Abstract
Complex environments may place constraints on animal sensory perception. However, little is known about how ecological constraints impact the formation of animal social groups, which ultimately necessitate that conspecifics detect one another. Here we studied the fission–fusion social groups of highly social terrestrial hermit crabs (Coenobita compressus), which frequently split and recombine, requiring individuals to actively sense the location of conspecifics. We manipulated the environment by relocating abundant natural fallen leaves on the beach, testing how and why this debris may constrain social group formation. Our experiments revealed that fallen leaves impose a fundamental limit on social grouping, with experimentally simulated groups attracting significantly fewer conspecifics (75% less) if they were surrounded by leaves. This constraint on social grouping was not the result of leaves acting as a physical barrier to movement. Furthermore, leaves only hindered visual perception of conspecifics and did not hinder other modalities besides vision. By experimentally moving leaves above the horizon, such that they no longer blocked animals’ field of view, we found that the impact of these visual constraints on grouping could be effectively abolished. Broadly, these experiments elucidate how complex environments impose sensory constraints on social animals’ ability to navigate toward groups.
Significance statement
Social animals must be able to detect and orient toward conspecifics if they are to form social groups. Features of the environment, however, may impose constraints on sensory perception that interfere with the detection of conspecifics. Here we studied social hermit crabs, which form social groupings within a complex habitat that contains abundant fallen leaves along the beach–forest interface. By experimentally manipulating leaves, we show that these seemingly insignificant ecological materials create visual constraints that block the cues free-wandering individuals normally use to orient toward social groups. Our study thus reveals how common aspects of the environment can exert major constraints on sensory perception, thereby severely limiting social group formation and ultimately sociality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms take in information about their environments through a variety of senses (Stevens 2013). Out of all sensory modalities, vision arguably provides some of the richest and most detailed environmental information (Land and Nilsson 2012). Indeed, vision functions across many ecological contexts, including predator avoidance, resource acquisition, mate detection, navigation, and communication (Zeil and Hemmi 2005; Smolka and Hemmi 2009). The ecological matrix, however, can place major constraints on visual perception, potentially restricting animals’ ability to find and acquire essential resources.
Eyes evolved to adaptively fit the environment in which they must function (Cronin et al. 2014). Yet many environments are dynamic, changing over time, for example, in terms of light levels (e.g., day versus night) and in the clarity of the environmental matrix (e.g., the amount of particulate matter in the air or water column) (Johnsen 2012). Indeed, the existence of certain ecological materials may completely block organisms’ view, such that even a “perfectly” evolved eye will be unable to overcome opaque barriers that are physically impossible to see through (Land and Nilsson 2012). Such opaque barriers are widespread, spanning a variety of biotic and abiotic features, including living trees as well as leftover plant matter, like fallen leaves. Ultimately, these ecological materials may impose limits on animals’ ability to see what they seek (Fleishman 1992).
For social animals, one of the most essential resources they seek is conspecifics (Ward and Webster 2016). Being around members of ones’ species can provide a variety of benefits, such as access to social information about food, mating opportunities, and protection against predators (Danchin 2004; Dall et al. 2005; Bonnie and Earley 2007; Rieucau and Giraldeau 2011). The obligate need for social animals to find conspecifics has generated strong selection pressures for visual and other sensory systems that are optimized for locating conspecifics (Bradbury and Vehrencamp 2011). Even less social species, which lack a need to constantly be around others, nevertheless must frequently operate in fission–fusion populations (Couzin and Laidre 2009), in which ephemeral social groupings dynamically split and reform over time. Such fluid arrangements, where individuals frequently transition from solitary to social, create selection pressures for finding conspecifics that can be as strong or even stronger than such selection pressures in more social species that live in permanent groups (Ward and Webster 2016).
Highly social terrestrial hermit crabs (Coenobita spp.) offer a manipulable experimental system for studying fission–fusion social dynamics in the field (Laidre 2010, 2013a; Bates and Laidre 2018). Individual crabs seek architecturally remodeled shells (Laidre 2012a; Laidre et al. 2012), which can only be acquired during social exchanges with conspecifics (Laidre 2014), in which one member of a social group dies or is evicted, and other members then capitalize on this resource opportunity (Laidre 2018a). Social groupings are ephemeral (Bates and Laidre 2018), since once individuals successfully acquire a remodeled shell they temporarily become anti-social to avoid losing their newly acquired shell. However, because individuals are constantly seeking to move up in the “housing market” (Laidre and Vermeij 2012) and acquire bigger remodeled shells that enhance their reproductive success (Laidre 2012b), they eventually seek out further social groupings after they have grown to accommodate their current shell. Vision has been found to be essential if individuals are to find these social groupings (Laidre 2010, 2013a; Bates and Laidre 2018). Yet critically, previous experiments have utilized idealistic field landscapes, clearing beforehand from the experimental arenas the abundant ecological materials, like fallen leaves, which could act as major sensory constraints on finding social groups.
