Abstract
Urban environments are novel landscapes that markedly alter animal behavior. Divergence in behavior in response to urbanization may provide advantages in navigation, exploiting resources, and surviving under a novel suite of selective pressures. Relatively few studies, however, have identified population-level behavioral changes in response to urbanization that are not confounded by rearing environment and prior experience (e.g., an urban upbringing). To address this, we used the Australian water dragon (Intellagama lesueurii) to test whether populations under varying levels of urbanization (urban, semi-natural, and natural populations) differ in their innate behavioral traits; acquired either heritably or due to population-specific maternal effects. Eggs were collected from wild mothers and hatched in the lab. Hatchlings were then reared in the lab under standardized conditions (a common-garden experiment). We then assayed individual behavioral traits (boldness, exploration, and neophilia) five times across their first year of development. We compared behavioral traits, as well as their expression (repeatability), between urban, semi-natural, and natural populations. Neophilia and explorative behavior was similar among all populations. However, dragons from semi-natural populations were significantly bolder than those from natural populations. Urban dragons were also bolder than dragons from natural populations, although this trend was not significant because of high variance in boldness. Dragons from semi-natural and urban populations had similar boldness scores, suggesting a potentially biologically relevant difference in boldness between them and natural populations. We also saw some differences in the consistency of the expression of behavior. Boldness in individuals from urban environments was also the only repeatable trait. Overall, our study suggests that boldness is an innate, urban-derived divergent behavioral trait that likely contributes to the success of these lizards in anthropogenically altered environments.
Significance statement
Lizards from human-modified areas are innately bolder than ones from natural habitats. To determine this, we raised lizards from eggs collected from urban, semi-natural, and natural populations in a standardized environment, removing the effects of prior experience and developmental environment, and examined their behavioral traits over time. The difference we found in boldness was related to their origin population, rather than being shaped through experience, suggesting this trait may be heritable and is being selected for in anthropogenic landscapes. Our study addresses an important gap in studies of urban behavioral ecology by examining behavioral differences among replicated, differently urbanized, sites after experimentally accounting for both rearing environment and prior experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Novel landscapes can expose individuals to challenges that may substantially alter their behavior (Sol et al. 2013; Alberti et al. 2017; Lapiedra et al. 2017). This action is typified by the behavioral shifts of animals living within urban environments (Shochat et al. 2006; Garroway and Sheldon 2013; Lowry et al. 2013; Sol et al. 2013), and has been documented in birds (Atwell et al. 2012), insects (Schuett et al. 2018), mammals (Lyons et al. 2017), reptiles (Peterman and Ryan 2009), and spiders (Kralj-Fišer et al. 2017). These urban-derived divergent behaviors can include altered anti-predator responses (McCleery 2009; Blumstein 2014), foraging behavior (Geggie and Fenton 1985; Shochat et al. 2004; Short and Petren 2008), increased problem-solving ability (Sol et al. 2011), behavioral thermoregulation (Peterman and Ryan 2009), and mate communication (Parris et al. 2009; Barnett 2015). Furthermore, changes in behavioral traits (e.g., boldness, neophilia, and exploration) may also provide advantages to navigating and exploiting urban environments (Kralj-Fišer et al. 2017). Boldness reflects an individual’s propensity to take risks; bolder individuals may be more active in novel, urban landscapes and situations, which could increase their time spent foraging, mate searching, or defending a territory (Réale et al. 2007; Sol et al. 2013; Sprau and Dingemanse 2017). Similarly, neophilia—an individual’s willingness to engage with novel stimuli or objects—could provide substantial advantages within an urban environment by increasing their ability to exploit novel resources (e.g., food sources or shelter; Bókony et al. 2012; Miranda et al. 2013). Finally, an individual’s propensity to explore could influence their success in urban environments by increasing their ability to disperse across novel landscapes (Damas-Moreira et al. 2019) and to gather important environmental information (e.g., identifying refuge, basking, and perching locations in novel environments; Lapiedra et al. 2017).
Although altered behavior and behavioral traits have been documented in numerous urban-living species, the specific mechanisms driving the formation of urban-derived divergent behavioral traits are largely unclear (but see Miranda et al. 2013). Behavioral plasticity has been suggested to aid urban-dwelling individuals increase their exploitation of urban resources and decrease the costs associated with urban habitats (Ditchkoff et al. 2006; Partecke et al. 2006; French et al. 2008; Atwell et al. 2012; Lucas and French 2012; Lampe et al. 2014; Kralj-Fišer et al. 2017). Alternatively, if these behavioral traits are heritable, and provide an advantage in urban environments, then selection may favor them in urban populations. Recent research has suggested that urban evolution is driving the persistence of species in heavily human-modified habitats (Johnson and Munshi-South 2017). Yet, even though divergent behavior in urban-living populations is likely adaptive, it is unclear if these behaviors are a result of selection or plasticity (Diamond 1986; Møller 2008; Lowry et al. 2013; Sol et al. 2013; Alberti et al. 2017). Recently, heritability of behavioral traits (e.g., boldness and aggression) has been documented for several urban-dwelling bird species (Evans et al. 2010; Müller et al. 2013; Holtmann et al. 2017; Sprau and Dingemanse 2017). Flight-capable birds, however, should experience weaker selection within urban environments compared with terrestrial species, because they are able to rapidly vacate urban habitats leading to increased gene flow. In contrast, less vagile terrestrial species are physically tied to specific locations within urban environments (Brown 1978; Wiens and Donoghue 2004; Lyons et al. 2017), and thus may experience stronger selection. Research into heritable behavioral traits in terrestrial urban species remains rare (but see Kralj-Fišer and Schneider 2012), but they are a study system that could greatly enhance our understanding of the full extent to which urban environments are shaping animal behavior.
