Abstract
Courtship interference occurs when dominant males hinder female assessment of prospective males during female mate choice, leading to a more complex distribution of mating success. In this study, we describe and evaluate courtship interference in underground mating of the fiddler crab Austruca lactea. When a mate-searching female enters the burrow of a courting male, neighboring males frequently interfere in the female-male interaction. We thus identified mate-searching females and observed their interactions until pairing. The duration until females’ final decision for pairing increased with the number of interferences by neighboring males. The females reappeared more frequently from the burrow of the finally selected male when neighboring males interfered. These results suggest that courtship interference by neighboring males delays pairing between the mate-searching female and the finally selected male in this species. The number of interferences by neighboring males increased with female size, implying that large females with high fecundity potential induce interference by neighboring males. Moreover, in approximately half of the cases in which interference occurred at the burrow of the immediate last male before the finally selected male, the finally selected male was the interfering one. The distribution of mating success was therefore biased toward males that combined attractiveness (according to female preference) and dominance (which is associated with courtship interference) in this species.
Significance statement
Courtship interference is a type of male-male competition and it may hamper female mate choice. In Austruca lactea, neighboring males often interfere in the interaction between a courting male and a mate-searching female. We demonstrated the effect of courtship interference by neighboring males in these interactions. The interferences prolonged the duration of female decision-making for pairing and temporarily expelled the female from the burrow of the finally selected male. These results imply that these interferences may potentially prevent pairing. Moreover, the finally selected males had often succeeded in interfering in the courtship interaction between the female and another male. Therefore, males with high mating success were not only attractive to the females of this species but also dominant in courtship interference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intra- and intersexual selection are primary components of sexual selection (Andersson 1994). Intrasexual selection typically occurs when males compete for females, whereas intersexual selection generally occurs when females choose males. Many empirical studies have shown the functions of sexually selected traits, such as weaponry/ornaments, territorial/courtship display, mating strategy, and mate preference, under each type of selection (Andersson 1994). However, the types of selection are not always independent; in fact, they often interact (Qvarnström and Forsgren 1998; Wong and Candolin 2005; Hunt et al. 2009). Male-male competition, for instance, can sometimes influence female mate choice through eavesdropping (Otter et al. 1999; Mennill et al. 2002) or courtship interference (Trail 1985; Severinghaus and Lin 1990; Sparreboom 1996; Howard et al. 1997; Sæther et al. 1999; Webster and Robinson 1999; Cooley and Marshall 2001; Kangas and Lindström 2001; Fisher et al. 2003; Wong 2004; Nieri et al. 2017). This interaction may lead to the two types of selection working in the same direction or in different directions, potentially causing evolutionary consequences to be more complex than expected (Wong and Candolin 2005; Hunt et al. 2009; Baxter et al. 2018).
Courtship interference is defined as when dominant males hamper female assessment of prospective males (Wong and Candolin 2005). It has been described for several taxa, such as mammals (Fisher et al. 2003), birds (Trail 1985; Sæther et al. 1999; Webster and Robinson 1999), amphibians (Sparreboom 1996; Howard et al. 1997), fishes (Kangas and Lindström 2001; Wong 2004), insects (Cooley and Marshall 2001; Nieri et al. 2017), and crustaceans (Severinghaus and Lin 1990). The distribution of mating success would be altered because of courtship interference by dominant males. In the Guianan cock-of-the-rock Rupicola rupicola, for example, interfering males disturb pairs physically and non-physically, disrupting 31% of courtship visits and terminating 32% matings per territorial male (Trail 1985). In the sword-tailed newt Cynops ensicauda popei, interfering males disturb pairs by shoving the other male aside and taking over the courtship; the frequency of interferences amounts to 70% on a certain stage of the courtship sequence (Sparreboom 1996). As reported by these studies, courtship interference sometimes reaches high frequencies and significantly influences the distribution of the male mating success. Such interferences are considered a type of male-male competition, and the intensity may be affected by dominant-subdominant relationships, which are associated with the fighting ability among males (Wong and Candolin 2005).