Here we conduct an experimental study that traces the origin, accumulation, and ultimate impact of ecological materials that might impose sensory constraints on animal social life. We use Coenobita compressus the “social hermit crab” (Laidre 2014; Bates and Laidre 2018) as a model system, both to characterize its complex natural environment and to manipulate this environment, testing the hypothesis that fallen leaves act as a major visual constraint on social group formation. In a series of experiments, we tested the following: (1) whether leaves constrain social group formation, (2) whether this constraint was due to the leaves being a physical barrier to movement or was instead due to constraints on sensory perception, (3) whether constraints on sensory perception applied across other modalities or were instead specifically linked to vision, and finally (4) whether visual constraints could be abolished by slight repositioning of the leaves above the visual horizon. Together, our experiments elucidate how complex environments ultimately impose visual constraints on animals’ ability to navigate toward social groups.
Methods
Study site and system
We conducted this study in Osa Peninsula, Costa Rica between January and March 2018 during the dry season. During this time of year, abundant leaves fall onto the beach–forest interface (Janzen 1983), an area that is dominated by terrestrial hermit crabs (Coenobita compressus) (Laidre 2013b). We measured the crabs and their habitat, including trees and fallen leaves. We then conducted experiments in which we manipulated fallen leaves to understand their impact on the crabs’ social group formation.
Habitat and animal measurements
Tree and fallen leaf transects
To examine the relative abundance of different tree species (the ultimate source of the leaves we hypothesized acted as visual constraints), we conducted a transect spanning the area in which all subsequent experiments were conducted. For this transect, we walked the beach 3 m from the edge of the beach–forest interface, recording every tree overhead. A GPS was used to map the exact route taken (2.18 km in length), which ran from Camp Crab to the north (08° 23′ 40″ N, 83° 20′ 10″ W) to Rio Los Sambos to the south (08° 23′ 13″ N, 83° 19′ 08″ W). To examine the relative concentration of fallen leaves, we also placed a 30 × 30 cm quadrat on the ground every 20 steps along this transect, sampling all leaves within each quadrat (for total of N = 155 quadrats along the entire transect).
Rate of fallen leaf accumulation
To examine the rate with which fallen leaves arrive and accumulate on the beach–forest interface over time, we randomly chose N = 5 locations along the transect (all locations had trees overhead when an observer stood 3 m from the embankment). At each of the five sites, we marked a 3 × 3 m quadrat at the beach–forest interface. For each site, we then collected every fallen leaf within the quadrat, recording the total number of leaves from each tree species. We collected leaves across all these sites every 2 days, spanning a full tidal cycle. After collection, leaves were stored away from the sampling sites, thereby ensuring further leaves that appeared in these quadrats were new and had not been previously counted.
Leaf height and eye height
To quantify the extent to which leaves may block individuals from seeing, we compared leaf height (the height of fallen leaves off the ground) to eye height (the height of hermit crab eyes off the ground), measuring both to the nearest millimeter. To quantify leaf height, we randomly selected N = 30 spots on the beach with one or more fallen leaves. Spots were selected as follows. At each of the N = 5 leaf fall rate quadrats, we started at the two quadrat corners closest to the ocean and then walked parallel to the embankment 3 m away from the quadrat. From there, we threw three rocks randomly onto the beach–forest interface. We then measured the height off the ground of the leaf (or leaves, if multiple were stacked) that were 10 cm toward the embankment from where each rock had landed. In cases where there was not a leaf at the 10 cm mark, we simply continued along the same line toward the embankment until encountering the first leaf. In addition to recording leaf height at these spots, we also recorded the number of leaves (if there was a stack of more than one).
