Abstract
Background
Hypertrophic scar is the abnormal appearance of wound healing that usually causes major physical, psychological, and cosmetic problems. Treatment of the hypertrophic scar still is a dilemma due to the lack of effective and excellent methods and agents. Recent reports show that botulinum toxin type A (BTX-A) improves wound healing. Therefore, the authors hypothesized that BTX-A may be favorable for the improvement of hypertrophic scars.
Methods
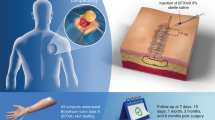
A total of 19 patients were randomly assigned to a prospective clinical study. At 1-month intervals, BTX-A (2.5 U per cubic centimeter of lesion) was injected in these patients for a total of 3 months. All the patients were followed up for at least half a year. Therapeutic satisfaction was recorded, and the lesions were assessed for erythema, itching sensation, and pliability.
Results
The study was completed by 19 patients. At the half-year follow-up visits, all the patients showed acceptable improvement, and the rate of therapeutic satisfaction was very high. The erythema score, itching sensation score, and pliability score after the BTX-A injection all were significantly lower than before the BTX-A injection. The differences all were statistically significant (P < 0.01).
Conclusion
For the treatment of hypertrophic scars, doctors and patients both found BTX-A acceptable because of its better therapeutic results. Its effect of eliminating or decreasing hypertrophic scars was promising.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hypertrophic scarring is a refractory skin disease. Patients with the disease often experience major physical deformities, restricted range of motion, pain, and pruritus. Because the basis for hypertrophic scar formation has not been fully elucidated, the clinical management of hypertrophic scarring remains problematic [1]. Numerous treatments are currently available including surgical excision, steroid injection, radiation therapy, laser, and pressure therapy, but these methods cannot obtain excellent therapeutic results. Hence, alternatives are needed.
Botulinum toxin type A (BTX-A) has become a useful tool for treating hyperactive muscles of facial expression. It is an agent that exerts its effect on the exocytosis of acetylcholine. This mechanism indirectly blocks neuromuscular transmission, resulting in muscle flaccidity. It has been used extensively to treat facial rhytides. Additionally, some papers have reported that BTX-A can minimize facial scarring because it reduces the muscle tension that acts on the healing wound. Besides the major factor that BTX-A relaxed muscle tension, the authors found that BTX-A could affect the cell cycle distribution of fibroblasts derived from the hypertrophic scar [2].
On the basis of these new findings about BTX-A, the authors hypothesized that BTX-A may be used for treating hypertrophic scars to alleviate or eliminate them. In this study, the we reported on 19 patients with persistent hypertrophic scars who were treated at our scar clinic using intralesional BTX-A injections. To our knowledge, this article is the first to report the use of BTX-A to treat hypertrophic scars in clinical work.
Patients and Methods
The study protocol for this prospective, uncontrolled trial conformed to the 1975 Declaration of Helsinki guidelines. It was approved by the review boards of the plastic surgery department at the authors’ hospital and the Research Administration of Harbin Medical University.
This study enrolled 19 patients 23 to 71 years of age referred to our department of plastic surgery. Informed consent was obtained from all the subjects. Patients treated with some other therapy within the preceding year were excluded. Apart from BTX-A, the patients received no other treatment of any kind after they had been entered into the study. Pregnant patients, patients planning a pregnancy in the near future, lactating women, patients with chronic renal failure, and patients showing any abnormalities in the liver function tests or blood cell counts were excluded from the study.
Every patient had only one lesion. Most importantly, the lesion in all the patients had persisted for at least 2 years and had maintained active hypertrophic characteristics due to individual susceptibility inherited from their families. Therefore, none of the lesions included in this study could mature spontaneously without some therapeutic measures during a period of several months.
