Abstract
Female mass in most altricial birds reaches its maximum during breeding at egg laying, which coincides temporally with the fertile phase when extra-pair paternity (EPP) is determined. Higher mass at laying may have two different effects on EPP intensity. On the one hand, it would lead to increased wing loading (body mass/wing area), which may impair flight efficiency and thereby reduce female’s capacity to resist unwanted extra-pair male approaches (sexual conflict hypothesis). On the other hand, it would enhance female condition, favouring her capacity to evade mate guarding and to search for extra-pair mates (female choice hypothesis). In both cases, higher female mass at laying may lead to enhanced EPP. To test this prediction, we reduced nest building effort by adding a completely constructed nest in an experimental group of female pied flycatchers (Ficedula hypoleuca). Our treatment caused an increase in mass and thereby wing loading and this was translated into a significantly higher EPP in the manipulated group compared with the control group as expected. There was also a significant negative relationship between EPP and laying date and the extent of the white wing patch, an index of female dominance. More body reserves at laying mean not only a higher potential fecundity but a higher level of EPP as well. This interaction had not previously received due attention but should be considered in future studies of avian breeding strategies.
Significance statement
While most research has been focused on determining possible criteria for extra-pair mate choice by females, less effort has been made on establishing if female traits are related to EPP and its intensity. One such trait is mass at laying which attains its highest level for breeding females of altricial birds. Our study indicates that a higher mass during the fertile phase not only has implications for female fecundity and predation risk but also for EPP in the resulting brood as more mass means a higher EPP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most female altricial birds show important changes in body mass in the course of the breeding cycle, increasing in mass before egg laying to a maximum just at laying, maintaining partly this high mass during incubation and losing it after hatching when feeding the chicks, thus returning to pre-breeding levels (Moreno 1989). This seasonal variation in female body mass has been interpreted as the result of a parental adaptive strategy and constitutes an important aspect of avian breeding biology. Firstly, a high body mass at laying would allow females to carry enough energetic reserves to lay high-quality eggs, and then to keep a good condition when activity is reduced during incubation, when foraging is compromised. Later on, body mass would have to be reduced to enhance flying efficiency during nestling provisioning (Norberg 1981). Those changes in female body mass have been observed even in experiments where parents were supplementary fed (Moreno 1989; Sanz and Moreno 1995; Lothery et al. 2014). Changes in mass affect crucially female flight ability during the breeding cycle through the modification of wing loading (body mass/wing area) (Videler 2005), a trait that has been theoretically and empirically negatively related to flight capacity at short distances (Pennycuick 1982; Kullberg et al. 2002).
In the last two decades, increasingly accurate molecular tools have revealed that 90% of socially monogamous bird species show extra-pair paternity (EPP), resulting from mating outside the social pair-bond (Petrie and Kempenaers 1998; Westneat and Stewart 2003). Given its influence on fitness, EPP must be an important factor in sexual selection (Møller and Birkhead 1994; Griffith et al. 2002; Garamszegi and Møller 2004). However, although great effort has been made to test adaptive explanations behind extra-pair copulation (EPC) behaviour within and across species, there is yet no consensus on the key factors that are behind it (Griffith et al. 2003; Forstmeier et al. 2014; Boulton et al. 2018).
EPP results from the complex interaction between a female, an extra-pair male and the social mate, so the behaviour and traits of each of those parties are of importance for the resulting EPP patterns. Most adaptive explanations propose that females may obtain indirect benefits from EP behaviour (Møller and Birkhead 1994; Forstmeier et al. 2014), e.g. through improving offspring viability trough the choice of more attractive extra-pair sires. Under this point of view, the outcome of EPP depends on the interplay of two factors. Firstly, on the social male’s capacity to guard their mates and fight off male intruders, which is a function of his aggressiveness and dominance (Moreno et al. 2010b). And secondly, on the female’s ability to evade mate guarding tactics (Alatalo et al. 1987), which may depend on her size, age (Bouwman and Komdeur 2005; Ramos et al. 2014), social dominance expressed through ornaments (Plaza et al. 2018) or flight ability (Stutchbury and Robertson 1987). In this respect, a high female body condition would favour the capacity of females to evade the attention of their mates and fly in search of extra-pair mates, roaming more easily through the breeding area.
In contrast with the above explanation, the sexual conflict hypothesis (Westneat and Stewart 2003; Arnqvist and Kirkpatrick 2005) derived from sexual selection, proposes that EPP results from a dynamic interplay in which both sexes strive towards conflicting ends. Under this scenario, strong selection in males to seek copulations independent of female choice would lead to higher incidence of EPP despite female costs to avoid EPCs (Arnqvist and Kirkpatrick 2005; Forstmeier et al. 2014). A consideration of female traits that relate to EPP may help us detect whether variation in female capacity to avoid EPCs explains EPP patterns. For instance, if EPCs are the result of male coercion (Westneat and Stewart 2003; Boulton et al. 2018), an increase in female mass would result in a higher wing loading which is translated into a reduced flight ability and a diminished capacity of the females to evade unwanted suitors. Indeed, such a negative relation between EPP and female flight ability has been found in some recent studies (Moreno et al. 2015; Plaza et al. 2019).
