Abstract
Background
Autologous fat grafting has rapidly become an important treatment for soft tissue defects in cosmetic and reconstructive surgery. However, consensus is lacking on the ideal donor site for harvesting and isolating stromal vascular fraction (SVF) cells to improve survival of fat grafts. We aimed to determine the best donor site for tissue harvesting and isolation of SVF cells for fat graft survival.
Methods
Adipose tissue samples were harvested from six women who underwent an aesthetic procedure. The samples were harvested by needle aspiration of five commonly used donor sites: flank, upper and lower abdomen, and lateral and inner thigh. The adipose tissue was injected subcutaneously into nude mice and grafts were harvested at 12 weeks. We evaluated graft volume, weight, and histologic parameters of the grafts: integrity, cysts/vacuoles, inflammation, fibrosis, and neovascularization. SVF cells isolated from donor sites were counted and assayed by flow cytometry.
Results
At 12 weeks post-transplantation, weight, volume, and histologic parameters did not differ among the grafts from the five tissue donor sites. Also, SVF and levels of cell surface markers did not differ by donor site.
Conclusions
This study revealed no ideal tissue donor site for fat grafting and SVF isolation. Choosing a site should be based on ease and safety of access and the preference and request of the patient.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
THE concept of fat grafting has existed since 1893 [1] and became popular for cosmetic and reconstructive surgery over the last 26 years with the development of liposuction techniques. Autologous fat is an ideal augmentation material for facial rejuvenation and soft-tissue augmentation because of its availability, low antigenicity, minimal donor morbidity, and comparability with foreign materials. However, the unpredictable absorption rate of autologous fat grafts may be the greatest obstacle to the use of fat as the best cosmetic filler. Recent publications show a resorption rate of 20–90 % [2–4].
Many attempts have been made to improve the long-term survival of fat tissue, including processing by washing, centrifugation after harvesting, and injection techniques. One technique involves centrifugation and atraumatic transfer of fat during surgery [5]. Recent attempts to improve graft survival have involved adding platelet-rich plasma [6], erythropoietin, and stromal vascular fraction (SVF) cells [7]. SVF is a heterogeneous cell mixture containing mostly stromal cells, vascular endothelial cells, and mural cells, which can be extracted from adipose tissue as a cell pellet through collagenase digestion. Some SVF cells have been identified as adipose-derived stem cells (ADSCs) [8]. ADSCs can improve long-term survival of transplanted adipose by secreting angiogenic factors such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor under hypoxic conditions. VEGF has been shown to enhance revascularization of ischemic tissues [9, 10]. ADSCs can also differentiate toward the osteogenic, chondrogenic, adipogenic, myogenic, neurogenic, and angiogenic lineages [11–13].
Many factors play a role in the success of autologous fat grafting. One factor is the selection of the donor site. Consensus is lacking on the best tissue donor site for fat grafting and isolation of SVFs. We aimed to analyze the role of the donor site in assessing the long-term survival of autologous fat grafts by examining the weight and volume of human grafted tissue transplanted in mice. Fat grafts harvested from the flank, upper and lower abdomen, and lateral and inner thigh areas of six women were implanted in mice and assessed for weight and histologic variables as well as viability and number of SVFs isolated to assess the optimal donor site for isolation of SVFs.
Materials and Methods
Fat Harvesting
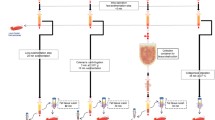
Fat samples were obtained from six female donors aged 26–37 years while they underwent cosmetic surgery. From each patient we harvested fat from the flank, upper and lower abdomen, and lateral and inner thigh. The women gave their informed consent for removal of the tissue and the study was approved by the local ethics committee. The donor sites were injected with a tumescent solution containing 0.08 % lidocaine and 1:500,000 epinephrine. Harvesting involved use of a blunt cannula with 3 mm suction and two holes in the tip attached to a 20 ml syringe with the plunger held back with 7–8 ml in order to minimize trauma to the fat grafts. After aspiration, the fat grafts were centrifuged at 1,000 rpm for 3 min to remove the lidocaine, oil, and erythrocytes.
Isolation and Counting of SVFs
Adipose tissue samples from the five donor sites were digested with 0.1 % collagenase in phosphate-buffered saline (PBS) for 30 min on a shaker at 37 °C. An equal volume of Dulbecco’s modified Eagle medium (DMEM) with 10 % fetal bovine serum was added to neutralize the collagenase. Mature adipocytes and connective tissues were separated from pellets by centrifugation (1,200×g for 5 min). The pellets were resuspended in erythrocyte lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and incubated for 5 min at room temperature. After centrifugation at 1,200×g for 5 min, the cell pellet was resuspended with PBS and number of nucleated cells was counted.