All the patients were treated once monthly with intralesional BTX-A (Lanzou Biochemical Company, Lanzou City, P.R. China) for a total of 3 months. The solutions were injected into the body of the lesion using a gauge needle until slight blanching was clinically visible. The BTX-A dosage was adjusted to 2.5 U per cubic centimeter of lesion, but did not exceed 100 U per patient in one injection. Table 1 presents more details on the amount of BTX-A injected in every patient.
All the patients were followed up for at least half a year. Assessments were performed based on the satisfaction of both the plastic surgeon and the patient, observations, and photographic records. In assessing patients, the authors compared the clinical symptoms before and after the BTX-A injections in terms of erythema, pliability, and itching sensation of the lesions. The observer also interviewed the patients and asked them about any adverse events encountered during the study.
After the 6-month follow-up period, the overall assessment information was obtained from the plastic surgeons and patients and graded subjectively on a 5-point scale as follows: no improvement, poor (up to 25% improvement), fair (26–50% improvement), good (51–75% improvement), or excellent (76–100% improvement).
Erythema was graded on a 5-point scale as follows: 0 (no erythema), 1 (mild erythema), 2 (moderate erythema), 3 (severe erythema), or 4 (very severe erythema). Lesion lightening was defined as the percentage of erythema reduction compared with the erythema before injection of BTX-A.
Pliability also was graded on a 5-point scale as follows: 0 (no induration), 1 (mild induration), 2 (moderate induration), 3 (severe induration), or 4 (very severe induration). Lesion softening was defined as the percentage of pliability reduction compared with the pliability before injection of BTX-A.
The severity of itching sensation was graded on a 5-point scale as follows: 0 (no itching sensation), 1 (mild itching sensation), 2 (moderate itching sensation), 3 (severe itching sensation), or 4 (very severe itching sensation). Itching improvement was defined as the percentage of itching reduction compared with the itching before injection of BTX-A.
Statistical analysis was performed using SPSS 10.0 software (SPSS Inc., Chicago, IL, USA). Paired t test analyses were conducted. All statistical tests were two-tailed, with a significance level set at a P value less than 0.05.
Results
The study enrolled 19 patients, 68% of whom were women. The mean age of the patients was 38.5 years, and the mean duration of the lesions was 39 months. The lesions were distributed as follows: three on the face and neck, five on the chest, six on the back, three on the earlobe, and two on the buttocks. The maximum lesion volume was approximately 35 cm3, and the minimum volume was 2.5 cm3. Table 1 presents more details. Besides injection pain, no other complications were detected in this study.
The overall assessment from the patients showed that 12 believed the improvement of their lesions reached the level of “good” (51–75% improvement), including two lesions on the neck, two on the chest, five on the back, one on the earlobe, and two on the buttocks. Seven patients believed the improvement of their lesions reached “excellent” (76–100% improvement), including one lesion on the face, three on the chest, one on the back, and two on the earlobe.
According to the overall assessment from the plastic surgeons, the improvement of 15 lesions reached “good,” including one lesion on the face, two on the neck, three on the chest, five on the back, two on the earlobe, and two on the buttocks. Four lesions in the assessment reached “excellent,” including one lesion on the back, two on the chest, and one on the earlobe (Figs. 1 and 2).
The mean erythema score decreased from 3.41 to 1.23 due to the use of BTX-A. The pliability score decreased from 3.85 to 0.78, and the itching score decreased from 3.50 to 0.83.
Discussion
Hypertrophic scarring is a common and intractable problem caused by all types of injuries. The current treatments focus on surgical operation, injection, radiation therapy, laser, and pressure therapy, but few perfect therapeutic results have been obtained due to high recurrence [3, 4]. Treatment of the hypertrophic scar has progressed very slowly, and understanding of the underlying mechanism remains poor. Hence, it is necessary to explore the etiology and therapeutic measures of hypertrophic scarring further.
Excellent measures for eliminating or decreasing hypertrophic scarring depend on the full comprehension of the molecular processes. A better understanding of the pathophysiology of hypertrophic scarring plays an important role in the development of novel therapeutic strategies. In this regard, the hypertrophic scar results from the excessive contraction of the traumatic skin edge and relative muscle.