The Australian water dragon (Intellagama lesueurii) is an agamid lizard species found throughout eastern Australia (Cogger 2014). Water dragons are common in urban areas and appear to have successfully exploited human-altered landscapes, where some populations have experienced rapid morphological evolution (Littleford-Colquhoun et al. 2017). This species is therefore a good model for testing whether behavior may play a role in their success in urban environments. Specifically, we used a common garden experiment, which removed the confounding effects of rearing environment and prior experience, to test whether urban environments are favoring particular heritable behavioral traits or whether behavior is best explained by experience. We raised hatchling dragons from eggs collected from mothers living in urban, semi-natural, and natural populations, and repeatedly quantified their behavioral traits (boldness, neophilia, and exploration) over the first year of life. We predicted higher levels of boldness, neophilia, and exploration in individuals from urban and semi–natural origin populations compared with their natural-living counterparts. If these predictions are upheld, this would constitute evidence for heritable behavioral divergence in urbanized populations. We also examined if behavioral traits were repeatable throughout development, and compared their consistency among origin population categories (urban, semi-natural, and natural). Repeatability of behavioral traits across time suggests strong, constant selection for a particular behavioral type within an environment (Dingemanse and Réale 2005; Bell 2012) and may give an indication of broad heritability of a trait (Dohm 2002). In contrast, lack of repeatability of behavioral traits may indicate plasticity; which may be beneficial in order to cope with changing, novel environments (Lampe et al. 2014; Griffin et al. 2016).
Methods
Study species
Australian water dragons are a large (maximum snout-vent length: 304 mm; Thompson 1993) agamid lizard. They are relatively long-lived species (28–40 years; Harlow and Harlow 1997; Griffiths 2006) with a generation time of 5 years (Littleford-Colquhoun et al. 2017). They are naturally found in forested areas associated with creeks, rivers, and other freshwater bodies (Cogger 2014); however, they are also common in urban parklands and other green spaces (Littleford-Colquhoun et al. 2017). These lizards are a dietary generalist (Baxter-Gilbert 2014), and are known to exploit anthropogenic food sources in urban areas (Baxter-Gilbert 2018).
Field collection and husbandry
In the spring (October and November) of 2015, we collected gravid female water dragons from 12 sites (four urban, four semi-natural, and four natural) within a 50-km radius within the greater Sydney area in New South Wales, Australia (see Supplementary Materials for exact location details, sample sizes, and SM Fig. 1). Urban sites had a dense local human population and a landscape that was widely human-modified (e.g., concrete, buildings, gardens, roads). Semi-natural sites were protected green spaces (national and regional parkland) that contained waterways adjacent to urban areas, and they had a moderate human presence (park visitors). Natural sites, although not completely free from human disturbance, were generally associated with native bushland, waterways with treed shorelines, and a relatively low human presence.
Upon capture, we transported females to Macquarie University (Sydney, NSW), or if captured at Taronga Zoo (Sydney, NSW) they were held there, and then oviposition was induced (for egg collection details see Baxter-Gilbert et al. 2018). Clutches of eggs were identically incubated throughout development (at a constant temperature of 26.5 °C allowing for an equal sex-ratio; Harlow 2001). Upon emerging, hatchlings were marked using a passive integrated transponder (PIT) tag and randomly allocated to one of 15 common garden enclosures (approximately 6–7 lizards per enclosure; initial experimental group N = 97 but group size varied over time due to seasonal differences in capture success; Table 1). The outdoor enclosures (6.2 m2 plastic tubs lined with sand, and containing tile refuges, hardwood dowel perches, and a small pool) were contained within a predator-exclusion net, which allowed for natural weather and photoperiods common to the Sydney region (see SM Fig. 2). Throughout the experiment, all dragons experienced identical housing, husbandry conditions (fed vitamin-supplemented crickets 3 times weekly), thermal conditions, and water ad libitum.
Behavioral assays
We assayed three behavioral traits (exploration, boldness, and neophilia) five times over the dragons’ first year of life (once every 2 months, excluding the winter brumation period of July and August). Dragon body size (snout-vent length) varied over this time period, averaging 48 mm (± 0.19 standard error (SE), min = 39 mm, max = 53 mm) initially, and growing to an average of 82 mm (± 1.21 SE, min = 64 mm, max = 133 mm) at the end of the year. Behavioral trait assays were conducted indoors over 3 days, and consisted of 1 assay per day. During each of the five rounds of assays, we were not always able to re-capture all lizards, resulting in some variation in sample sizes (Table 1). Our experimental room was not large enough to house all dragons at once, so we conducted assays in four batches (maximum of 32 individuals per batch, two batches per day, and 6 days total). Assays took place in a temperature-controlled room, set to the dragon’s preferred body temperature of 30 °C (Hosking 2010), unless otherwise stated. During assays, dragons were individually housed and behaviors were remotely video recorded using a security camera system (CCTV Security Systems, Melbourne, Victoria). Each behavioral assay (exploration, boldness, neophilia) was scored from the videos by a single researcher to ensure consistency, with the video scorer being blind to the lizard’s origin population (see below for scoring criteria).