Bird nests have traditionally been considered as a simple receptacle for eggs and nestlings (Deeming 2013), while their functional characteristics in relation to avian reproduction have recently been taken into account (Cantarero et al. 2015b; Bailey et al. 2016). The costs of nest building have largely been documented (Hansell 2000) in terms of physiological stress for the builders (Morales et al. 2008; Moreno et al. 2008), their health and body condition (Tomás et al. 2006) or survival (Gill and Stutchbury 2005). The effort spent on this task may constrain reproductive behaviour during subsequent breeding phases, particularly so for the sex that is mainly involved in nest building. We have shown in a previous experiment that females whose nest construction costs are experimentally reduced, display improved body condition that results in a higher reproductive success (Moreno et al. 2010a). In many species, nest building precedes or overlaps in time with the fertile period and the time when reserves are accumulated in preparation for egg laying. Thus, we may expect that experimentally reducing or eliminating the cost of nest building may lead to an enhanced accumulation of reserves prior to laying (Moreno 1989), resulting in a higher condition but also in a higher wing loading during the fertile phase.
In the present study, we manipulated female body condition and wing loading, by drastically reducing female nest building effort in order to investigate the effect of this manipulation on EPP in pied flycatchers (Ficedula hypoleuca), a model species in studies of genetic polyandry, e.g. (Ellegren et al. 1995). The manipulation involved adding a completely built nest to an experimental set of nest-boxes. In this species, nest building is conducted mainly (Gelter and Tegelström 1992; Martínez-de la Puente et al. 2009) or exclusively (Curio 1959) by the female. Our previous evidence shows that this modification of nest building effort exclusively increases female body condition (Moreno et al. 2010a), whereas a food supplementation experiment would have also affected males (Moreno et al. 1999). We test the hypothesis that increases in female body mass at this sensitive period will lead to increased EPP levels through enhanced condition or reduced flight efficiency. To take into account female quality and dominance, we included laying date and the extent of a female social plumage signal as independent variables, as well as a plumage signal of the social mate’s dominance.
Material and methods
General field methods
This study was conducted during the spring of 2016 in a deciduous forest of Pyrenean oak Quercus pyrenaica, at 1200 m.a.s.l. near Valsaín, central Spain (40° 54′ N, 4° 01′ W). A total of 450 nest-boxes have been installed in this area since 1991, leading to a series of long-term studies of pied flycatchers breeding in them (the bottom area of the nest-box was 175 cm2 and the distance from the bottom to the entrance hole was 12.5 cm, Lambrechts et al. 2010). The breeding season of this species lasts from the middle of April when the first birds arrive from migration, to the beginning of July when all chicks have fledged. We clean all nest-boxes every year after breeding is over. Daily checking was done from April 15 to detect the initiation and progress of nest building until the end. Afterwards, all occupied nest-boxes were checked every 2–3 days to record laying date (Julian calendar), clutch size, hatching date and brood size. The modal clutch size in the population is 6, and most females begin incubation on the laying of the penultimate egg (Ruiz-de-Castañeda et al. 2012) so we considered incubation to begin on the laying of the fifth egg (mean incubation period is 14 days).
It was not possible to record data blind because our study involved focal animals in the field.
Nest manipulation
The average reported time spent in nest building by flycatchers is 4 to 11 days (Curio 1959; Lundberg and Alatalo 1992; Moreno et al. 2008). Although intra-pair copulations have been reported 9 days before the laying of the first egg (Von Haartman 1956), experiments by Lifjeld et al. (1997a) showed that only inseminations occurring from day − 2 before the laying of the first egg until the day the penultimate egg is laid result in fertilizations. This short fertilization window coincides in time with most observed copulations, which are confined to this relatively short period immediately before the start of egg laying (Von Haartman 1956; Alatalo et al. 1987; Chek et al. 1993). In the year in which this study was conducted (2016), a cold spell in May at the time of nest building led to delays in laying (the average time between the end of nest construction and laying date was 11 ± SE 0.57 days). This is in contrast with the typical pattern in which only a few days elapse between nest completion and laying (Moreno et al. 2010a). Thus, nest building did not overlap the period when females were fertile, so the effects of the experiment in terms of changes in EPP cannot be due to behavioural changes occurring during nest building. There was no association between the length of the interval from finished nest building to start of laying and EPP (Spearman’s rank correlation: r57 = 0.15, P = 0.23). This suggests that the degree of overlap between nest building activities and the fertile phase did not affect the results of our experiment.
We randomly assigned nests to either control or experimental treatments on the first building day, which was detected by the presence of a few nest material pieces placed in a circle (Cistus laurifolius bark strips and oak leaves). We discarded nests if they were more advanced than this early stage. In total, 36 control nests and 23 experimental nests were included in the experiment. A full description of nest material composition for pied flycatchers in our study area is provided in Moreno et al. (2009). The manipulation consisted in placing a completed flycatcher nest inside the nest-box on the day when the treatment was assigned to the experimental group. Control nests on the contrary were not manipulated until they were naturally completed and simply exchanged for other completed flycatcher nests. In this way, we made sure that all active nests (where eggs were laid) had experienced the same level of human disturbance, with the difference that in the experimental group, female building costs were greatly reduced with respect to the control group. Nest completion was determined by the same observer following the standard criteria of the presence of a rounded compact nest cup (Moreno et al. 2010a). All added (experimental) or exchanged (control) nests were obtained from freshly completed Pied flycatcher nests that we had previously found abandoned in the study area before hatching of nestlings in previous reproductive seasons, since when they had been frozen at − 20 °C until use. We weighed all of them once defrosted and shortly before their usage, as well as all the substituted nests in the control group. No differences in mass between introduced (21.80 ± SE 1.63 g) and substituted (24.20 ± SE 1.45 g) nests were found (F1,57 = 1.27, P = 0.48). As in both groups females added some material after the manipulation, all nests were also weighed after laying so the amount of material collected by females was known for both groups (difference in mass between the supplemented nests and the final ones). Accordingly, the average total amount of material collected by control and experimental females was 24.72 ± SE 1.50 and 5.46 ± SE 1.88 g respectively, showing that control females provided almost five times as much material as experimental females, with the difference being significant between the two treatments (F1,57 = 63.5, P < 0.01). After manipulation, no nest desertion was detected.