Female quality may also affect courtship interference. As the time and energy that males can invest in courtship and mating are limited, it is sometimes adaptive for males to prudently choose mates. Theoretical and empirical studies have supported that male mate preference may be based on female fecundity, which increases the number of offspring, and on maturity, which affects future male mating opportunities (Goshima et al. 1996; Bonduriansky 2001; Härdling and Kokko 2005; Reading and Backwell 2007; Booksmythe et al. 2011; Edward and Chapman 2011; Wada et al. 2011). In the fiddler crab Austruca mjoebergi, for example, males spend more time courting and waving to large females, who can produce large clutches (Reading and Backwell 2007; Booksmythe et al. 2011). In Gelasimus tetragonon, males often reject females who are in early reproductive stages (Goshima et al. 1996). In the hermit crab Pagurus middendorffii, male mate preference for female fecundity and duration until breeding varies with male body size, which is associated with fighting ability (Wada et al. 2011). Considering that males are able to identify these reproductive traits of females, it is logical to suppose that interfering males may also selectively disrupt mate-searching females based on these traits. In other words, if the mate-searching female is relatively superior to other females regarding these traits (i.e., fecundity and maturity), rival males would attempt to more vigorously and persistently hamper the male courtship and female assessment, although males are known to possibly prefer females of any size when male mating opportunities are extremely limited (Reading and Backwell 2007; Booksmythe et al. 2011).
Fiddler crab is one of the most suitable organisms to examine both intra- and intersexual selection in the field. Males fight for territory and for the burrows where females oviposit (Jennions and Backwell 1996; Pratt et al. 2003; Morrell et al. 2005; Muramatsu and Koga 2016). During the reproductive season, males court mate-searching females by using multiple sexual signals, such as conspicuous coloration, large claw, courtship structure, waving displays, and courtship vibrations (Salmon and Atsaides 1968; Yamaguchi 1971; Crane 1975; Christy 1983; Murai et al. 1987; Christy 1995; Backwell and Passmore 1996; deRivera 2005; Murai and Backwell 2006; Takeshita and Murai 2016; Mowles et al. 2017; Takeshita et al. 2018; Takeshita 2019). In underground mating, which is one of the two mating tactics of the fiddler crab (Yamaguchi 1971; Murai et al. 1987; Reaney et al. 2012), males engage in waving display around their burrow using an exaggerated claw. Mate-searching females then visit and assess these potential mates. When a mate-searching female approaches a male, the male alters its waving display intensely (Pope 2005). In this stage, Austruca perplexa females prefer males with high waving height (Murai and Backwell 2006). In A. mjoebergi, males with higher waving rate are preferred by females (Reaney 2009; Callander et al. 2012; Sanches et al. 2017). When the female further approaches the male and the burrow, the male emits vibrational signals on the ground and/or from inside the burrow (Salmon and Atsaides 1968; Takeshita and Murai 2016; Mowles et al. 2017). In Austruca lactea, males first enter the burrow and then they emit vibrations from the inside; the females, which prefer males with high rates of vibrational pulses, then move into the burrow (Takeshita and Murai 2016). If the pairing is successful, the male then goes up the burrow entrance and closes it (Yamaguchi 1971; Christy 1983; Murai et al. 1987). In A. lactea, when a female follows the prospective male into the burrow, neighboring males often interfere (see Results and Electronic Supplementary Material [ESM] 1).
An interference behavior (rubbing) by a neighboring male (MOV 91049 kb)
We herein describe the female mate-searching process and social interactions in A. lactea, and we reveal the potential effect of courtship interference by rival males of this species. We additionally demonstrate whether body size of prospective males and mate-searching females and the period until female oviposition influence the courtship interferences. Body size is generally considered an index of the male’s fighting ability in fiddler crabs (Jennions and Backwell 1996; Pratt et al. 2003; Morrell et al. 2005; Muramatsu and Koga 2016). Fecundity increases with female size in A. lactea (Murai et al. 1987; Yamaguchi 2003) as well as in other fiddler crab species (e.g., Minuca rapax: Greenspan 1980; G. tetragonon: Goshima et al. 1996; A. mjoebergi: Reading and Backwell 2007; Tubuca arcuata: Aoki et al. 2010). Duration until female oviposition limits male mating opportunities because males employ postcopulatory guarding in the burrow during this period (Goshima and Murai 1988). Therefore, all three factors may affect the interferences. Our aim was to estimate the role of courtship interference and understand how this interference affects male mating success in this species.