To quantify eye height, we randomly selected N = 30 hermit crabs from a bag of 400 hermit crabs collected along the beach–forest interface. We measured each individual’s shell diameter (Laidre 2012b). Shell diameter was a highly conservative measure of eye height: it always exceeded the maximum height off the ground of individuals’ eyes, even when individuals were in their highest walking posture (paired t test of eye height versus shell diameter for N = 30 crabs: t = 13.69, df = 29, p < 0.0001; mean ± SE for eye height 10.1 ± 0.54 mm; for shell diameter 13.9 ± 0.50 mm).
Behavioral tests: general experimental design
To test the impact of fallen leaves, we conducted a series of four experiments (detailed below) in which we manipulated the distribution of leaves at the beach–forest interface. All experiments were conducted between 0500 and 1000, a time of peak activity in which individuals wander the beach in search of social groups to join. The general design for these experiments followed Laidre (2010) and involved constructing temporary experimental quadrats (60 × 60 cm), with a wooden dowel being used to make light (3 mm deep) imprints in the sand.
Each of the four experiments had two different conditions: in one condition (the “no leaves” condition), the entire perimeter of the quadrat was open and free of leaves (Figure S1), while in the other conditions (the “leaves” condition), the entire perimeter of the quadrat was surrounded by leaves (Figure S2). To conduct a given experiment, we alternated randomly between the no leaves and the leaves conditions (N = 20 replicates per condition), constructing new quadrats for each replicate. For quadrats with leaves, we gathered fallen leaves from another location and then stacked them three high around the exterior of the quadrat (mean ± SE of leaf height around quadrat 48.6 ± 1.6 mm, which fell within the natural range of our measurements of leaf height on the beach). For the quadrats without leaves, we allocated the same time to construction and performed the same movements as we did for the quadrats with leaves, thereby controlling for these factors. Once a quadrat was fully setup and the relevant stimuli (see below) had been placed inside it, we then observed from 5 m away with binoculars for 10 min while recording individuals’ behavior. Note that it was not possible to record data blind because our study involved clearly visible conditions (leaves versus no leaves) in the field.
Experiment 1: constraints on social group formation?
To test if leaves constrain social group formation, we examined the attractiveness of simulated social groups that were placed in the center of quadrats for the leaves versus the no leaves conditions. We simulated social groups using three crabs tethered with fishing line to a dowel (see Laidre 2010). To quantify attractiveness, we recorded how many individuals entered the quadrat over the course of the entire 10 min of observation and also how many individuals (excluding tethered crabs) ultimately accumulated within the quadrat by the end of the 10 min experiment.
Experiment 2: blockade or sensory constraints?
To test if leaves act as a blockade that is a physical barrier to movement, we examined how readily individuals could escape when placed in the center of quadrats of the leaves versus the no leaves conditions. Individuals were highly motivated to escape in these experiments, since they had just been handled before being put in this new location. For each test, we placed N = 10 individuals simultaneously in the center of a quadrat, giving them 10 min to exit the quadrat. A total of 400 hermit crabs were tested across the N = 20 replicates for both conditions.
Experiment 3: chemical or visual constraints?
To test if leaves constrain other sensory modalities (namely the chemical modality and so not exclusively vision), we provided an odor attractant in the center of quadrats of the leaves versus the no leaves conditions. For the odor, we chose coconut oil, which is known to be highly attractive (Laidre 2013b) (note: live conspecific odor was not used, since it is known to be only weakly attractive at best; Laidre 2010). For both conditions, we buried a 16-oz. plastic cup in the center of the quadrat, with the top of the cup arranged even with the ground, so that the cup was hidden but its opening was still accessible to the air (Figure S3). We then pipetted 3 ml of coconut oil (“Tico Natura,” Heredia, Costa Rica) into the cup and used gloves, so as to avoid spreading any oil on leaves or other parts of the quadrat. To quantify attractiveness of the chemical cues, we recorded how many individuals were present within the quadrat at the end of the 10 min. Visual cues were effectively absent from this experiment, given that attracted individuals fell into the cups.
Experiment 4: can visual constraints be abolished?
To test if leaves’ impact as a visual constraint could be abolished, we again employed simulated social groups (as in experiment 1), except in this experiment we elevated leaves just above the visual horizon—to a point where they could no longer obscure crabs’ direct line of sight along the ground. For the leaves condition in this experiment, we used wooden BBQ skewers, interwoven to connect leaves and hold them in the air above the sand substrate (Figure S4). The gap of a few centimeters between the sand substrate and the raised leaves meant these leaves were above even the tallest tethered crab, thus leaving an unobscured line of sight for free wandering individuals outside the quadrat to see the tethered conspecifics inside the quadrat. For the no leaves condition in this experiment, we used an identical setup of wooden BBQ skewers, but without any leaves attached (Figure S5). Attractiveness was quantified as in experiment 1.