Capture and sampling
All females were captured on day 7 of incubation in order to weigh them after laying (capturing them sooner may lead to desertion), by simply blocking the nest-box entrance and catching them during daytime. Later in the season, all adults were captured in their nest-boxes while feeding nestlings of 7–8 days (nestlings fledge 16–19 days after hatching) by using a conventional nest-box trap set at the entrance of the nest-box (Cantarero et al. 2016b). The trap was active for a maximum of 1 h to minimize disturbance to adult birds and nestlings, and it was removed earlier if both adults were trapped before that time. No individual remained more than 5 min inside the nest-box after the trap closed. All birds were identified by their rings or ringed if necessary and mass was recorded with a Pesola spring balance (accuracy 0.25 g). Females were aged by their rings, and for the ones that were not ringed, we assigned the age of 2 years (typical age at which females are recruited to the breeding population in our studies). We also measured wing length with a stopped ruler to the nearest mm. As a measure of female plumage ornaments, a digital photograph of the white wing patch was taken from above at a height of 10 cm from the animal by placing the wing in its natural folded position on a flat surface with a ruler besides for reference, and forming a roughly 135° angle with the wing. The same photographic technique has been used in previous studies (Moreno et al. 2014; Cantarero et al. 2016a). All digital photos were later analysed with Adobe Photoshop CS5 v.11.0. to estimate surfaces with the reference to the ruler. A zoom of 400% and a paintbrush of 17 pixels, with 100% hardness and 25% spacing were used to estimate white wing patch areas estimated in cm2 (Sirkiä et al. 2015). The percentage of male dorsal blackness was estimated by scoring black feathers in the head and mantle at 10-point intervals from 5 (0–10%) to 95 (90–100%) (Canal et al. 2011). A small sample of blood from the brachial vein (10–20 μl) was taken and stored on Flinders Technology Associates reagent loaded cards (Whatman Bioscience, Florham Park, NJ, USA) until needed for the paternity analyses. All captures were performed between 8 and 10 a.m. in the morning.
We ringed all chicks when they were 13 days old (hatching day = day 1), and we similarly collected a small blood sample from the brachial vein for paternity analyses. All carcasses and abandoned eggs found inside the nest-boxes during regular checks were collected and frozen on the same day for later paternity analyses through tissue extraction. Hatching failure affected 20 of 348 eggs in 33% of the nests (N = 20). However, 13 eggs did not show any trace of embryonic development suggesting that they were infertile (this can easily be visually detected by examining the egg in contrast to the light). Moreover, 10 chicks (of two different nests) were predated so we left those nests out of the experiment.
Genotyping
We obtained samples from 59 families, including the two social mates and their whole brood at 12 days of age (112 adults, 325 nestlings). DNA was obtained from blood samples using a standard extraction protocol that digests the cards where the blood was fixed and animal tissues from the carcasses and eggs. We used BioSprint Blood kits (QiaGen, Duren, Germany) to extract and purify genomic DNA from the blood samples and Type-it kits (QiaGen, Duren, Germany) to amplify approximately 5 ng of template DNA in the PCR.
We used 10 pied flycatcher microsatellite loci for genotyping, following published primer sequences described in Leder et al. (2008). Two multiplex PCR reactions were designed as described before (Moreno et al. 2015), in which we amplified loci Fhy301, Fhy466, Fhy336, Fhy370 and Fhy452 in one reaction (set I) and Fhy328, Fhy223, Fhy236, Fhy304 and Fhy407 in the other (set II). The PCR program consisted in a denaturing step of 94 °C during 2 min, then 30 cycles with 30 s at 94 °C, 30 s at 55 °C and 30 s at 72 °C, finally an extension step of 2 min at 72 °C. Conditions were the same for both multiplex sets. With 13, 14, 18, 17, 15, 25, 17, 29, 10 and 15 alleles respectively, all loci were polymorphic and a combined non-exclusion probability of second parent of 0.00000114 was calculated by CERVUS 3.0.7 (Kalinowski et al. 2007). Three loci (Fhy336, Fhy236 and Fhy452) significantly deviated from Hardy–Weinberg equilibrium after Bonferroni correction, but in only one locus (Fhy452), CERVUS estimated a null allele frequency that was higher than 0.05.