Additionally, at the cellular level, the abnormal proliferation of cutaneous fibroblasts is an important reason for formation of hypertrophic scars. Recent studies have elucidated that BTX-A not only can inhibit the muscle tension but also can facilitate the reasonable distribution of the cell cycle of the fibroblasts derived from hypertrophic scarring.
Some scholars have reported that BTX-A can prevent scar formation in the treatment of facial trauma [5, 6]. But has BTX-A eliminated or decreased hypertrophic scarring in the human body? No report on this problem has been found.
On the basis of these findings, the authors could logically hypothesize that BTX-A represents a novel therapeutic agent for treating hypertrophic scars. After intensive preparation, the authors carried out the clinical study to prove this hypothesis. According to the results of this study, BTX-A can decrease the volume of hypertrophic scars; eliminate the syndrome-like erythema, pliability, and itching sensation; and improve the appearance of the hypertrophic scar. Therefore, the authors firmly believe that BTX-A is promising for the treatment of hypertrophic scars.
At this writing, due to the lack of large-scale clinical and experimental research, we cannot elucidate a mechanism of action that explains precisely why BTX-A has the effect of controlling hypertrophic scarring. However, the following factors may be responsible in part for BTX-A reduction of hypertrophic scarring.
First, BTX-A prevents contraction of muscle and skin near the hypertrophic scar tissue, which decreases the tensile force during formation of the hypertrophic scar [5]. Second, BTX-A may correct the imbalance in cellular dynamics caused by the overabundance of cellular proliferation and the lack of cellular apoptosis in the tissue of the hypertrophic scar by affecting the cell cycle of fibroblasts [2]. Of course, further research is needed to obtain more theoretical support for this explanation.
This study had some limitations. First, it did not include a control condition and was not double blinded, which may affect the degree of confidence in the research. Second, the follow-up period was only half a year. Therefore, this study lacked data on long-term therapeutic effects. Recurrence may occur at later times. Third, the results of this study were obtained for only 19 patients. The authors believed the number of subjects was very small.
These limitations cannot be ignored. We will next use random, contrast, and double-blinded methods to conduct a clinical investigation on a large scale to obtain more data about this problem.
Conclusion
The clinical study investigating the use of BTX-A to treat hypertrophic scars has led to the rudimentary conclusion that BTX-A represents a novel, promising agent for the therapy for these scars. As further research is conducted, the precise role of BTX-A in the molecular mechanism for controlling hypertrophic scarring should emerge. This process should result in the development of novel methods to prevent and treat hypertrophic scarring.
References
Gassner HG, Sherris DA, Otley CC (2000) Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 105:1948–1953
Xiao ZB, Zhang MB (2008) Botulinum toxin type A affects cell cycle distribution of fibroblasts derived from hypertrophic scar. Plast Reconstr Aesth Surg 61:1128–1129.2
David AS, Holger GG (2002) Botulinum Toxin to minimize facial scarring. Facial Plast Surg 18:35–39
Richardson WS, Willis GW, Smith JW (2003) Evaluation of scar formation after botulinum toxin injection or forced balloon dilation to the lower esophageal sphincter. Surg Endosc 17:696–698
Holger GG, Anthony EB, Clark CO, Derek KB, Andy JB, Amy LW, David AS (2006) Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. Mayo Clin Proc 81:1023–1028
Dhawan S, Chopra S (2007) Nonsurgical approaches for the treatment of anal fissures. Am J Gastroenterol 102:1312–1321
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhibo Xiao and Fengmin Zhang are the primary authors of this article.
Rights and permissions
About this article
Cite this article
Xiao, Z., Zhang, F. & Cui, Z. Treatment of Hypertrophic Scars With Intralesional Botulinum Toxin Type A Injections: A Preliminary Report. Aesth Plast Surg 33, 409–412 (2009). https://doi.org/10.1007/s00266-009-9334-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-009-9334-z