Materials and methods
Field observations
All observations were conducted daily during low tide for approximately 4–6 h in the daytime between 2 July and 18 August 2016, on a sandy mudflat in Nagaura Island, Kami-Amakusa, Kumamoto, Japan (32° 32′ N, 130° 24′ E). This location has little vegetation cover and few large rocks. We first looked for mate-searching females and then recorded their searching process using a video camera (NEX-VG20H, SONY, Japan) with telephoto zoom lens (18–200 mm, SEL18200, SONY, Japan) until pairing (N = 92). A pairing was considered successful when the male appeared at the entrance of the burrow after the female followed the male into the burrow (and the female remained inside) or when both sexes remained inside the burrow for over 10 min. After pairing, we enclosed the entrance of each burrow with a metal semispherical cage (diameter, 10 cm) to determine the elapsed days until oviposition as males emerge from the burrow after oviposition (Yamaguchi 1971; Murai et al. 1987; Goshima and Murai 1988). We checked these burrows for male emergence every day; emerging males were immediately caught. We then collected the ovigerous females by digging the burrow. Carapace width of both sexes was measured with a caliper (to the nearest 0.1 mm). Of the 92 pairs that we pursued, the entire behavioral interaction sequence between female and finally selected male was observed in 79 of them; the remaining pairs were observed from the middle of the sequence of interactions on. Sixty pairs were successfully collected and their carapace widths and duration until oviposition were recorded; for the remaining pairs, we failed to collect the individuals of either or both sexes. Fifty pairs had their entire sequence of interactions observed and their carapace widths (for both sexes) and duration until oviposition recorded. Unfortunately, we were unable to determine the duration until female final decision (see below) for one pair because the female positioned herself behind substrates. Air temperature was recorded using a data logger (TidbiT v2 UTBI-001, Onset Computer Corporation, USA) every 30 min during the field observation period. Average temperature including that of nighttime was 29.26 ± 3.81 °C (mean ± SD) (range 22.54–40.66 °C).
Behavioral analyses
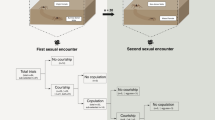
All movie files were analyzed to quantitatively assess the interactions among mate-searching females, finally selected males, and neighboring males. Courtship interference was herein defined as when neighboring males approached the entrance of the burrow of a male visited by a female without performing the waving display. This approach often involved several specific behaviors such as “rubbing” (rubbing their large claw on the substrate), “tapping” (tapping their ambulatory legs on the entrance of the burrow), and “claw insertion” (inserting their large claw, and sometimes their entire body, into the burrow). These behaviors seemed to disturb the female assessments (see Discussion). The approaching behavior mostly happened after females entered the burrow of courtship males. We first focused on mate-searching females and counted the number of males whose burrows had been visited by females from the beginning of each video until pairing. Second, we identified the finally selected male and checked if this male had approached (i.e., interfered) the burrow of the male that the female had visited immediately before the finally selected male. Third, we addressed the interaction at the burrow of the finally selected male; we counted the number of times that a female had repeatedly entered and exited the burrow of the respective finally selected male after the female reached the burrow. We also counted the number of interferences in which neighboring males disrupted the courtship interactions between a female and the respective finally selected male; interference was counted as one event even when multiple neighboring males simultaneously approached the pair (i.e., the number of interferences represents the cumulative number of times that interferences occurred in different moments). Moreover, we measured the duration from females’ arrival at the burrow of the finally selected male until the females’ final decision to determine pairing (i.e., the final movement of the female into the burrow).
To investigate the effect of interference by neighboring males on pairing, we focused only on the first movement of the females into the burrow immediately after female arrival at the burrow of the finally selected male; we checked whether the male appeared on the ground immediately after the first movement (i.e., pairing) or the female reappeared on the ground (i.e., the female delayed pairing), besides recording whether interference occurred at that time.
To describe the behavioral aspects of the interferences, the position of the interfering male regarding the burrow of the finally selected male (i.e., approaching the burrow, visiting its entrance, and invading the burrow; see Results) and the details of the behaviors during interferences (i.e., rubbing, tapping, and claw insertion) were recorded. In case when multiple males simultaneously interfered, the position and the specific behaviors were represented by the neighboring male that most nearly approached the burrow.