Paternity analysis
We determined genetic parentage by comparing the genotypes of chicks with those of female and male nest owners. We considered that chicks were the offspring of the adults if their genotypes were compatible for the loci typed. To confirm this, we ran a paternity analysis using CERVUS (v 3.0.7. Field Genetics), specifying for all chicks the identity of the mother and allowing the software to assign the genetic father from the whole sample of adult males. In the paternity analyses, we used a level of confidence of 95%, we allowed a proportion of 5% mistyped loci and assumed that the proportion of candidate parents sampled was 85%, with a minimum number of 6 loci typed. CERVUS assigned paternity to the male with the highest LOD score (obtained by taking the natural log of the overall likelihood ratio; the likelihood ratio is the probability for the candidate parent to be the true parent divided by the probability for the candidate parent of not being the true parent). We accepted this as the genetic father of a given nestling only when the difference between the LOD scores of the first and the second most probable fathers was statistically significant (Kalinowski et al. 2007). We considered as extra-pair offspring those nestlings (82 in total) with two or more mismatched loci with respect to their social fathers by CERVUS (the mismatch never involving markers that deviated from Hardy–Weinberg equilibrium). From all these nestlings, 46 cases were assigned to a male which was not included in the population male pool (most probably a non-territorial floater). However, when the difference in LOD score between the first and the second most probable father was not significant, we did not assign a genetic father (36 cases in total). We also visually checked if those males assigned by CERVUS as fathers of extra-pair offspring matched the genotypes of the nestlings they were assigned to. We took a conservative rule, and considered as a father–offspring pair in 9 out of the 46 cases of extra-pair chicks assigned by the program, since these mismatched the social male in only one locus.
We considered that a single locus mismatch between the genotypes of the male and a chick could be due to mutation or genotyping mistakes, and for this reason, we overruled the CERVUS decision of considering these as extra-pair offspring. One mismatch between females and offspring occurred in 14 cases (8 cases in the control group and 6 in the experimental group), and in 16 cases with fathers (7 cases in the control group and 9 in the experimental group).
Statistical analyses
We first investigated possible differences between groups in breeding variables (hatching date and clutch size) and relevant female and male traits which could influence the effect of our treatment. When they were not normally distributed, we performed Mann–Whitney U tests.
We then examined the effect of our treatment on female wing loading by performing an unpaired T test as it was normally distributed. Following Moreno et al. (2015), we extracted an index of wing loading (g/dm2) by dividing female body mass by the square of wing length (n = 47). This index was validated with direct measurements of wing areas in the field in a pilot study conducted on birds not included in the experiment. In this study conducted in 2017, wing area was estimated from photographs (n = 41) of the contour of flattened wings against a sheet of paper with a ruler for reference as described above. The correlation of the two measures shows that our index was an acceptable proxy for wing loading (Spearman’s rank correlation: r71 = 0.77; P < 0.001). As we wanted to examine potential changes in wing loading caused by the effect of our treatment on female body mass, we first checked for differences in female wing length and then also in female mass. To that end, we performed two unpaired t tests as both variables were normally distributed. As these mentioned traits are related to age, we also examined differences between groups in female age by performing a Mann–Whitney U test, due to its lack of normality. All analyses mentioned were done with the STATISTICA package, v 10.0 (StatSoft, Inc., Tulsa, Oklahoma, USA).
The incidence of EPP was analysed in two ways. On the one hand, as a binary response (occurrence vs. absence of EPP) within nests by a univariate generalized linear model using the GENMOD procedure in SAS v9.4 (StatSoft, Inc., Tulsa, Oklahoma, USA), with a binomial distribution, to test the effect of our treatment on EPP occurrence. Three additional potentially relevant independent variables were also taken into account, trying not to add unnecessary complexity to the analyses. First, we included laying date since extra-pair behaviour could be influenced by the availability of reproductive individuals, which varies throughout the season as reproductive pairs are established. Second, we took into account the extent of the area of the female white wing patch (since we have previously shown that it is a predictor of individual social signalling capacity (Plaza et al. 2018) and territorial defence behaviour, through testosterone levels (Cantarero et al. 2016a). Finally we also included the social male dorsal blackness as a measure of his dominance which is positively related to the mate guarding effect, and significantly negatively related to EPP in previous published studies (Moreno et al. 2015). We also examined potential differences in those female and male characteristics between groups. On the other hand, we conducted a similar analysis using a different univariate generalized lineal model following the same procedure, but using instead the proportion of EPY (number of extra-pair young divided by brood size with “event/trial” syntax) as a measure of extra-pair paternity. All values are presented with standard error.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author MP on reasonable request.
Results
EPP occurred in 21 out of 59 broods (35.59%) and affected 82 of 325 nestlings (25.23%). We found non-identified extra-pair sires in five nests. The number of EPY in nests with EPP ranged from 1 to 7 nestlings, being on average 3.90 ± 0.42 EPY, which represents 67.76 ± 0.40% of the broods on average.
The two experimental groups were similar in hatching date and clutch size (Table 1, both P > 0.40). We did not find differences in female wing length and age between experimental and control groups (Table 1). However, we did find significant differences in female mass (Table 1), which was higher in the experimental than in the control group. Also the experiment was successful in inducing differences in female wing loading during incubation between treatments due to higher values in the experimental group with respect to the control one (Table 1).
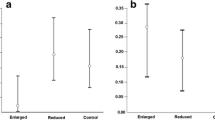
Our manipulation also caused an effect on the occurrence of EPP, which was significantly higher in the experimental group (Table 2, Fig. 1). The final model also included significant negative associations between EPP occurrence and the extent of the female white wing patch (Table 2; mean wing patch area for nests with EPP and without EPP were 1.28 ± 0.07 and 1.42 ± 0.05 cm2 respectively; t = 1.41; P = 0.16), and between EPP and laying date (Table 2; mean laying date for nests with EPP and without EPP were 49.10 ± 0.46 and 49.92 ± 0.33 respectively, day 1 = April 1; t = 1.45; P = 0.15).