Predictions and statistical analyses
If leaves impose a major visual constraint on social group formation, then the following predictions should be met:
-
(1)
Fallen leaves should be abundant at the beach–forest interface, a hot spot of social group formation among terrestrial hermit crabs.
-
(2)
After these leaves are removed, more fallen leaves should steadily accumulate in their place.
-
(3)
Leaves should effectively block individuals’ view, with the height of leaves off the ground exceeding the eye height of crabs.
-
(4)
Leaves should constrain social group formation, with fewer individuals entering and accumulating in the leaves versus the no leaves condition in experiment 1.
-
(5)
Leaves should not be a physical barrier to movement, with individuals being equally capable of escaping the leaves versus the no leaves condition in experiment 2.
-
(6)
Leaves should not constrain chemical sensing, with equal numbers of individuals accumulating in the leaves versus the no leaves condition in experiment 3.
-
(7)
Elevating leaves in the air should effectively abolish visual constraints, with equal numbers of individuals entering and accumulating in the raised leaves versus the no leaves condition in experiment 4.
To test these predictions, we first quantified the abundance and accumulation of fallen leaves in the habitat. We then used t tests (with unequal variance) to (i) compare eye height versus leaf height and (ii) compare the leaves versus no leaves conditions across experiments 1 through 4. All statistical analyses were performed in JMP® Pro 12.1.0 (SAS Institute, Cary, NC, USA), with the overall alpha level controlled at 0.05 and with the Bonferroni method used when undertaking multiple tests.
Results
Habitat and animal measurements
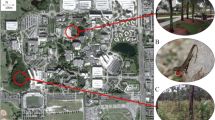
Two tree species, beach almond (Terminalia catappa) and coconut (Cocos nucifera), dominated the coastline (Fig. 1a), comprising 33.2% and 60.6% of trees respectively, with only 6.2% of other tree species. Fallen leaves along the beach–forest interface (Fig. 1b) were represented disproportionately (75.1%) by beach almond leaves. The raw number of fallen beach almond leaves was substantial (mean ± SE 207.8 ± 134.6 leaves; range 44–745 leaves) on day 1 in the 3 × 3 m quadrats. After being cleared of their starting concentration of leaves, new fallen leaves continued to accumulate within the quadrats on subsequent days (Fig. 2), at a rate of 31.7 ± 4.8 (mean ± SE) leaves every 2 days. Notably, the height of fallen leaves dwarfed that of crabs’ eye height (t test: t = 7.95, df = 30.00, p < 0.0001; Fig. 1c, d), thus presenting a potential major visual constraint.
Habitat dominated by highly social terrestrial hermit crabs (Coenobita compressus) along the beach–forest interface of Osa Peninsula, Costa Rica. a Trees along the coastline are composed predominately of beach almond (Terminalia catappa) and coconut (Cocos nucifera). b Fallen leaves are abundant in this area, particularly beach almond leaves. c Social hermit crabs must navigate around these fallen leaves while forming social groups. d The height of fallen leaves (N = 30) exceeds the eye height of the crabs (N = 30), presenting a possible visual constraint for detecting conspecifics
Number of fallen beach almond leaves (mean ± SE) across N = 5 quadrats along the beach–forest interface. Day 1 represents the initial leaf count, after which all leaves were removed. Then, every 2 days, new fallen leaves were counted and these leaves were likewise removed, revealing the rate with which new fallen leaves arrived in the quadrats across 21 days
Experiment 1: constraints on social group formation?
Compared to the no leaves condition, the leaves condition attracted significantly fewer individuals to simulated social groups (t = 5.55, df = 21.37, p < 0.0001; Fig. 3a) and resulted in significantly smaller accumulations around those social groups (t = 5.50, df = 22.49, p < 0.0001; Fig. 3b). Leaves thus act as a constraint on social group formation, ultimately reducing group sizes by over 75% their original size.
Experiment 2: blockade or sensory constraints?
Individuals placed within quadrats surrounded by leaves readily escaped by maneuvering above and below these leaves, easily climbing over or crawling under them. For both the leaves and the no leaves condition, all individuals easily escaped in under 10 min (Fig. 4). Leaves thus do not present an insurmountable physical barrier to movement, but at most constrain sensory perception.