Statistical analysis
First, to investigate the relationship between the number of interferences by neighboring males and the number of times that females repeatedly entered the burrow of finally selected males, a Pearson’s correlation test was conducted. Second, we applied a regression analysis to analyze the effect of interference on the duration from the arrival of a mate-searching female at the burrow of the finally selected male until the female’s final decision for pairing; the response variable was the duration until the female’s final decision, and the explanatory variable was the number of interferences. Third, to demonstrate the factors that affect the number of interferences, we applied a generalized linear model (GLM) with negative binominal distribution and log link function and the Wald test; the response variable was the number of interferences, and the explanatory variables were carapace width of the finally selected male and of the female and elapsed days until oviposition. Log-transformed duration until the female final decision was added as an offset term in the model. Although all interaction terms were considered, these interactions were removed from the model because they were not significant. Because there was also no significance in all three main effects (see Results), we then used each single variable as the explanatory one. Fourth, to examine the effect of courtship interference on pairing, we focused on the female’s first movement into the burrow of a finally selected male; the frequency in which the male/female emerged after the movements with and without the interference by neighboring males was compared using Fisher’s exact test. Finally, we examined size assortative pairing by using Pearson’s correlation test and regression analysis. For the regression analysis, the response variable was carapace width of the paired males, and the explanatory variable was carapace width of the paired females. All analyses were conducted using the “MASS” packages in R 3.5.2 (R Core Team 2018).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
The effect of interference by neighboring males
Mate-searching females visited 7.41 ± 10.06 males and their burrows (range 1–78 males, N = 79). Searching duration was 453.91 ± 620.11 s (range 11–4,377 s, N = 79); note that the number of visited males and the duration of each visit were calculated from the beginning of each observation and thus do not represent the entire mate-searching trip of the females.
The finally selected males frequently interfered in the courtship of the immediate last male, sometimes employing several specific behaviors (see below). Of the 67 cases in which the entire interaction between female and last male were observed, interferences occurred in 71.6% (48/67). In the remaining cases, no interferences occurred at the last male’s burrow, and the females then visited the finally selected male (19/67). In 52.08% of the cases in which interferences occurred (25/48), one or more interferences were caused by the finally selected male. In the other cases, females refused all interfering males and chose a non-interfering finally selected male (23/48).
The finally selected males were also frequently disturbed by neighboring males. After the female reached the burrow of the finally selected male, females often repeatedly entered and exited the burrow (2.99 ± 2.89 times, range 1–16, N = 79). Neighboring males generally interfered when the female entered the burrow (ESM 1). The number of interferences by neighboring males was 2.46 ± 2.78 (range 0–16, N = 79). The number of interferences was strongly correlated with the number of times a female entered a burrow (r = 0.98, Pearson’s correlation test: t77 = 38.89, P < 0.0001, Fig. 1).
The elapsed duration from visiting the burrow until the female’s final decision was 86.73 ± 228.22 s (range 1–1,880 s, N = 78). The elapsed duration increased with the number of interferences by neighboring males (regression analysis: coefficient ± SE= 44.08 ± 7.94, t1,76 = 5.55, P < 0.001, Fig. 2).
The number of interferences by neighboring males increased with female size. Although the number of interferences was not influenced by male size and elapsed days until oviposition, the effect of female size was marginal (Table 1). Considering each variable separately, the number of interferences significantly increased with female size (GLM: coefficient ± SE = 0.39 ± 0.17, Z = 2.32, P < 0.05, Fig. 3), but there was no significant variation regarding the other two variables (GLM, male size: Z = 1.15, P = 0.25; duration until oviposition: Z = − 1.17, P = 0.24).
Regarding the females’ first movement into the burrow of the finally selected male, the probability of the female reappearing on the ground immediately after the movement increased with the occurrence of an interference (Fisher’s exact test, P < 0.0001, Table 2).
In the pairs, size assortative pairing was detected (r = 0.50, Pearson’s correlation test: t58 = 4.39, P < 0.0001, N = 60, Fig. 4). The elapsed days from pairing until oviposition was 1.75 ± 0.99 (range 1–5 days, N = 60).
Interference behavior by neighboring males
In all observed pairs (N = 92), the cumulative number of repeated movements of all females into the burrow of the finally selected male was 296. Interference by neighboring males occurred in 84.80% of the 296 cases (251/296).