We also found a significant effect of our treatment on the proportion of EPY (Table 2) which was higher in the experimental than in the control group (means for each group were 0.31 ± 0.07 and 0.15 ± 0.05, effect size was 67% following Nakagawa and Cuthill (2007)). We also found significant negative relationships between the proportion of EPY and both the extent of female white wing patch and laying date (Table 2), although the associations on their own were not significant (white wing patch Spearman’s rank correlation: r57 = − 0.19, P > 0.05; laying date Spearman’s rank correlation: r57 = − 0.22, P > 0.05) (Table 2). There were no differences between groups in the extent of the female white wing patch and male dorsal blackness (Table 1).
Discussion
The experimental reduction of nest building effort resulted in a significant increase in female body mass and wing loading and a subsequent increase in the occurrence of EPP and EPY in the experimental group. We also found that the probability of a nest containing EPP and the proportion of EPY were negatively related to the extent of the female white wing patch and laying date.
In the present study, control females took an average of 3 days to build the nest and collected almost 24 g of nest material, which is similar to values reported in other studies of populations breeding in central Spain (Moreno et al. 2008, 2010a). Females constructed their nests at a rate of 6 g/day. These high rates may imply important energy costs as indicated by associations of building rate with female physiological costs in this species (Moreno et al. 2008), causing a significant effect on female body mass and therefore on wing loading, as we detected when comparing this variable between groups. Predation on adult females has been found to be high during nest building and egg laying, caused by vulnerability when collecting nest materials due to the increased female mass during this stage (Slagsvold and Dale 1996). However there was no predation in our study population in either of the experimental groups as deduced from the absence of cases of early nest abandonment.
Our experimental results showed that females of the experimental group displayed a higher condition and wing loading as well as higher EPP levels. These results are in accordance with a previous study reported by Plaza et al. (2019), in which handicapped females with a diminished flying ability caused by an increased wing loading, also displayed higher EPP levels. Wing loading has previously been negatively correlated with flying capacity and a reduced manoeuvring ability (van den Hout et al. 2010; Salewski et al. 2014). In our treatment, we found a higher body mass (translated into higher wing loading) in the experimental group during the incubation period (soon after our nest manipulation treatment was applied). Assuming that mass at incubation reflects mass during nest building, these results suggest that females that did not have to build a complete nest before laying could dedicate more time to feed themselves and increase their reserves to better provision their eggs with resources. Moreno et al. (2010a) found that a reduction in nest building effort was translated into increased offspring fitness.
One interpretation of our results would support the role of sexual conflict in the evolution of EPP. This interpretation would explain the patterns as caused by experimental females being less able to escape from unwanted copulations with extra-pair males, thereby increasing their EPP rate (Plaza et al. 2019). This is in agreement with a scenario in which the levels of EPP would be influenced by male coercion instead of female choice (Björklund and Westman 1983) and it is consistent with the results found in a non-experimental study by Moreno et al. (2015), where a positive association between wing loading and EPP was reported. In contrast, an adaptive mate choice explanation would support the interpretation that improved body condition in experimental females led to increases in female condition and extra time, allowing them to seek out EPC by spending more time in extra-territorial forays and evading their social mate’s guarding. None of these two options can be discarded. An alternative explanation would predict a potentially enhanced experimental female attractiveness due to the improved body condition translated into a higher capacity to lay a large number of high-quality eggs (increased fecundity and fitness perception). In this case, males paired to experimental females would increase mate guarding and copulation rate (Pilastro et al. 2002; Griggio et al. 2003, 2005) leading to lower levels of EPP. We can now rule out this hypothesis as our results do not support it.
During the fertile period of the female, the social male would face a compromise between mate guarding and searching for potential EPCs. We consider that this compromise would not be affected by our treatment, as female fertility (Lifjeld et al. 1997b) could be easily perceived by the male through female behavioural signals (e.g. solicitations) rather than from the state of nest completion. Some individual characteristics expressing phenotypic quality may influence a male’s ability or willingness to perform mate guarding. However, male dorsal blackness as an index of social dominance did not affect EPP. Furthermore, the extent of the male wing patch as another potential male social signal showed no association with EPP (Spearman’s rank correlation: r57 = − 0.06, P = 0.63).
Previous studies in pied flycatchers did not detect differences between extra-pair and within-pair males in age, size or ornamentation (Moreno et al. 2010b) and there is no evidence of indirect benefits for extra-pair offspring in terms of good genes, as measured by microsatellite heterozygosity or body condition (Lifjeld et al. 1997a; Moreno et al. 2013). Although there is evidence of good-gene effects in other species accrued thorough EPP (e.g. Kempenaers et al. 1992; Blomqvist et al. 2002), the picture is not so clear and recent analyses of the evolution of infidelity in monogamous passerines suggest that EPP is not adaptive for females in some species and that it may be the result of strong selection in males (Arnqvist and Kirkpatrick 2005; Forstmeier et al. 2011). However, there might be benefits for female extra-pair behaviour that researchers just have not investigated or thought of yet (Mennerat et al. 2018).
The extent of white on female pied flycatcher wings has been proposed as a signal of dominance through its association with testosterone levels (Moreno et al. 2014; Cantarero et al. 2015a). Moreover, female vigilance and dominance behaviours are positively associated with the size of this patch (Plaza et al. 2018). Thus, dominant females with larger patches may enforce their dominant status through signalling, being more able to resist unwanted males and thereby negatively interacting with EPP occurrence. This result supports previous evidence in the same population regarding female age (Moreno et al. 2015). That old and dominant females (more experienced) exhibit lower EPP values contradicts the presumption that EPP is the result of adaptive female choice as precisely these females would be in a better position to select extra-pair sires and resist mate guarding by their social mates.