Day 1: Explorative behavior
Our measure of explorative behavior quantified the amount of time (s) a dragon spent moving (i.e., exploring) in a novel arena. To assay these behaviors, we introduced dragons into a novel environment, similar to an open-field test (Perals et al. 2017; Riley et al. 2017; Damas-Moreira et al. 2019). The testing arenas were always the same size (rectangular arenas; 690 W × 470 L × 455 H mm) and had two black refuge boxes (120 W × 175 L × 38 H mm) at opposite ends. We varied the substrate between each of the five repeated measures (plain paper, eucalyptus mulch, sugar cane mulch, topsoil, and pine-bark mulch; see SM Fig. 3A) to ensure the arena was novel each time. At the beginning of each trial, we introduced the dragon into the arena within a central, containment refuge. The dragon was allowed to acclimate within this refuge for 5 min, whereupon the refuge was lifted, and the assay began. Each exploration assay ran for 30 min. The dragons then remained in these enclosures for the duration of the assay period (3 days). From video recordings, we scored the time a lizard spent moving within the trial (s). This value was used as our “exploration score”; as the value increases, it reflects more explorative behavior (Riley et al. 2017; Damas-Moreira et al. 2019).
Day 2: Boldness
Our measure of boldness was the amount of time (s) it took a dragon to leave an unfavorable refuge after a simulated predatory attack. We created a thermal difference within the testing arena by lowering the temperature in the experimental room to 22 °C, and positioning a heat lamp directly over one of the refuge boxes, creating a “hot” refuge (Carazo et al. 2014; Riley et al. 2017; Damas-Moreira et al. 2019). We also positioned an ice pack beneath the enclosure, directly under the other refuge, creating a “cold” refuge (Carazo et al. 2014; Riley et al. 2017; Damas-Moreira et al. 2019; see SM Fig. 3B). By doing this, we created a high- and low-quality refuge. At the beginning of each trial, we introduced the dragon into the arena within a central, containment refuge and left it to acclimate for 5 min. We then simulated a predatory attack by removing the containment refuge and “chasing” the dragon with a blue, gloved hand until it entered the “cold” refuge (Riley et al. 2017; Damas-Moreira et al. 2019). We then remotely video recorded the dragon’s behavior for 1 h. We measured a lizard’s boldness as the amount of time (s) it took the dragon to leave the “cold” refuge. This value was our “boldness score”, with lower times indicating higher boldness. If the dragons did not exit the refuge within the duration of the trial, we assigned it a value of 3600 s.
Day 3: Neophilia
Our neophilia assay quantified how close (cm) a dragon would approach a novel object. Within each enclosure, a bullseye (10-, 20-, 30-, and 40-cm diameter rings surrounding a central 5-cm diameter circle) was printed on paper and taped to the base of the arena (prior to all behavioral assays beginning; see SM Fig. 3). The two refuge boxes from the previous assay were removed, and a novel object was placed at the center of the bullseye (see SM Fig. 3C). During the five neophilia assays, each individual saw a different novel object each time. The objects chosen are common refuse items found in urban areas that were similarly sized (between 6 and 8 cm diameter), and was different across each assay period. The novel objects were presented in this order: (1) unused 350-ml paper coffee cups, (2) unused aluminum 160-ml pie tins, (3) empty 600-ml water bottle, (4) unopened bag of 19-g potato chips, and (5) unopened 330-ml soft drink can. Similar to the previous two assays, each dragon was placed within a central containment refuge at the start of an assay, and left for 5 min to acclimate. To begin the assay, the central containment refuge was removed, and individuals were left for 30 min to interact with the novel object.
From the videos of the neophilia assay, we noted the proximity of the individual to the novel object using the rings of the bullseye to indicate distance to the object (e.g., outer-most ring = 20 cm and inner-most ring = 5 cm). Dragons that climbed the novel object were given a score of 0 cm, and individuals beyond the outermost ring were assigned a score of 25 cm. The closest distance a dragon approached the novel object over the 30-min period represented its “neophilia score” and was the value used in our analysis; lower scores indicate a higher level of neophilia.
Statistical analyses
Behavioral traits
Before analysis, we explored our data following the protocol detailed in Zuur et al. (2010). Two of our three behavioral traits, boldness and neophilia, followed a normal distribution and had no outliers. We used a rank transformation to normalize our exploration score (Kar et al. 2016). Before analyses, we also ensured there was no strong collinearity between model predictor variables (i.e., a R2 of greater than 0.70).
We examined differences in dragon behavioral traits using linear mixed effect models (LMM, using the function lmer in the lme4 R package; Bates et al. 2015; R Core Team 2016). We ran separate LMMs for each of the three behavioral traits. The LMMs with exploration and neophilia as the response variable included the fixed effects of dragon age (days since hatching; continuous), origin population type (categorical: natural, semi-natural, or urban), and batch (categorical: 1, 2, 3, or 4). The LMM with boldness as the response variable had the additional continuous fixed factor of time spent scaring the lizard (s). Continuous fixed factors were mean-centered using a z-transformation before analysis, which standardizes the variables and facilitates interpretation of main effects in the presence of interactions (Schielzeth 2010). In all LMMs, we accounted for dependencies within our data from sampling each lizard repeatedly (random intercept and slope for lizard identity across age), sampling individuals from the same clutch (random intercept for lizard clutch), the same captive enclosures (random intercept for tub identity), and the same study population (random intercept for study site). To allow comparisons among all origin site types, we re-leveled the reference for origin population category and re-ran the model (Nakagawa 2004). The assumptions of normality of residuals, for both fixed and random effects, and heterogeneity of variance were verified for all LMMs (Zuur et al. 2009), α was set at 0.05, and the R function confint was used to bootstrap 95% confidence intervals for parameter estimates. We also calculated unconditional means and 95% CIs (corrected for non-independence) for each origin population type using the function Effect in the R package effects (Fox 2003; Fox and Hong 2009). Assessment of unconditional means and the magnitude of their differences (i.e., effect size) can reflect biological significance (Nakagawa and Cuthill 2007; Gerstner et al. 2017).