The interferences by neighboring males were separated into three levels regarding the position of the neighboring males in relation to the burrow of the finally selected male: approaching the burrow, visiting the entrance, and invading the burrow. Since we defined that interference occurred when the neighboring males approached a burrow, approaching occurred in 100% of the cases of interference (251/251). Visits to the entrance and invasion of the burrow occurred in 40.24% (101/251) and 2.79% (7/251) of the cases, respectively.
The interference behaviors were also categorized into three types: rubbing (see ESM 1), tapping, and insertion of the claw (see Materials and Methods). Frequencies of rubbing, tapping, and insertion of the claw were 49.40% (124/251), 10.36% (26/251), and 3.59% (9/251), respectively. These behaviors were often performed by the same individuals in an interference sequence.
Discussion
Courtship interference may have an important role in preventing the pairing between mate-searching females and their prospective males. In this species, there are two types of mating tactics: underground and surface mating (Yamaguchi 1971; Murai et al. 1987; Severinghaus and Lin 1990). The present study shows the effect of courtship interference on the former tactic. However, interferences by other males in this species have also been reported regarding the latter tactic. Studies on a Taiwanese population in this species showed that, in surface mating where males copulate with neighboring females at the entrance of females’ burrow, males are sometimes intercepted by other neighboring males (Severinghaus and Lin 1990). This, together with the results of our present study, suggests that courtship interference in this species is common and that it may bias the distribution of mating success toward dominant males more than expected.
Interferences occurred during a certain stage of the courtship sequence in underground mating. The number of times that females repeatedly entered and exited the burrow was strongly correlated with the number of interferences (Fig. 1). This supports that interferences occurred when mate-searching females entered burrows, implying that visual disappearance of the female acts as a cue that causes neighboring males to disturb them.
Two explanations for the effectiveness of interferences in underground mating may be suggested. The first is that interferences would seismically prevent signal transmission. Males of some fiddler crab species use vibrational signals through the substrate for courtship (Salmon and Horch 1972; Aicher and Tautz 1990; Takeshita and Murai 2016; Mowles et al. 2017). Also in this species, males employ vibrational signals when females visit the entrance and the inside of their burrows (Takeshita and Murai 2016). The interferences occurred when females were inside the burrows (ESM 1). It is possible that the rubbing and tapping behaviors, which were frequently observed during the interference behaviors, made some noises and intercepted the sexual communication. However, in A. lactea, females have been shown to not have any preference for the components of the vibrational signals when they are inside burrows (Takeshita and Murai 2016), that is, during the stages in which interferences occur. Therefore, it is currently hard to understand how interferences actually affect communication through noises. Nevertheless, the possibility of females using some components of the signals to gather information on males from the inside of the burrow remains. In A. mjoebergi, for example, peak frequency acoustic signals are correlated with burrow volume (Mowles et al. 2017), which is in turn associated with timing of larval hatching in several fiddler crab species (Christy 1983; Backwell and Passmore 1996; deRivera 2005; Reaney and Backwell 2007).
The second explanation is that, because females reject mating with non-selected males, interferences may consequently succeed. When neighboring males invade burrows and take the mating opportunity from the preferred males, females may suffer from having to mate with the non-preferred mate because of a relative reduction of the indirect benefit. In underground mating, as copulation occurs inside the burrow, females may not be able to visually distinguish between preferred and intruder males. Therefore, females may exit the burrow, at least temporarily, to reject the interfering males, thereby postponing pairing.
There are two possible reasons that may explain why neighboring males persisted to disturb larger females (Fig. 3). First, as clutch size increases with carapace width in this species (Murai et al. 1987; Yamaguchi 2003), larger females may have higher fecundity than smaller females. Therefore, because mating with larger females would contribute to a higher fitness, the neighboring males would interfere when the female is especially larger. Second, because of their high visual detectability, larger females are preferred. However, as the operational sex ratio of fiddler crabs generally skews to males, male mating opportunity may be very rare. Therefore, a male would interfere in the courtship of any female that it can identify. In fact, males of A. mjoebergi do not forgo any mating opportunities with small females, although they can discriminate between large and small females and preferentially court large females (Reading and Backwell 2007; Booksmythe et al. 2011). Although these two explanations are not mutually exclusive, the latter seems to be more reasonable.