Values found in brood EPP occurrence are similar to others in the same population and slightly higher in the percentage of nestlings affected (22.4 and 7.5% in 2003, Moreno et al. 2010a; 28.8 and 13.1% in 2010, Moreno et al. 2013; 38.3 and 17.6% in 2015, Moreno et al. 2015). They are also similar to those found in another Iberian population studied by Canal et al. (2011) (39 and 20% respectively), and to the medium EPP rate in socially monogamous passerines which is above 25%. The importance of breeding synchrony and density on the interspecific variation in EPP has previously been reported (Stutchbury and Morton 1995; Griffith et al. 2002). It is assumed that temporal availability of reproductively active individuals may differ across the breeding season. In our highly synchronous breeding population (Griffith et al. 2002; Moreno et al. 2013), density of males not yet involved in parental duties may markedly decline throughout the season. As a consequence, the pressure of males seeking EPC may decrease, resulting in the negative relation between laying date and the incidence of EPP. Previous studies in the same population showed no relation (Moreno et al. 2015) or a negative relation (Moreno et al. 2013) between EPY and laying date. In fact, Canal et al. (2012) described for the same species a decrease in EPP values during the days before the laying date, followed by an increase during egg laying and incubation, and no EPC occurring after those periods, suggesting that the demands of paternal care decreased the availability of males for EPCs. This pattern is in accordance with the general negative relation we found.
To conclude, we have found that females with a higher body mass during the fertile period display higher EPP levels. The evolution of mass change strategies in breeding altricial birds (Moreno 1989) has thus implications for EPP patterns. More body reserves at laying mean not only a higher potential fecundity but a higher level of EPP as well. This interaction had not previously received due attention but should be considered in future studies of avian breeding strategies. If female condition at laying denotes a high EPP for their partners, the possible negative consequences of a good breeding condition for females in terms of reduced mate incubation feeding (Cantarero et al. 2014) or help with nestling provisioning would merit further studies (Arnqvist and Kirkpatrick 2005). We also found that females with signals of higher social dominance show lower EPP values. Those results underline the role of female social traits in the evolution of avian EPP.
References
Alatalo RV, Gottlander K, Lundberg A (1987) Extra-pair copulations and mate guarding in the polyterritorial pied flycatcher, Ficedula hypoleuca. Behaviour 101:139–155
Arnqvist G, Kirkpatrick M (2005) The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am Nat 165:S26–S37
Bailey IE, Morgan KV, Oschadleus HD, DeRuiter SL, Meddle SL, Healy SD (2016) Nest-building males trade off material collection costs with territory value. Emu 116:1–8
Björklund M, Westman B (1983) Extra-pair copulations in the pied flycatcher (Ficedula hypoleuca). Behav Ecol Sociobiol 13:271–275
Blomqvist D, Andersson M, Küpper C, Cuthill IC, Kis J, Lanctot RB, Sandercock BK, Székely T, Wallander J, Kempenaers B (2002) Genetic similarity between mates and extra-pair parentage in three species of shorebirds. Nature 419:613–615
Boulton RA, Zuk M, Shuker DM (2018) An inconvenient truth: the unconsidered benefits of convenience polyandry. Trends Ecol Evol 33:904–915
Bouwman KM, Komdeur J (2005) Old female reed buntings (Emberiza schoeniclus) increase extra-pair paternity in their broods when mated to young males. Behaviour 142:1449–1463
Canal D, Potti J, Dávila JA (2011) Male phenotype predicts extra pair paternity in pied flycatchers. Behaviour 148:691–712
Canal D, Jovani R, Potti J (2012) Male decisions or female accessibility? Spatiotemporal patterns of extra pair paternity in a songbird. Behav Ecol 23:1146–1153
Cantarero A, Laaksonen T, Järvistö PE, Gil D, López-Arrabé J, Redondo AJ, Moreno J (2015a) Nest defense behaviour and testosterone levels in female pied flycatchers. Ethology 121:946–957
Cantarero A, Laaksonen T, Järvistö PE, López-Arrabé J, Gil D, Moreno J (2016a) Testosterone levels in relation to size and UV reflectance of achromatic plumage traits of female pied flycatchers. J Avian Biol 48:243–254
Cantarero A, López-Arrabé J, Moreno J (2015b) Selection of nest-site and nesting material in the eurasian nuthatch Sitta europaea. Ardea 103:91–94
Cantarero A, López-Arrabé J, Palma A, Redondo AJ, Moreno J (2014) Males respond to female begging signals of need: a handicapping experiment in the pied flycatcher Ficedula hypoleuca. Anim Behav 94:167–173
Cantarero A, López-Arrabé J, Plaza M, Saavedra-Garcés I, Moreno J (2016b) Males feed their mates more and take more risks for nestlings with larger female-built nests: an experimental study in the nuthatch Sitta europaea. Behav Ecol Sociobiol 70:1141–1150
Curio E (1959) Beitrage zur populations okologie des trauerschnappers (Ficedula h. hypoleuca Pallas). Zool Jahrb (Systematik) 87:185–230
