6 months later, they switch it for a definitive breast implant. They thereby decrease the capsular contracture rate to 10 % [8, 12].
In this report, only two patients (14 %) experienced capsular contracture: one with Baker grade 2 contracture (patient 2) and one with Baker grade 3 contracture (patient 7). This rate of capsular breast contracture is similar to that in staged breast reconstruction.
The described reconstruction technique also has been used for breast reconstruction secondary to modified radical mastectomy. In these cases, the reconstruction was performed in two surgical procedures. During the first procedure, a breast expander was placed and covered with the dorsi muscle. The reported results were good [2, 17]. Nonetheless, Güeven et al. [17] did not report the contracture rate or evaluate the breast reconstruction.
We used this technique for patients 7 and 10, with both experiencing a modified radical mastectomy. Despite this, however, they had sufficient amounts of skin, and we thus decided to perform the reconstruction in a single surgical procedure.
Our results and those of other authors show that the seroma rate, surgical bleeding, hematomas, hospitalization, and reoperations are similar between endoscopic [3, 7, 11] and open harvesting of the latissimus dorsi muscle flap [8, 12, 14, 18, 19].
Various concepts have been taken into consideration in evaluating breast reconstruction results [5]. Each author has described his or her personal concept. The surgeon’s evaluation takes into consideration the shape, position, and volume of the reconstructed breast [20]. However, the patient provides the best evaluation. In this study, the patients evaluated their satisfaction with the appearance of the reconstructed breast and donor site scar as well as the limitations in their social, working, and sport activities.
Our results show that breast reconstruction with a latissimus dorsi muscle flap harvested using the endoscopic technique offers the same quality of breast reconstruction as the open harvesting technique, with the advantage of a smaller scar at the donor site. Based on our results, we consider the described technique to be useful and valid.
References
Fine NA, Orgill DP, Pribaz JJ (1994) Early clinical experience in endoscopic-assisted muscle flap harvest. Ann Plast Surg 33:465–472
Ramakrishnan V, Southern SJ, Tzafetta R (2000) Reconstruction of the high-risk chest wall with endoscopically assisted latissimus dorsi Harvest and expander placement. Ann Plast Surg 44:250–258
Losken A, Schefer GT, Carlson GW, Jons GE, Styblo TM, Bostwick J III (2004) Immediate endoscopic lastissimus dorsi flap risk of benefit in reconstructing partial mastectomy defects. Ann Plast Surg 53:1–5
Nakajima H, Fujiwara I, Mizuta N, Sakaguchi K, Ohashi M, Nishiyama A et al (2010) Clinical outcomes of video-assisted skin-sparing partial mastectomy for breast cancer and immediate reconstruction with latissimus dorsi muscle flap as breast-conserving therapy. World J Surg 34:2197–2203
Missana MC, Pomel C (2007) Endoscopic latissimus dorsi flap harvesting. Am J Surg 194:164–169
Nakajima H, Sakaguchi K, Mizuta N, Hachimine T, Ohe S, Sawai K (2002) Video-assisted total glandectomy and immediate reconstruction for breast cancer. Biomed Pharmacother 56:205S–208S
Pomel C, Missana MC, Atallah D, Lasser P (2003) Endoscopic muscular latissimus dorsi flap harvesting for immediate breast reconstruction after skin sparing mastectomy. Eur J Surg Oncol 29:127–131
Menke H, Erkens M, Olbrish RR (2001) Evolving concepts in breast reconstruction with latissimus dorsi flaps: results and follow-up of 121 consecutive patients. Ann Plast Surg 47:107–114
Aly A, Avila E, Cram AE (2000) Endoscopic plastic surgery. Surg Clin North Am 80:1373–1382
Vasconez LO (2007) Endoscopic latissimus dorsi flap harvesting. Am J Surg 194:170–171
Lin CH, Wei F-W, Levin LS, Chen MC (1999) Donor-site morbidity comparison between endoscopically assisted and traditional harvest of free latissimus dorsi muscle flap. Plast Reconstr Surg 104:1070–1078
Hammond CD (2009) Latissimus dorsi flap breast reconstruction. Plast Reconstr Surg 124:1055–1063
Adams WP Jr, Lipschitz AH, Ansari M et al (2004) Functional donor site morbidity following latissimus dorsi muscle flap transfer. Ann Plast Surg 53:6–11
Güemes A, Sousa R, Cachón R, Valcarreres P, Rufas M, Gonzalo A, Gil I, Lozano R (2008) Minimally invasive breast surgery: breast reconstruction using pure muscular latissimus dorsi flap. Cir Esp 83:85–88
Salzberg AC (2012) Focus on technique: one-stage implant-based breast reconstruction. Plast Reconstr Surg 130(Suppl 2):95S–103S
Kim JYS, Davila AA, Persing S, Connor CM, Jovanovic B, Khan AS et al (2012) A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 129:28–41
Güeven E, Basaran K, Yazar M, Özden BC, Kuvat SV, Aydin H (2010) Electrothermal bipolar vessel sealer in endoscope-assisted latissimus dorsi flap harvesting. J Laparoendosc Adv Surg Tech A 20:735–742
Taghizadeh R, Shoaib T, Hart AM, Weiler-Mithoff EM (2008) Triamcinolone reduces seroma re-accumulation in the extended latissimus dorsi donor site. J Plast Reconstr Aesthet Surg 61:636–642
Dancey AL, Cheema M, Thomas SS (2010) Prospective randomized trial of the efficacy of marginal quilting suture and fibrin sealant in reducing the incidence of seromas in the extended latissimus dorsi donor site. Plast Reconstr Surg 125:1309–1317
Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ (2009) Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 124:345–353
Acknowledgments
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iglesias, M., Gonzalez-Chapa, D.R. Endoscopic Latissimus Dorsi Muscle Flap for Breast Reconstruction After Skin-Sparing Total Mastectomy: Report of 14 Cases. Aesth Plast Surg 37, 719–727 (2013). https://doi.org/10.1007/s00266-013-0131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-013-0131-3