Finally selected males often succeeded to interfere in the courtship of the immediate last male visited by the mate-searching female, implying that the dominant males in courtship interference obtain relatively high mating success. Our results show that the proportion in which finally selected males had taken over the female of the last (previous) male through interference was of approximately 50% of the cases in which interference occurred. This proportion seems high as mate-searching females generally receive attention from multiple surrounding males. The proximate explanation for interfering males to often succeed at attracting females is that these males may be closely located to the female after the interference occurs, in a position that is suitable for courtship. Thus, if the interfering male is more attractive than the courting one, females may change the prospective mate to the interfering male.
Because courtship interference may be considered a type of male-male competition, population parameters (e.g., sex ratio and density) and environmental factors (e.g., predation pressure) may also influence the intensity and persistence of interfering behaviors. For example, Webster and Robinson (1999) showed that the frequency of interferences increases with colony size in the female-defense polygamy birds. The reason for courtship interference to have been frequently observed in the present study may be derived from features of the population, such as male-biased operational sex ratio in reproductive season, high population density, and low predation pressure. Such situations may intensify the strength of male-male competition.
The inhibition of interferences may also contribute to mating success. Dominant males have been reported to often reduce the territory of neighboring males in some ocypodid crabs. In A. lactea, Yamaguchi and Tabata (2004) observed that male territory size reduced when the large claw, which acts as a weapon, was removed. In the dotillid crab Ilyoplax pusilla, larger males construct barricades against smaller neighbors and often plug the burrow to restrict the territory of the neighbors (Wada 1984, 1987); males that succeeded in mating built barricades more frequently than non-mating males (Ohata and Wada 2008). However, the present study shows that male carapace width, which is an index of fighting ability (Jennions and Backwell 1996; Pratt et al. 2003; Morrell et al. 2005; Muramatsu and Koga 2016), did not influence the number of the interferences (Table 1). This was possibly a result of the relatively low amount of opportunities to encounter mate-searching females, which may have generated non-mate preference in interfering males. However, it is necessary to compare the fighting ability of successful and unsuccessful interfering males to reveal the actual effects of the dominant-subdominant relationship. Thus, further investigation on male traits associated with territory and inhibition of interferences is required.
How does intrasexual selection influence intersexual selection through courtship interference in this species? The finally selected males can, by definition, be assumed to be attractive. Besides, the males are also dominant for the courtship interference. Therefore, it is possible to estimate that males with high mating success possess both high attractiveness and ability of interference. Such an evolutionary consequence is also known in other fiddler crab species. For example, the large claw of fiddler crabs can function under both intrasexual and intersexual selection: the position of the tubercle mechanically compensates the closing force of the longer claw, which is attractive for mates (Dennenmoser and Christy 2013). In addition, courtship interference extends mate-searching duration for females, which may incur in costs such as increased risk of predation and dehydration. These costs may reduce the threshold criterion of female preference (Real 1990). We therefore emphasize that intra- and intersexual selection are mutually associated through courtship interference and would complementarily favor several sexual traits of fiddler crabs. To thoroughly elucidate the distribution of mating success in this species, it is necessary to understand the effects of both courtship display and interference.
References
Aicher B, Tautz J (1990) Vibrational communication in the fiddler crab, Uca pugilator. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 166:345–353