Chek AA, Lifjeld JT, Robertson RJ (1993) Captive study of copulation in the pied flycatcher Ficedula hypoleuca. Fauna Norvegica Ser C Cinclus 16:67–73
Deeming C (2013) Feathering the nest. Biologist 60:26–30
Ellegren H, Lifjeld JT, Slagsvold T, Primmer CR (1995) Handicapped males and extrapair paternity in pied flycatchers: a study using microsatellite markers. Mol Ecol 4:739–744
Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B (2011) Female extrapair mating behavior can evolve via indirect selection on males. P Natl Acad Sci USA 108:10608–10613
Forstmeier W, Nakagawa S, Griffith SC, Kempenaers B (2014) Female extra-pair mating: adaptation or genetic constraint? Trends Ecol Evol 29:456–464
Garamszegi LZ, Møller AP (2004) Extrapair paternity and the evolution of bird song. Behav Ecol 15:508–519
Gelter HP, Tegelström H (1992) High frequency of extra-pair paternity in Swedish pied flycatchers revealed by allozyme electrophoresis and DNA fingerprinting. Behav Ecol Sociobiol 31:1–7
Gill SA, Stutchbury BJM (2005) Nest building is an indicator of parental quality in the monogamous neotropical buff-breasted wren (Thryothorus leucotis). Auk 122:1169–1181
Griffith SC, Blomqvist D, Andersson M et al (2003) Why do birds engage in extra-pair copulation? Nature 422:833
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Griggio M, Matessi G, Pilastro A (2003) Male rock sparrow (Petronia petronia) nest defence correlates with female ornament size. Ethology 109:659–669
Griggio M, Valera F, Casas A, Pilastro A (2005) Males prefer ornamented females: a field experiment of male choice in the rock sparrow. Anim Behav 69:1243–1250
Hansell M (2000) Bird nests and construction behaviour. Cambridge University Press, Cambridge
Kalinowski S, Taper M, Marshall T (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1009–1106
Kempenaers B, Verheyen GR, den Broeck MV, Burke T, Broeckhoven CV, Dhondt A (1992) Extra-pair paternity results from female preference for high-quality males in the blue tit. Nature 357:494–496
Kullberg C, Metcalfe NB, Houston DC (2002) Impaired flight ability during incubation in the pied flycatcher. J Avian Biol 33:179–183
Lambrechts M, Adriaensen F, Ardia DR et al (2010) The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol 45:1–26
Leder EH, Karaiskou N, Primmer CR (2008) Seventy new microsatellites for the pied flycatcher, Ficedula hypoleuca and amplification in other passerine birds. Mol Ecol Resour 8:874–880
Lifjeld JT, Slagsvold T, Dale S, Ellegren H (1997a) A sexually selected paradox in the pied flycatcher: attractive males are cuckolded. Auk 114:112–115
Lifjeld JT, Slagsvold T, Ellegren H (1997b) Experimental mate switching in pied flycatchers: male copulatory access and fertilization success. Anim Behav 53:1225–1232
Lothery CJ, Thompson CF, Lawler ML, Sakaluk SK (2014) Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS One 9:e106260
Lundberg A, Alatalo RV (1992) The pied flycatcher. Poyser, London
Martínez-de la Puente J, Merino S, Lobato E, Moreno J, Tomás G, Morales J (2009) Male nest-building activity influences clutch mass in pied flycatchers Ficedula hypoleuca. Bird Stud 56:264–267
Mennerat A, Charmantier A, Jørgensen C, Eliassen S (2018) Correlates of complete brood failure in blue tits: could extra-pair mating provide unexplored benefits to females? J Avian Biol 49:e01701
Møller AP, Birkhead TR (1994) The evolution of plumage brightness in birds is related to extrapair paternity. Evolution 48:1089–1100
Morales J, Velando A, Moreno J (2008) Pigment allocation to eggs decreases plasma antioxidants in a songbird. Behav Ecol Sociobiol 63:227–233
Moreno J (1989) Body-mass variation in breeding northern wheatears: a field experiment with supplementary food. Condor 91:178–186
Moreno J, Gil D, Cantarero A, López-Arrabé J (2014) Extent of a white plumage patch covaries with testosterone levels in female pied flycatchers Ficedula hypoleuca. J Ornithol 155:639–648
Moreno J, Lobato E, González-Braojos S, Ruiz-de-Castañeda R (2010a) Nest construction costs affect nestling growth: a field experiment in a cavity-nesting passerine. Acta Ornithol 45:139–145
Moreno J, Martínez J, Corral C, Lobato E, Merino S, Morales J, Martínez-de la Puente JM, Tomás G (2008) Nest construction rate and stress in female pied flycatchers Ficedula hypoleuca. Acta Ornithol 43:57–64
Moreno J, Martínez JG, González-Braojos S, Cantarero A, Ruiz-de-Castañeda R, Precioso M, López-Arrabé J (2015) Extra-pair paternity declines with female age and wing length in the pied flycatcher. Ethology. 121:501–512
Moreno J, Martínez JG, González-Braojos S, Ruiz-de-Castañeda R, Cantarero A, Sánchez-Tojar A (2013) Extra-pair matings, context-dependence and offspring quality: a brood manipulation experiment in pied flycatchers. Behaviour 150:359–380
Moreno J, Martínez JG, Morales J, Lobato E, Merino S, Tomás G, Vásquez RA, Möstl E, Osorno JL (2010b) Paternity loss in relation to male age, territorial behaviour and stress in the pied flycatcher. Ethology 116:76–84
Moreno J, Merino S, Lobato E, Ruiz-De-Castañeda R, Martínez-De La Puente J, Del Cerro S, Rivero-De Aguilar J (2009) Nest-dwelling ectoparasites of two sympatric hole-nesting passerines in relation to nest composition: an experimental study. Ecoscience 16:418–427