Consistency in behavior
We examined the consistency of an individual’s behavioral traits to investigate if repeatability was affected by origin population type. To accomplish this, we first subset the data by origin population category, resulting in three separate datasets. For urban-origin dragons, we had 126 observations from 28 individuals across 17 clutches, 14 enclosures, and 4 populations. For semi-natural-origin dragons, we had 225 observations of exploration, and 228 observations of boldness and neophilia from 52 individuals across 32 clutches, 15 enclosures, and 4 populations. For natural-origin dragons, we had 82 observations of exploration, and 83 of boldness and neophilia from 23 individuals across 14 clutches, 13 enclosures, and 4 populations.
We calculated adjusted repeatability (Radj|age; Biro and Stamps 2015) for each origin population type while controlling for the same covariates that were within their respective LMMs (Nakagawa and Schielzeth 2010; Biro and Stamps 2015). We calculated 95% confidence intervals by bootstrapping the data 1000 times with the boot function from the R package boot (Davison and Hinkley 1997; Canty and Ripley 2017). Radj|age was considered significantly more than what would occur by chance alone if the 95% confidence intervals did not overlap 0. We compared Radj|age between treatments by visually examining overlap between 95% CIs and the Radj|age value for natural, semi-natural, and urban sites. Being conservative, we considered a difference as significant if the 95% CIs did not overlap. Theoretically, Radj|age ranges between 0 (individuals never expressing the same trait value over repeated measures) and 1 (individuals always expressing the same trait value over repeated measures; Nakagawa and Schielzeth 2010), although the average repeatability observed in the field of animal behavior is 0.37 (Bell et al. 2009).
Results
Behavioral traits
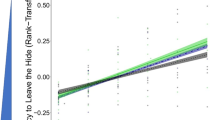
Exploration did not significantly differ among origin population category (Table 2; comparison between semi-natural and urban: β = 0.118, 95% CI = − 0.612, 0.427, t = − 0.444, P = 0.657), and neither did neophilia (Table 2; comparison between semi-natural and urban: β = − 0.628, 95% CI = − 3.173, 2.130, t = − 0.445, P = 0.656). Dragons that originated from semi-natural sites were significantly bolder than natural sites (Table 2). Boldness did not significantly differ between urban and natural sites (Table 2), nor semi-natural and urban sites (β = 10.810, 95% CI = − 408.829, 448.212, t = 0.048, P = 0.962). However, both the semi-natural and urban dragons exited the hide (the metric for boldness) about 8.3 min (492 s and 502 s, respectively) sooner than individuals in the natural-origin population category (Table 2; Fig. 1).
Australian water dragons from semi-natural sites were significantly bolder than those from natural sites (b). Here we present unconditional means and 95% CIs of each behavioral trait, a exploration, b boldness, and c neophilia, for each origin population category (black, gray, and white points represent natural (NAT), semi-natural (SEMI-NAT), and urban (URB) populations, respectively) of Australian water dragons (Intellagama lesueurii)
Consistency in behavior
Explorative behavior of dragons was not significantly repeatable in any origin population (urban: Radj|age = 0.44, 95% CI = 0, 0.68; semi-natural: Radj|age = 0.14, 95% CI = 0, 0.40; natural: Radj|age = 0.02, 95% CI = 0, 0.45). Similarly, neophilia of dragons was not significantly repeatable in any origin population (urban: Radj|age = 0.05, 95% CI = 0, 0.34; semi-natural: Radj|age = 0.06, 95% CI = 0, 0.30; natural: Radj|age = 0, 95% CI = 0, 0.45).
Dragons originating from urban populations had moderate repeatability in boldness (Radj|age = 0.32, 95% CI = 0.02, 0.63), while boldness was not significantly repeatable in dragons from semi-natural (Radj|age = 0.18, 95% CI = 0, 0.41) and natural (Radj|age = 0.25, 95% CI = 0, 0.61) populations. Repeatability was not different among origin population types in any of the behavioral traits measured.
Discussion
We found that water dragons from semi-natural populations were significantly bolder than those from natural-origin populations. Also, a difference between urban and natural-origin populations, although non-significant, trended in the same direction. We expected lizards to be bolder in relation to the extent of urbanization experienced by their origin population (i.e., urban dragons to be most bold, semi-natural dragons to be moderately bold, natural dragons to be least bold). Interestingly, the parameter estimates reflect this logic. The difference in boldness between urban and natural populations (parameter estimates and effect sizes) was actually slightly greater (by 10 s) than the difference in boldness between semi-natural and natural environments (Table 2; Fig. 1). The lack of significance is likely a consequence of greater variance in boldness among individuals within the urban environment. We suggest that the difference in boldness between dragons from urban and natural-origin population categories is likely still biologically relevant, because both the semi-natural and urban dragons exited the hide at approximately the same time, which was substantially earlier than individuals in the natural-origin population type.