Flow Cytometry
Freshly isolated SVF cells were examined for surface molecule expression by flow cytometry. At least 1,000,000 cells were incubated with the antibodies anti-CD90 fluorescein isothiocyanate (FITC), anti-CD44 allophycocyanin (APC), anti-CD31 phycoerythrin (PE), anti-CD34 PE, and anti-CD45 PE for 30 min and washed twice in PBS. Mouse isotype antibodies served as controls. Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Fat Transplantation to Nude Mice
Thirty 6-week-old BALB/c-nu nude mice were divided into five groups (n = 6 each) for injection with fat from one of the five donor sites. All animal procedures were performed in accordance with the guidelines of the Southern Medical School Animal Care and Use Committee. The backs of nude mice were the recipient sites for fat injection. Each mouse underwent subcutaneous injection in two locations, with 0.5 ml fat injected per site by use of a 14-gauge needle.
Weight and Volume of Transplanted Fat and Histology
Animals were killed 12 weeks after fat transplantation (Fig. 1). The grafted fat tissues were excised and measured for weight and volume. Harvested samples were fixed and processed for histology. Each sample was embedded in paraffin, cut into sections, and stained with hematoxylin and eosin. In total, 12 slides were randomly chosen from each group, with five fields per slide randomly chosen for microscopy evaluation by two blinded reviewers. Each slide was evaluated for the presence of intact and nucleated fat cells; cysts and vacuoles; inflammation, as evidenced by infiltration of lymphocytes and macrophages; fibrosis; and neovascularization by capillary density. The presence of each variable was graded on a scale from 0 to 5: 0, absent; 1, minimal; 2, minimal to moderate; 3, moderate; 4, moderate to extensive; and 5, extensive [14].
Statistical Analysis
Results are expressed as mean ± SD. Statistical analyses involved one-way ANOVA, with post-hoc least significant difference tests in cases of nonhomogeneous variances across groups. A value of p < 0.05 was considered statistically significant.
Results
Long-term Survival of Fat Grafts
No animals died during the study. All grafted adipose tissue was surrounded by a thin envelope that isolated the donor adipose tissue from the recipient tissue (Fig. 1). Graft weight and volume did not differ among the five tissue donor-site grafts (p = 0.556 and p = 0.979, respectively; Table 1; Fig. 2). Tissue from the lateral thigh showed the best weight and volume but not significantly. As compared with pretransplantation fat volume, the average maintenance volume of all fat grafts was 46 %.
Histology
At 12 weeks post transplantation, mouse grafts with fat tissue from the five donor sites did not differ in histologic variables, including tissue integrity, cyst/vacuole formation, inflammation, fibrosis, and neovascularization (Table 2; Fig. 3). All of the grafts were surrounded by a collagen and fibrous capsule. The peripheral zone was composed of viable mature adipocytes. Also, a few cysts and inflammatory cell infiltration and fibrosis were observed. The central zone showed an inflammatory process with fatty cysts and collagen condensation (Fig. 3).
Histology of mouse tissue injected with fat tissue from five human donor sites 12 weeks after transplantation (hematoxylin and eosin staining, original magnification ×200). A few cysts, inflammatory cell infiltration, and fibrosis were observed. Donor sites are a flank, b upper abdomen, c lower abdomen, d inner thigh, and e lateral thigh
Number of SVFs and Characterization of SVFs by Flow Cytometry
Fat tissue harvested from the five donor sites did not differ in number of SVF cells isolated (p = 0.608). The mean number of nucleated cells in the SVFs was 1.31 × 106 per ml adipose tissue (Table 3). Fat tissue harvested from the five donor sites did not differ in the proportion of cells positive for CD31, CD34, CD44, CD90, or CD45 (Table 4; Fig. 4). The mean percentages of cells positive for CD31, CD34, CD44, CD90, and CD45 were 19.3, 62.9, 74.2, 55.1, and 6.52 %, respectively.
Discussion
With developments in liposuction techniques, liposuction has become one of the most commonly performed operations for removing unwanted fat or for autologous fat grafting. Autologous fat grafting to fill soft tissue defects or augment tissue is a common procedure that is safe and effective. However, the absorption rate of the fat grafts is the most contentious issue, and the long-term survival of fat grafts is usually disappointing for both the patient and the surgeon. Thus, surgeons have performed many clinical and experimental studies to find the source and a solution to this problem. Research into autologous fat grafts has focused on optimizing graft viability at each step of the process: choosing candidates for surgery and donor sites, fat harvesting, fat processing, and transplantation techniques. Moreover, the effect of adding vitamins, insulin, or growth factors on fat graft survival has been evaluated [15, 16]. SVF cells isolated from liposuction aspirates without any manipulation such as cell sorting or culture has recently become the focus of attention because fat transplantation with SVFs could enhance the survival rate of the graft fat [17]. However, consensus is lacking on the best donor site for isolation of SVFs.