Moreno J, Merino S, Potti J, de León A, Rodríguez R (1999) Maternal energy expenditure does not change with flight costs or food availability in the pied flycatcher (Ficedula hypoleuca): costs and benefits for nestlings. Behav Ecol Sociobiol 46:244–251
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Norberg RA (1981) Temporary weight decrease in breeding birds may result in more fledged young. Am Nat 118:838–850
Pennycuick CJ (1982) The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Philos Trans R Soc B 300:75–106
Petrie M, Kempenaers B (1998) Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol Evol 13:52–58
Pilastro A, Griggio M, Biddau L, Mingozzi T (2002) Extrapair paternity as a cost of polygyny in the rock sparrow: behavioural and genetic evidence of the ‘trade-off’ hypothesis. Anim Behav 63:967–974
Plaza M, Cantarero A, Cuervo JJ, Moreno J (2018) Female incubation attendance and nest vigilance reflect social signaling capacity: a field experiment. Behav Ecol Sociobiol 72:24
Plaza M, Cantarero A, Gil D, Moreno J (2019) Experimentally flight-impaired females show higher levels of extra-pair paternity in the pied flycatcher Ficedula hypoleuca. Biol Lett 15:20190360
Ramos AG, Nunziata SO, Lance SL, Rodríguez C, Faircloth BC, Gowaty PA, Drummond H (2014) Interactive effects of male and female age on extra-pair paternity in a socially monogamous seabird. Behav Ecol Sociobiol 68:1603–1609
Ruiz-de-Castañeda R, Burtt EH, González-Braojos S, Moreno J (2012) Bacterial degradability of an intrafeather unmelanized ornament: a role for feather-degrading bacteria in sexual selection? Biol J Linn Soc 105:409–419
Salewski V, Hochachka WM, Flinks H (2014) Changes in stonechat Saxicola torquata morphology: a response to climate change? J Ornithol 155:601–609
Sanz JJ, Moreno J (1995) Mass loss in brooding female pied flycatchers Ficedula hypoleuca: no evidence for reproductive stress. J Avian Biol 26:313–320
Sirkiä PM, Adamík P, Artemyev AV et al (2015) Fecundity selection does not vary along a large geographical cline of trait means in a passerine bird. Biol J Linn Soc 114:808–827
Slagsvold T, Dale S (1996) Disappearance of female pied flycatchers in relation to breeding stage and experimentally induced molt. Ecology 77:461–471
Stutchbury BJ, Robertson RJ (1987) Behavioral tactics of subadult female floaters in the tree swallow. Behav Ecol Sociobiol 20:413–419
Stutchbury BJ, Morton ES (1995) The effect of breeding synchrony on extra-pair mating systems in songbirds. Behaviour 132:675–690
Tomás G, Merino S, Moreno J, Sanz JJ, Morales J, García-Fraile S (2006) Nest weight and female health in the blue tit (Cyanistes caeruleus). Auk 123:1013–1021
van den Hout PJ, Mathot KJ, Maas LRM, Piersma T (2010) Predator escape tactics in birds: linking ecology and aerodynamics. Behav Ecol 21:16–25
Videler JJ (2005) Avian flight. Oxford University Press, Oxford
Von Haartman L (1956) Territory in the pied flycatcher. Ibis 98:460–475
Westneat DF, Stewart IRK (2003) Extra-pair paternity in birds: causes, correlates, and conflict. Annu Rev Ecol Evol Sci 34:365–396
Acknowledgements
This study is a contribution to the research developed at “Ventorrillo” field station. We are very grateful to D. Gil for improving the manuscript with his comments and to A. Machordom for helping at the molecular Lab of Museo Nacional de Ciencias Naturales. We are also grateful to the referees, whose feedback substantially improved the manuscript.
Funding
This study was financed by project CGL2013-48193-C3-3-P and CGL2017-83843-C2-1-P to JM from Spanish ‘Ministerio de Ciencia, Innovación y Universidades’. MP was supported by FPI grant from ‘Ministerio de Ciencia, Innovación y Universidades’. AC is supported by a postdoctoral fellowship from Fundación Ramón Areces.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MP has received research FPI grant from ‘Ministerio de Ciencia, Innovación y Universidades’. AC declares that he has no conflict of interest. JM declares that he has no conflict of interest.
Ethical approval
We were legally authorized to capture and handle pied flycatchers by Consejería de Medio Ambiente de Castilla y León (competent regional authority, protocol number EP/SG/706/2016, according to Royal Decree 53/2013), and by J. Donés, director of “Centro Montes de Valsaín”, to work in the study area. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experiments comply with current Spanish laws, and grant holder and field researchers were officially licensed for animal manipulation following current EU regulations on animal manipulation (authorization types C and D with reference numbers CAP-T-0123-15 and CAP-T-0121-15). The study was ethically approved by the Ethical Committee of the ‘Consejo Superior de Investigaciones Científicas’ (CSIC).
Additional information
Communicated by S. Pruett-Jones
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plaza, M., Cantarero, A. & Moreno, J. An experimental increase in female mass during the fertile phase leads to higher levels of extra-pair paternity in pied flycatchers Ficedula hypoleuca. Behav Ecol Sociobiol 73, 161 (2019). https://doi.org/10.1007/s00265-019-2771-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2771-z