Our findings support idea that wildlife persisting in, or colonizing, urban environments have a tendency to be bolder (reviewed in Lowry et al. 2013; Miranda et al. 2013; Sol et al. 2013) and suggests increased boldness in our urbanized water dragons is innate, either as a heritable trait or because of maternal effects. These findings further align with recent studies that demonstrate urbanization may be selecting for heritable behavioral traits (e.g., increased aggression and boldness, Evans et al. 2010; Müller et al. 2013; Holtmann et al. 2017; Sprau and Dingemanse 2017). If heritable, then increased boldness in urban areas may promote fitness, through facilitating increased foraging and mating opportunities. This fitness benefit may drive selection for enhanced boldness within novel environments (Dingemanse and Réale 2005; Réale et al. 2007). The design of our common garden experiment was able to remove a host of potential confounding factors, such as nest environment, prior experience, or habituation (Evans et al. 2010; Lampe et al. 2014; Vincze et al. 2016; Siviter et al. 2017), which does support our assentation that increased boldness is an urban-derived heritable trait. Yet, we cannot rule out the possibility of site-specific maternal effects. We recommend future research into the behavioral traits of urban and natural populations take into account any differences in the allocation of nutrients and maternal hormones into developing eggs, as this may alter hatchling behavioral traits (Groothuis et al. 2005; Räsänen and Kruuk 2007; Bertin et al. 2009). Maternal effects on behavioral traits may also include differences in maternal basking opportunities between populations, as seen in other Australian agamids (Amphibolurus muricatus; Schwanz 2016).
With respect to neophilia and exploration, we did not find differences in among origin population categories. These behavioral traits may not be strongly favored for within Sydney urban environments. Alternatively, there may have been a flaw in the trial design (e.g., testing arena size or means of measuring) or there may be ontogenetic changes in the timing of expression of these behaviors (e.g., perhaps dragons do not express variation in neophilia or exploration behavior until they are older). Urban, wild-caught brown anoles (Anolis sagrei) are bolder, less aggressive, and more explorative compared with natural-origin populations (Lapiedra et al. 2017); however, in this study, they could not rule out the effects of rearing environment and prior experience on the behaviors they were observing. Expression of behavioral traits like exploration and neophilia may be highly plastic in urban habitats (Bókony et al. 2012; Sol et al. 2013). For example, there is a positive correlation between boldness and aggression in song sparrows (Melospiza melodia), but this relationship breaks down in urban areas (Scales et al. 2011), which may result from individuals modulating their behavior based on specific costs and benefits associated with differing habitats (Sol et al. 2014). Overall, more research is necessary to understand the selective forces that are shaping the behavior of urban wildlife (Lowry et al. 2013) and what determines the roles that both plastic and fixed behavioral traits play for species persisting in urban landscapes.
The only behavioral trait we observed to be consistent was boldness in individuals from urban environments which was significantly, but moderately, repeatable. Repeatability, or consistency, in behavior across time generally reflects selection for the expression of that trait within that environment (Dingemanse and Réale 2005). For this reason, the repeatable boldness in urban populations supports our assertion that urban selection favors consistently bolder individuals. For all other behavioral trait and environment type combinations, dragons exhibited low within-individual repeatability of behavioral traits, which suggests that plasticity in their expression may be favorable. However, an alternative hypothesis for the general lack of repeatability in behavior is that the behavior of juveniles may simply be more plastic (Favati et al. 2016; Riley et al. 2017), with more fixed behaviors traits forming as they mature. This would also suggest that rearing environment and prior experience may inform the development of behavior in these dragons. Formation of consistent behavior, related to urban-derived behavioral syndromes, has been documented in adult brown anoles (Lapiedra et al. 2017), and we suggest that further research on adult dragon behavioral expression across urban populations is required to determine if they would yield comparable results. Potentially, practical issues with study design may be another explanation for why repeatability of dragon behavioral traits was not found across habitats and environments. For example, we may have not selected an ecologically relevant time frame for the quantification of trait consistency (Dohm 2002). Overall, further investigation into the differences in behavior and the expression of behavioral traits in anthropogenic landscapes will shed light into how selective forces act on individuals during urban evolution.
In summary, urban landscapes are both expanding globally and a major contributor to biodiversity loss (McKinney 2002; Seto et al. 2012). In light of the novel landscapes humanity has created (Ellis and Ramankutty 2008), it is imperative we understand the role of urban evolution in allowing wildlife to adapt to an increasingly urban world (Dingemanse and Réale 2005; Lowry et al. 2013; Holtmann et al. 2017; Johnson and Munshi-South 2017). Furthermore, we need to understand how these naturally evolving divergent behavioral traits can be applied to conservation actions and wildlife management to enhance our ability to protect species that are less capable at persisting in urban areas, which is currently a major threat to wildlife worldwide (Greggor et al. 2016). Our study provides experimental evidence of an innate, urban-derived divergent behavioral trait (boldness) in a vertebrate, removed from the confounding effects of developmental environment and prior experience, and advances our understanding of both urban evolution (Johnson and Munshi-South 2017) and the role of behavior in evolution, particularly in novel environments.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to logistical constraints, but are available from the corresponding author on reasonable request.