We aimed to determine the best donor site for tissue harvesting and isolation of SVF cells. Adipose tissue samples were harvested by needle aspiration from five commonly used donor sites in six healthy women undergoing an aesthetic procedure, then injected subcutaneously into nude mice. At 12 weeks post transplantation, graft weight, volume, and histologic parameters did not differ by tissue donor site, not only for grafts from a single patient but also across all six patients. Also, the SVF fraction and cell surface marker levels did not differ by tissue donor site on flow cytometry.
Rohrich et al. [18] first attempted to find the ideal donor site for fat grafting. Quantitative in vitro colorimetric assay of cell proliferation to analyze viability revealed no body part that provided any advantage as a donor site because abdominal, thigh, flank, and knee fat were all equivalent in terms of cell viability [18]. Ullmann et al. [19] used the nude mice model to study the long-term survival of human fat in vivo. At 16 weeks post transplantation in mice, the three donor sites evaluated—thigh, abdomen, and breast—did not differ in vascularization, cyst formation, fibrosis, necrosis, or inflammation [19]. In our study we used the nude mouse model to study the long-term survival of human fat in vivo and examined five commonly used donor sites: flank, upper and lower abdomen, and lateral and inner thigh. We also did not find a statistical difference in volume, weight, and histologic variables by tissue donor site.
In determining the best donor site for SVF isolation, we did not find significant differences in SVF cell number by tissue donor site. Also, the SVFs did not differ in percentages of cells positive for CD31, CD34, CD44, CD90, or CD45 expression. A mean of 62.9 % of the SVFs expressed the stem cell-associated marker CD34, and stromal cell marker expression of CD44 and CD90 was present in 74.2 and 55.1 %, respectively, of the SVF cells. The percentages of cells positive for the endothelial cell-associated marker CD31 was 19.3 %. These results are similar to those of Mitchell et al. [20].
Conclusion
The findings in this study will be encouraging to surgeons in choosing a donor site for fat grafting and SVF isolation, which should be based on ease and safety of access and the preference and request of patients. However, long-term survival for SVF-assisted lipotransfer requires investigation in animal models.
References
Neuber F (1893) Fett transplantation. Chir Kongr Verhandl Deutsche Gesellsch Chir 22:66
Nguyen A, Pasyk KA, Bouvier TN (1990) Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg 85:378–386
Niechajev I, Sevchuk O (1994) Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg 94:496–506
Boschert MT, Beckert BW, Puckett CL, Concannon MJ (2002) Analysis of lipocyte viability after liposuction. Plast Reconstr Surg 109:761–765
Coleman SR (1997) Facial recontouring with lipostructure. Clin Plast Surg 24:347–367
Hong SJ, Lee JH, Hong SM, Park CH (2010) Enhancing the viability of fat grafts using new transfer medium containing insulin and β-fibroblast growth factor in autologous fat transplantation. J Plast Reconstr Aesthet Surg 63:1202–1208
Yoshimura K, Asano Y, Aoi N (2010) Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J 16:169–175
Zuk PA, Zhu M, Mizuno H (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228
Rehman J, Traktuev D, Li J (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298
Miranville A, Heeschen C, Sengenes C (2004) Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110:349–355
Zuk PA, Zhu M, Ashjian P (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
De Ugarte DA, Alfonso Z, Zuk PA (2003) Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett 31:267–270
Planat-Benard V, Silvestre JS, Cousin B et al (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109:656–663
Yi C, Pan Y, Zhen Y (2006) Enhancement of viability of fat grafts in nude mice by endothelial progenitor cells. Dermatol Surg 32:1437–1443
Markey AC, Glogau R (2000) Autologous fat grafting: comparison of techniques. Dermatol Surg 26:1135–1139
Nishimura T, Hashimoto H, Nakanishi I, Furukawa M (2000) Microvascular angiogenesis and apoptosis in the survival of free fat grafts. Laryngoscope 110:1333–1338
Yoshimura K, Sato K, Aoi N (2008) Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 34:1178–1185
Rohrich RJ, Sorokin ES, Brown SA (2004) In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg 113:391–395
Ullman Y, Shoshani O, Fodor A (2005) Searching for the favorable site for fat injection: in vivo study using the nude mice model. Dermatol Surg 31:1304–1307
Mitchell JB, McIntosh K, Zvonic S (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24:376–385
Acknowledgments
The authors declare that they have no conflicts of interest to disclose. This work was financially supported by the National Nature Science Foundation of China (30901566, 81171834).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, K., Gao, J., Zhang, Z. et al. Selection of Donor Site for Fat Grafting and Cell Isolation. Aesth Plast Surg 37, 153–158 (2013). https://doi.org/10.1007/s00266-012-9991-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-012-9991-1