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Aoki M, Watanabe Y, Imai H, Kamada M, Wada K (2010) Interpopulation variations in life history traits in the fiddler crab Uca arcuata. J Crustac Biol 30:607–614
Backwell PRY, Passmore NI (1996) Time constraints and multiple choice criteria in the sampling behaviour and mate choice of the fiddler crab, Uca annulipes. Behav Ecol Sociobiol 38:407–416
Baxter C, Mentlik J, Shams I, Dukas R (2018) Mating success in fruit flies: courtship interference versus female choice. Anim Behav 138:101–108
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Booksmythe I, Jennions MD, Backwell PRY (2011) Male fiddler crabs prefer conspecific females during simultaneous, but not sequential, mate choice. Anim Behav 81:775–778
Callander S, Jennions MD, Backwell PRY (2012) The effect of claw size and wave rate on female choice in a fiddler crab. J Ethol 30:151–155
Christy JH (1983) Female choice in the resource-defense mating system of the sand fiddler crab, Uca pugilator. Behav Ecol Sociobiol 12:169–180
Christy JH (1995) Mimicry, mate choice, and the sensory trap hypothesis. Am Nat 146:171–181
Cooley JR, Marshall DC (2001) Sexual signaling in periodical cicadas, Magicicada spp. (Hemiptera: Cicadidae). Behaviour 138:827–855
Crane J (1975) Fiddler crabs of the world: Ocypodidae: genus Uca. Princeton University Press, Princeton
Dennenmoser S, Christy JH (2013) The design of a beautiful weapon: compensation for opposing sexual selection on a trait with two functions. Evolution 67:1181–1188
deRivera CE (2005) Long searches for male-defended breeding burrows allow female fiddler crabs, Uca crenulata, to release larvae on time. Anim Behav 70:289–297
Edward DA, Chapman T (2011) The evolution and significance of male mate choice. Trends Ecol Evol 26:647–654
Fisher HS, Swaisgood R, Fitch-Snyder H (2003) Countermarking by male pygmy lorises (Nycticebus pygmaeus): do females use odor cues to select mates with high competitive ability? Behav Ecol Sociobiol 53:123–130
Goshima S, Murai M (1988) Mating investment of male fiddler crabs, Uca lactea. Anim Behav 36:1249–1251
Goshima S, Koga T, Murai M (1996) Mate acceptance and guarding by male fiddler crabs Uca tetragonon (Herbst). J Exp Mar Biol Ecol 196:131–143
Greenspan BN (1980) Male size and reproductive success in the communal courtship system of the fiddler crab Uca rapax. Anim Behav 28:387–392
Härdling R, Kokko H (2005) The evolution of prudent choice. Evol Ecol Res 7:697–715
Howard RD, Moorman RS, Whiteman HH (1997) Differential effects of mate competition and mate choice on eastern tiger salamanders. Anim Behav 53:1345–1356
Hunt J, Breuker CJ, Sadowski JA, Moore AJ (2009) Male–male competition, female mate choice and their interaction: determining total sexual selection. J Evol Biol 22:13–26
Jennions MD, Backwell PRY (1996) Residency and size affect fight duration and outcome in the fiddler crab Uca annulipes. Biol J Linn Soc 57:293–306
Kangas N, Lindström K (2001) Male interactions and female mate choice in the sand goby, Pomatoschistus minutus. Anim Behav 61:425–430
Mennill DJ, Ratcliffe LM, Boag PT (2002) Female eavesdropping on male song contests in songbirds. Science 296:873–873
Morrell LJ, Backwell PRY, Metcalfe NB (2005) Fighting in fiddler crabs Uca mjoebergi: what determines duration? Anim Behav 70:653–662
Mowles SL, Jennions MD, Backwell PRY (2017) Multimodal communication in courting fiddler crabs reveals male performance capacities. R Soc Open Sci 4:161093
Murai M, Backwell PRY (2006) A conspicuous courtship signal in the fiddler crab Uca perplexa: female choice based on display structure. Behav Ecol Sociobiol 60:736–741
Murai M, Goshima S, Henmi Y (1987) Analysis of the mating system of the fiddler crab, Uca lactea. Anim Behav 35:1334–1342
Muramatsu D, Koga T (2016) Fighting with an unreliable weapon: opponent choice and risk avoidance in fiddler crab contests. Behav Ecol Sociobiol 70:713–724
Nieri R, Mazzoni V, Gordon SD, Krugner R (2017) Mating behavior and vibrational mimicry in the glassy-winged sharpshooter, Homalodisca vitripennis. J Pest Sci 90:887–899
Ohata M, Wada K (2008) Is barricade building behavior linked to pair formation in the dotillid crab Ilyoplax pusilla? Crust Res 37:63–66
Otter K, McGregor PK, Terry AM, Burford FR, Peake TM, Dabelsteen T (1999) Do female great tits (Parus major) assess males by eavesdropping? A field study using interactive song playback. Proc R Soc Lond B 266:1305–1309
Pope DS (2005) Waving in a crowd: fiddler crabs signal in networks. In: McGregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge, pp 252–276