References
Alberti M, Marzluff J, Hunt VM (2017) Urban driven phenotypic changes: empirical observations and theoretical implications for eco-evolutionary feedback. Philos Trans R Soc B 372:20160029. https://doi.org/10.1098/rstb.2016.0029
Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23:960–969. https://doi.org/10.1093/beheco/ars059
Barnett CA (2015) Urban hymns how urban environments affect bird populations and avian singing behavior. In: Mahala G (ed) Seabirds and songbirds: habitat preferences, conservation and migratory behaviour. Nova Science Publisher Inc., Hauppauge, pp 115–134
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Baxter-Gilbert J (2014) Heterospecific coprophagy in an eastern water dragon, Intellagama lesueurii lesueurii, (Gray 1831). Herpetofauna 44:34–37
Baxter-Gilbert JH (2018) Behavioural and biological responses of Australian water dragons (Intellagama lesueurii) to urbanisation. PhD thesis, School of Biological Sciences, Macquarie University, Sydney, AU
Baxter-Gilbert J, Riley JL, Whiting MJ (2018) Runners and fighters: clutch effects and body size drive innate antipredator behaviour in hatchling lizards. Behav Ecol Sociobiol 72:97. https://doi.org/10.1007/s00265-018-2505-7
Bell AM (2012) Animal behaviour: personality in the wild. Nature 491:341–342. https://doi.org/10.1038/491341a
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783. https://doi.org/10.1016/j.anbehav.2008.12.022
Bertin A, Richard-Yris MA, Houdelier C, Richard S, Lumineau S, Kotrschal K, Möstl E (2009) Divergent selection for inherent fearfulness leads to divergent yolk steroid levels in quail. Behaviour 146:757–770. https://doi.org/10.1163/156853909X446190
Biro PA, Stamps JA (2015) Using repeatability to study physiological and behavioural traits: ignore time-related change at your peril. Anim Behav 105:223–230. https://doi.org/10.1016/j.anbehav.2015.04.008
Blumstein DT (2014) Attention, habituation, and antipredator behaviour: implications for urban birds. In: Gil D, Brumm H (eds) Avian urban ecology: Behavioural and physiological adaptations. Oxford University Press, Oxford, pp 41–53
Bókony V, Kulcsár A, Tóth Z, Liker A (2012) Personality traits and behavioral syndromes in differently urbanized populations of house sparrows (Passer domesticus). PLoS One 7:e36639. https://doi.org/10.1371/journal.pone.0036639
Brown JH (1978) The theory of insular biogeography and the distribution of boreal birds and mammals. Great Basin Nat Mem 2:209–227 https://www.jstor.org/stable/23376568
Canty A, Ripley B (2017) boot: Bootstrap R (S-Plus) functions R package version 1:3–19, https://cran.r-project.org/web/packages/boot/index.html
Carazo P, Noble DW, Chandrasoma D, Whiting MJ (2014) Sex and boldness explain individual differences in spatial learning in a lizard. Proc R Soc B 281:20133275. https://doi.org/10.1098/rspb.2013.3275
Cogger HG (2014) Reptiles and amphibians of Australia, 7th edn. Reed Books, Chatswood
Damas-Moreira I, Riley JL, Harris DJ, Whiting MJ (2019) Can behaviour explain invasion success? A comparison between sympatric invasive and native lizards. Anim Behav 151:195–202. https://doi.org/10.1016/j.anbehav.2019.03.008
Davison AC, Hinkley DV (1997) Bootstrap methods and their applications. Cambridge University Press, Cambridge
Diamond JM (1986) Natural selection: rapid evolution of urban birds. Nature 324:107–108. https://doi.org/10.1038/324107a0
Dingemanse NJ, Réale D (2005) Natural selection and animal personality. Behaviour 142:1159–1184. https://doi.org/10.1163/156853905774539445
Ditchkoff SS, Saalfeld ST, Gibson CJ (2006) Animal behaviour in urban ecosystems: modifications due to human-induced stress. Urban Ecosyst 9:5–12. https://doi.org/10.1007/s11252-006-3262-3
Dohm MR (2002) Repeatability estimates do not always set an upper limit to heritability. Funct Ecol 16:273–280. https://doi.org/10.1046/j.1365-2435.2002.00621.x
Ellis EC, Ramankutty N (2008) Putting people in the map: anthropogenic biomes of the world. Front Ecol Environ 6:439–447. https://doi.org/10.1890/070062
Evans J, Boudreau K, Hyman J (2010) Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116:588–595. https://doi.org/10.1111/j.1439-0310.2010.01771.x
Favati A, Zidar J, Thorpe H, Jensen P, Løvlie H (2016) The ontogeny of personality traits in the red junglefowl, Gallus gallus. Behav Ecol 27:484–493. https://doi.org/10.1093/beheco/arv177
Fox J (2003) Effect displays in R for generalised linear models. J Stat Softw 8:1–27. https://doi.org/10.18637/jss.v008.i15
Fox J, Hong J (2009) Effect displays in R for multinomial and proportional-odds logit models: extensions to the effects package. J Stat Softw 32:1–24. https://doi.org/10.18637/jss.v032.i01
French SS, Fokidis HB, Moore MC (2008) Variation in stress and innate immunity in the tree lizard (Urosaurus ornatus) across an urban–rural gradient. J Comp Physiol B 178:997–1005. https://doi.org/10.1007/s00360-008-0290-8
Garroway CJ, Sheldon BC (2013) Urban behavioural adaptation. Mol Ecol 22:3430–3432. https://doi.org/10.1111/mec.12351
Geggie JF, Fenton MB (1985) A comparison of foraging by Eptesicus fuscus (Chiroptera: Vespertilionidae) in urban and rural environments. Can J Zool 63:263–266. https://doi.org/10.1139/z85-040