Experiment 3: chemical or visual constraints?
Equal numbers of individuals accumulated in the leaves and the no leaves condition (t = 0.57, df = 36.63, p = 0.57; Fig. 5), indicating that leaves do not constrain odor perception, only visual perception.
Experiment 4: can visual constraints be abolished?
The raised leaves and the no leaves condition attracted equal numbers of conspecifics to simulated social groups (t = 0.08, df = 37.89, p = 0.94; Fig. 6a) and ultimately resulted in equal accumulations around social groups (t = 0.53, df = 35.29, p = 0.60; Fig. 6b). Thus, if leaves are elevated above the visual horizon, they no longer present a visual constraint on social grouping.
Discussion
Few studies have experimentally tested the extent to which common ecological materials, especially fallen leaves, act as major visual constraints on social group formation (reviewed in Stevens 2013; Cronin et al. 2014). Here we used a system in which we were able to track the origin, accumulation, and ultimate impact of ecological materials and also experimentally manipulate these materials to directly test their role as visual constraints on social grouping. Social hermit crabs (Laidre 2012b, 2014; Bates and Laidre 2018) rely primarily on vision to detect conspecifics (Laidre 2010). Vision provides rapid and detailed information on social groupings, which occur over short temporal periods (Laidre 2013a, 2013b) and across vast spatial scales along the beach (Laidre and Vermeij 2012). Vision, however, has limitations (Cronin et al. 2014). Our results reveal that naturally abundant ecological materials, in the form of fallen leaves, strongly constrain social group formation. While fallen leaves do not hinder movement through the environment or chemical detection, they do obfuscate visual information, blocking the line of sight toward conspecifics. By simply raising fallen leaves above crabs’ horizontal field of view, we found that these visual constraints could effectively be abolished, thereby returning social group formation to its original high level.
Ecological materials, like leaves, as well as many forms of environmental noise, like wind and vibrations (Wignall et al. 2011; Mcnett et al. 2010), can cause a partial or complete degradation of the physical transmission of sensory information (Fleishman 1992). Degradation can also occur across any modality, not just vision, ultimately confounding relevant cues and signals used by many social animals (Ward and Webster 2016). The level of such degradation, and of environmental noise generally, may change temporally in either predictable or random fashion (Johnsen 2012). For social hermit crabs, wind and tidal forces constantly move fallen leaves randomly, thus creating a complex matrix along the beach–forest interface, which can block visual cues usually given off by socially grouped conspecifics. Critically, the challenge posed by such environmental noise ultimately generates selection pressures, which can favor multiple evolutionary strategies by which individuals might overcome environmentally confounded sensory information.
One strategy by which social animals can overcome environmental noise is altering their signaling behavior (Laidre and Johnstone 2013), especially increasing the conspicuousness of signals (Uy and Endler 2004). For example, passerines call at higher frequencies and for shorter durations in urban areas with increased levels of acoustic noise pollution (Bermudez-Cuamatzin et al. 2010). Similarly, during periods of high wind, which causes increased vegetation movement, lizards increase their signaling behavior, thereby continuing to effectively attract mates and keep rivals at bay despite the greater visual noise and background distractions (Fleishman 1992; Peters and Evans 2003; Ord et al. 2007; Peters et al. 2007). For social hermit crabs, conspecifics may only rarely actively signal their presence, which means that free wandering individuals must instead rely on passive social cues to opportunistically detect social groupings (Laidre 2010). Consequently, free wandering individuals may need to adjust their locomotion speed or travel distances in areas with abundant fallen leaves, exploring these areas more thoroughly if they are to maximize their chances of finding and joining social groups. Plasticity in wandering behavior might therefore provide a key solution by which individuals could continue to locate social groupings despite variable amounts of fallen leaves.