Pratt AE, McLain DK, Lathrop GR (2003) The assessment game in sand fiddler crab contests for breeding burrows. Anim Behav 65:945–955
Qvarnström A, Forsgren E (1998) Should females prefer dominant males? Trends Ecol Evol 13:498–501
R Core Team (2018) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Reading KL, Backwell PRY (2007) Can beggars be choosers? Male mate choice in a fiddler crab. Anim Behav 74:867–872
Real L (1990) Search theory and mate choice. I. Models of single-sex discrimination. Am Nat 136:376–405
Reaney LT (2009) Female preference for male phenotypic traits in a fiddler crab: do females use absolute or comparative evaluation? Anim Behav 77:139–143
Reaney LT, Backwell PRY (2007) Temporal constraints and female preference for burrow width in the fiddler crab, Uca mjoebergi. Behav Ecol Sociobiol 61:1515–1521
Reaney LT, Maurer G, Backwell PRY, Linde CC (2012) Paternity analysis of two male mating tactics in the fiddler crab, Uca mjoebergi. Behav Ecol Sociobiol 66:1017–1024
Sæther SA, Fiske P, Kålås JA (1999) Pushy males and choosy females: courtship disruption and mate choice in the lekking great snipe. Proc R Soc Lond B 266:1227–1234
Salmon M, Atsaides SP (1968) Visual and acoustical signalling during courtship by fiddler crabs (genus Uca). Am Zool 8:623–639
Salmon M, Horch KW (1972) Acoustic signalling and detection by semiterrestrial crabs of the family Ocypodidae. In: Winn HE, Olla BL (eds) Behavior of marine animals, current perspective in research volume 1: invertebrates. Plenum Press, New York, pp 60–96
Sanches FHC, Costa TM, Barreto RE, Backwell PRY (2017) Faster male displays and less complex choice are more attractive to female fiddler crabs as they reduce search costs. Anim Behav 124:119–123
Severinghaus LL, Lin HC (1990) The reproductive behaviour and mate choice of the fiddler crab (Uca lactea lactea) in mid-Taiwan. Behaviour 113:292–307
Sparreboom M (1996) Sexual Interference in the Sword-tailed Newt, Cynops ensicauda popei (Amphibia: Salamandridae). Ethology 102:672–685
Takeshita F (2019) Color changes of fiddler crab between seasons and under stressful conditions: patterns of changes in lightness differ between carapace and claw. J Exp Mar Biol Ecol 511:113–119
Takeshita F, Murai M (2016) The vibrational signals that male fiddler crabs (Uca lactea) use to attract females into their burrows. Sci Nat 103:49
Takeshita F, Murai M, Matsumasa M, Henmi Y (2018) Multimodal signaling in fiddler crab: waving to attract mates is condition-dependent but other sexual signals are not. Behav Ecol Sociobiol 72:140
Trail PW (1985) Courtship disruption modifies mate choice in a lek-breeding bird. Science 227:778–780
Wada K (1984) Barricade building in Ilyoplax pusillus (De Haan)(Crustacea: Brachyura). J Exp Mar Biol Ecol 83:73–88
Wada K (1987) Neighbor burrow-plugging in Ilyoplax pusillus (Crustacea: Brachyura: Ocypodidae). Mar Biol 95:299–303
Wada S, Arashiro Y, Takeshita F, Shibata Y (2011) Male mate choice in hermit crabs: prudence by inferior males and simple preference by superior males. Behav Ecol 22:114–119
Webster MS, Robinson SK (1999) Courtship disruptions and male mating strategies: examples from female-defense mating systems. Am Nat 154:717–729
Wong BBM (2004) Male competition is disruptive to courtship in the Pacific blue-eye. J Fish Biol 65:333–341
Wong BBM, Candolin U (2005) How is female mate choice affected by male competition? Biol Rev 80:559–571
Yamaguchi T (1971) Courtship behavior of a fiddler crab, Uca lactea. Kumamoto J Sci Biol 10:13–37
Yamaguchi T (2003) Seasonal changes in the energy content of females of the fiddler crab, Uca lactea, especially during the reproductive period. Crustaceana 76:1371–1397
Yamaguchi T, Tabata S (2004) Territory usage and defence of the fiddler crab, Uca lactea (De Haan)(Decapoda, Brachyura, Ocypodidae). Crustaceana 77:1055–1080
Acknowledgments
We thank Prof. Yasuhisa Henmi and members of Aitsu Marine Station, Kumamoto University, for the valuable discussion.
Funding
This study was supported by JSPS KAKENHI Grant Numbers 15K18613 and 19K06857 to FT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Breithaupt
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Takeshita, F., Murai, M. Courtship interference by neighboring males potentially prevents pairing in fiddler crab Austruca lactea. Behav Ecol Sociobiol 73, 164 (2019). https://doi.org/10.1007/s00265-019-2774-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2774-9