Gerstner K, Moreno-Mateos D, Gurevitch J, Beckmann M, Kambach S, Jones HP, Seppelt R (2017) Will your paper be used in a meta-analysis? Make the reach of your research broader and longer lasting. Methods Ecol Evol 8:777–784. https://doi.org/10.1111/2041-210X.12758
Greggor AL, Berger-Tal O, Blumstein DT, Angeloni L, Bessa-Gomes C, Blackwell BF, St Clair CC, Crooks K, de Silva S, Fernández-Juricic E, Goldenberg SZ, Mesnick SL, Owen M, Price CJ, Saltz D, Schell CJ, Suarez AV, Swaisgood RR, Winchell CS, Sutherland WJ (2016) Research priorities from animal behaviour for maximising conservation progress. Trends Ecol Evol 31:953–964. https://doi.org/10.1016/j.tree.2016.09.001
Griffin AS, Guez D, Federspiel I, Diquelou M, Lermite F (2016) Invading new environments: a mechanistic framework linking motor diversity and cognition to establishment success. In: Weis JS, Sol D (eds) Biological invasions and animal behaviour. Cambridge University Press, Cambridge, pp 26–46
Griffiths K (2006) Frogs and reptiles of the Sydney region. Reed New Holland, Sydney
Groothuis TG, Müller W, von Engelhardt N, Carere C, Eising C (2005) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352. https://doi.org/10.1016/j.neubiorev.2004.12.002
Harlow PS (2001) Ecology of sex-determining mechanisms in Australian agamid lizards. PhD thesis, School of Biological Sciences, Macquarie University, Sydney, AU
Harlow PS, Harlow MF (1997) Captive reproduction and longevity in the eastern water dragon (Physignathus lesueurii). Herpetofauna. 27:14–19
Holtmann B, Santos ESA, Lara CE, Nakagawa S (2017) Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype-environment covariance. Proc R Soc B 284:20170943. https://doi.org/10.1098/rspb.2017.0943
Hosking C (2010) Husbandry guidelines for Australian water dragon, Physignathus lesueurii (Reptilia: Agamidae). Australian Museum, Sydney http://www.nswfmpa.org/Husbandry%20Manuals/Published%20Manuals/Reptilia/Water%20Dragon%20(Hosking).pdf
Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Science 358:eaam8327. https://doi.org/10.1126/science.aam8327
Kar F, Whiting MJ, Noble DWA (2016) Influence of prior contest experience and level of escalation on contest outcome. Behav Ecol Sociobiol 70:1679–1687. https://doi.org/10.1007/s00265-016-2173-4
Kralj-Fišer S, Schneider JM (2012) Individual behavioural consistency and plasticity in an urban spider. Anim Behav 84:197–204. https://doi.org/10.1016/j.anbehav.2012.04.032
Kralj-Fišer S, Hebets EA, Kuntner M (2017) Different patterns of behavioural variation across and within species of spiders with differing degrees of urbanisation. Behav Ecol Sociobiol 71:125. https://doi.org/10.1007/s00265-017-2353-x
Lampe U, Reinhold K, Schmoll T (2014) How grasshoppers respond to road noise: developmental plasticity and population differentiation in acoustic signalling. Funct Ecol 28:660–668. https://doi.org/10.1111/1365-2435.12215
Lapiedra O, Chejanovski Z, Kolbe JJ (2017) Urbanisation and biological invasion shape animal personalities. Glob Chang Biol 23:592–603. https://doi.org/10.1111/gcb.13395
Littleford-Colquhoun BL, Clemente C, Whiting MJ, Ortiz-Barrientos D, Frère CH (2017) Archipelagos of the Anthropocene: rapid and extensive differentiation of native terrestrial vertebrates in a single metropolis. Mol Ecol 26:2466–2481. https://doi.org/10.1111/mec.14042
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549. https://doi.org/10.1111/brv.12012
Lucas LD, French SS (2012) Stress-induced tradeoffs in a free-living lizard across a variable landscape: consequences for individuals and populations. PLoS One 7:e49895. https://doi.org/10.1371/journal.pone.0049895
Lyons J, Mastromonaco G, Edwards DB, Schulte-Hostedde AI (2017) Fat and happy in the city: eastern chipmunks in urban environments. Behav Ecol 28:1464–1471. https://doi.org/10.1093/beheco/arx109
McCleery RA (2009) Changes in fox squirrel anti-predator behaviours across the urban–rural gradient. Landsc Ecol 24:483–449. https://doi.org/10.1007/s10980-009-9323-2
McKinney ML (2002) Urbanisation, biodiversity, and conservation. BioScience 52:883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
Miranda AC, Schielzeth H, Sonntag T, Partecke J (2013) Urbanisation and its effects on personality traits: a result of microevolution or phenotypic plasticity? Glob Chang Biol 19:2634–2644. https://doi.org/10.1111/gcb.12258
Møller AP (2008) Flight distance of urban birds, predation, and selection for urban life. Behav Ecol Sociobiol 63:63–75. https://doi.org/10.1007/s00265-008-0636-y
Müller JC, Partecke J, Hatchwell BJ, Gaston KJ, Evans KL (2013) Candidate gene polymorphisms for behavioural adaptations during urbanisation in blackbirds. Mol Ecol 22:3629–3637. https://doi.org/10.1111/mec.12288
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045. https://doi.org/10.1093/beheco/arh107
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605. https://doi.org/10.1111/j.1469-185X.2007.00027.x
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Parris K, Velik-Lord M, North J (2009) Frogs call at a higher pitch in traffic noise. Ecol Soc 14:1–24 http://www.jstor.org/stable/26268025
Partecke J, Schwabl I, Gwinner E (2006) Stress and the city: urbanisation and its effects on the stress physiology in European blackbirds. Ecology 87:1945–1952. https://doi.org/10.1890/0012-9658(2006)87%5B1945:SATCUA%5D2.0.CO%3B2