Another strategy for overcoming the challenge of environmental noise is to exploit additional sensory modalities. Once one sensory modality becomes ineffective at acquiring the necessary information (e.g., vision is constrained due to opaque materials), then detection can be enhanced through the use of a different modality or by using multiple modalities at once (Girard et al. 2015). Barn owls, for example, are less effective hunters if they rely on one modality, but if they utilize both vision and hearing, their likelihood of capturing prey at night increases (Whitchurch and Takahashi 2006). Social hermit crabs are capable of exploiting several modalities to orient to social groupings (Laidre 2010, 2018b). Notably though, chemical cues are far more salient if derived from freshly deceased individuals rather than living ones (Valdes and Laidre 2019). The olfactory modality is therefore an insufficient “backup” for locating living conspecifics. Interestingly, social hermit crabs also exploit high levels of collective commotion to orient toward especially active social groupings (Laidre 2013a), and this greater commotion and movement—a by-product of the whole group’s activity—could potentially transmit substrate-borne vibrations that free wandering individuals pick up on. Vision though appears to reign supreme in the hierarchy of modalities for social hermit crabs: once vision is blocked by ecological materials, social grouping is dramatically impaired, as we have shown in the present study. Future research can test visual cues in even finer detail by isolating social groups in clear glass jars, which completely trap chemical cues and are also soundproof. Complementary experiments could then dissect the importance of multiple modalities (chemical, vibrational, visual, and tactile), singly and in combination, to determine how these modalities interact and whether multi-modal cues might more effectively spur social group formation.
Yet another way to overcome environmental noise is through an evolutionary modification of the sensory system itself. Sensory organs are adapted to meet the demands of the environment, providing organisms with the necessary capabilities to glean information that is most pertinent to survival and reproductive success (Stevens 2013). Some environments favor extreme specialization of certain aspects of the sensory system over others. As an example, the Mexican tetra is found in caves with no light, which has resulted in the complete loss of its eyes, but an increased sensitivity to pressure changes in the water (Yoshizawa et al. 2010). For social hermit crabs, despite their strong reliance on vision for detecting social groupings, little is known about the detailed mechanistic properties of their compound eyes. It is likely these eyes have been fine-tuned for detecting and orienting toward course-grained movements along the horizon of the beach, which would maximize chances for shell acquisition from conspecifics. Intriguingly, in our experiments, even when simulated social groups were surrounded by leaves, some free wandering individuals consistently made direct beelines into the quadrat from a distance, apparently homing in visually on the social group through tiny openings in the otherwise unbroken wall of crumpled leaves. A multitude of potential visual cues could alert free wandering individuals to social groupings (Cronin et al. 2014), including movement, color, contrast, or polarization of the conspecifics’ bodies or shells. Future research could isolate precisely what visual cues are attractive, as well as how the compound eyes of social hermit crabs may have been specialized (relative to other, less social hermit crabs) for detecting conspecific cues.
Perhaps surprisingly, environmental noise may not always be something to overcome or cope with but may instead be useful to exploit, for the purpose of biological privacy (Strassmann and Queller 2014). By its very definition, environmental noise confounds relevant sensory information, effectively masking an individual’s presence or appearance, thereby making it more challenging to detect (Stevens 2013). The exploitation of environmental noise for concealment is well documented in predator–prey interactions (Bear and Hasson 1997), where, for example, assassin bugs become more effective at hunting web-building spiders by exploiting wind-induced vibration to disguise their approach along the web (Wignall et al. 2011). Less is known, however, about how social animals might exploit environmental noise, either to control social group size or to increase individual privacy. Privacy is especially important for social animals that possess external “property” (Strassmann and Queller 2014), as hermit crabs do—in the form of a shell, which must be defended against conspecifics (Alcaraz and Jofre 2017). Individuals already in possession of an optimal shell could potentially hide, within or beneath leaves, thereby reducing their likelihood of encountering conspecifics who might steal their shell. Social hermit crabs—with their intense property competition for highly valuable, architecturally remodeled shells; their dynamic social groupings that continually break and reform; and their complex environmental matrix, containing abundant fallen leaves, with many conceivable hiding places—could prove a useful system for future explorations of whether social animals exploit environmental noise for personal privacy.
Data availability
The datasets analyzed during the current study are available from the corresponding authors.