Perals D, Griffin AS, Bartomeus I, Sol D (2017) Revisiting the open-field test: what does it really tell us about animal personality? Anim Behav 123:69–79. https://doi.org/10.1016/j.anbehav.2016.10.006
Peterman WE, Ryan TJ (2009) Basking behaviour of emydid turtles (Chysemys picta, Graptemys geographica, and Trachemys scripta) in an urban landscape. Northeast Nat 16:629–636. https://doi.org/10.1656/045.016.n412
R Core Team (2016) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.r-project.org/
Räsänen K, Kruuk LEB (2007) Maternal effects and evolution at ecological time-scales. Funct Ecol 21:408–421. https://doi.org/10.1111/j.1365-2435.2007.01246.x
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Riley JL, Noble DW, Byrne RW, Whiting MJ (2017) Early social environment influences the behaviour of a family-living lizard. R Soc Open Sci 4:161082. https://doi.org/10.1098/rsos.161082
Scales J, Hyman J, Hughes M (2011) Behavioural syndromes break down in urban song sparrow populations. Ethology 117:887–895. https://doi.org/10.1111/j.1439-0310.2011.01943.x
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. https://doi.org/10.1111/j.2041-210X.2010.00012.x
Schuett W, Delfs B, Haller R, Kruber S, Roolfs S, Timm D, Willmann M, Drees C (2018) Ground beetles in city forests: does urbanization predict a personality trait? PeerJ 6:e4360. https://doi.org/10.7717/peerj.4360
Schwanz LE (2016) Parental thermal environment alters offspring sex ratio and fitness in an oviparous lizard. J Exp Biol 2016:2349–2357. https://doi.org/10.1242/jeb.139972
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci U S A 109:16083–16088. https://doi.org/10.1073/pnas.1211658109
Shochat E, Lerman SB, Katti M, Lewis DB (2004) Linking optimal foraging behaviour to bird community structure in an urban-desert landscape: field experiments with artificial food patches. Am Nat 164:232–243. https://doi.org/10.1086/422222
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191. https://doi.org/10.1016/j.tree.2005.11.019
Short KH, Petren K (2008) Boldness underlies foraging success of invasive Lepidodactylus lugubris geckos in the human landscape. Anim Behav 76:429–437. https://doi.org/10.1016/j.anbehav.2008.04.008
Siviter H, Deeming DC, Rosenberger J, Burman OH, Moszuti SA, Wilkinson A (2017) The impact of egg incubation temperature on the personality of oviparous reptiles. Anim Cogn 20:109–116. https://doi.org/10.1007/s10071-016-1030-1
Sol D, Griffin AS, Bartomeus I, Boyce H (2011) Exploring or avoiding novel food resources? The novelty conflict in an invasive bird. PLoS One 6:e19535. https://doi.org/10.1371/journal.pone.0019535
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. https://doi.org/10.1016/j.anbehav.2013.01.023
Sprau P, Dingemanse NJ (2017) An approach to distinguish between plasticity and non-random distributions of behavioural types along urban gradients in a wild passerine bird. Front Ecol Evol 5:92. https://doi.org/10.3389/fevo.2017.00092
Thompson MB (1993) Estimate of the population structure of the eastern water dragon, Physignathus lesueurii (Reptilia: Agamidae), along riverside habitat. Wildl Res 20:613–619. https://doi.org/10.1071/WR9930613
Vincze E, Papp S, Preiszner B, Seress G, Bókony V, Liker A (2016) Habituation to human disturbance is faster in urban than rural house sparrows. Behav Ecol 27:1304–1313. https://doi.org/10.1093/beheco/arw047
Wiens JJ, Donoghue MJ (2004) Historical biogeography, ecology and species richness. Trends Ecol Evol 19:639–644. https://doi.org/10.1016/j.tree.2004.09.011
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgments
We would like to thank P. Bolton, C. Fryns, F. Kar, S. Klopper, and D. Noble for their assistance in field. We are grateful to T. Damasio, M. Mühlenhaupt, C. Wilson, and the husbandry volunteers at Macquarie University’s Lizard Lab for their assistance in the lab, and P. Harlow and O. Lapiedra for providing their insights into this topic. Finally, we would like to thank the anonymous reviewers for their comments and suggestions.
Funding
This research was supported by scholarship from Macquarie University (JBG) and Natural Sciences and Engineering Research Council of Canada (JBG). JLR was supported by an Endeavor and Claude Leon Foundation postdoctoral fellowship during this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval for lizard captures and our experimental protocols followed animal ethics guidelines that were approved by both the Macquarie University Animal Ethics Committee (ARA no. 2015/023) and Taronga Zoo Animal Ethics Committee (ARA no. 3b/08/15). Our research was approved by the New South Wales National Parks and Wildlife Service, Office of Environment and Heritage (License no. SL100570).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by T. Madsen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 5764 kb)
Rights and permissions
About this article
Cite this article
Baxter-Gilbert, J., Riley, J.L. & Whiting, M.J. Bold New World: urbanization promotes an innate behavioral trait in a lizard. Behav Ecol Sociobiol 73, 105 (2019). https://doi.org/10.1007/s00265-019-2713-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2713-9