References
Alcaraz G, Jofre GI (2017) Aggressiveness compensates for low muscle strength and metabolic disadvantages in shell fighting: an outcome of the individual’s past. Behav Ecol Sociobiol 71:87
Bates KM, Laidre ME (2018) When to socialize: perception of time-sensitive social structures among social hermit crabs. Anim Behav 138:19–27
Bear A, Hasson O (1997) The predatory response of a stalking spider, Plexippus paykulli, to camouflage and prey type. Anim Behav 54:993–998
Bermudez-Cuamatzin E, Rios-Chelen AA, Gil D, Garcia CM (2010) Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol Lett 8:320–320
Bonnie KE, Earley RL (2007) Expanding the scope for social information use. Anim Behav 74:171–181
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, MA
Couzin ID, Laidre ME (2009) Fission-fusion populations. Curr Biol 19:R633–R635
Cronin TW, Johnsen S, Marshall NJ, Warrant EJ (2014) Visual ecology. Princeton University Press, Princeton, NJ
Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193
Danchin E (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
Fleishman LJ (1992) The influence of the sensory system and the environment on motion patterns in the visual displays of Anoline lizards and other vertebrates. Am Nat 139:S36–S61
Girard MB, Elias DO, Kasumovic MM (2015) Female preference for multi-modal courtship: multiple signals are important for male mating success in peacock spiders. Proc R Soc B 282:20152222
Janzen DH (1983) Costa Rican natural history. University of Chicago Press, Chicago
Johnsen S (2012) The optics of life: a biologist’s guide to light in nature. Princeton University Press, Princeton, NJ
Laidre ME (2010) How rugged individualists enable one another to find food and shelter: field experiments with tropical hermit crabs. Proc R Soc B 277:1361–1369
Laidre ME (2012a) Niche construction drives social dependence in hermit crabs. Curr Biol 22:R861–R863
Laidre ME (2012b) Homes for hermits: temporal, spatial and structural dynamics as transportable homes are incorporated into a population. J Zool 288:33–40
Laidre ME (2013a) Eavesdropping foragers use level of collective commotion as public information to target high quality patches. Oikos 122:1505–1511
Laidre ME (2013b) Foraging across ecosystems: diet diversity and social foraging spanning aquatic and terrestrial ecosystems by an invertebrate. Mar Ecol 34:80–89
Laidre ME (2014) The social lives of hermits. Nat Hist 122:24–29
Laidre ME (2018a) Evolutionary ecology of burrow construction and social life. In: Wellborn GA, Thiel M (eds) Life histories. Oxford University Press, New York, NY, pp 279–301
Laidre ME (2018b) Social cognition in the wild: from lab to field in hermit crabs. In: Bueno-Guerra N, Amici F (eds) Field and laboratory methods in animal cognition: a comparative guide. Cambridge University Press, New York, NY, pp 237–239
Laidre ME, Johnstone RA (2013) Animal signals: a primer. Curr Biol 23:R829–R833
Laidre ME, Vermeij GJ (2012) A biodiverse housing market in hermit crabs: proposal for a new biodiversity index. Cuadernos de Investigación UNED 4:175–179
Laidre ME, Patten E, Pruitt L (2012) Costs of a more spacious home after remodelling by hermit crabs. J Roy Soc Interface 9:3574–3577
Land MF, Nilsson D (2012) Animal eyes. Oxford University Press, New York, NY
Mcnett GD, Luan LH, Cocroft RB (2010) Wind-induced noise alters signaler and receiver behavior in vibrational communication. Behav Ecol Sociobiol 64:2043–2051
Ord TJ, Peters RA, Clucas B, Stamps JA (2007) Lizards speed up visual displays in noisy motion habitats. Proc R Soc B 274:1057–1062
Peters RA, Evans CS (2003) Design of the Jacky dragon visual display: signal and noise characteristics in a complex moving environment. J Comp Physiol A 189:447–459
Peters RA, Hemmi JM, Zeil J (2007) Signaling against the wind: modifying motion-signal structure in response to increased noise. Curr Biol 17:1231–1234
Rieucau G, Giraldeau L-A (2011) Exploring the costs and benefits of social information use: an appraisal of current experimental evidence. Phil Trans Roy Soc B 366:949–957
Smolka J, Hemmi JM (2009) Topography of vision and behaviour. J Exp Biol 212:3522–3532
Stevens M (2013) Sensory ecology, behaviour, and evolution. Oxford University Press, New York, NY
Strassmann JE, Queller DC (2014) Privatization and property in biology. Anim Behav 92:305–311
Uy JAC, Endler JA (2004) Modification of the visual background increases the conspicuousness of golden-collared manakin displays. Behav Ecol 15:1003–1010
Valdes L, Laidre ME (2019) Scent of death: evolution from sea to land of an extreme collective attraction to conspecific death. Ecol Evol 9:2171–2179. https://doi.org/10.1002/ece3.4912
Ward A, Webster M (2016) Sociality: the behaviour of group-living animals. Springer, New York, NY
Whitchurch EA, Takahashi TT (2006) Combined auditory and visual stimuli facilitate head saccades in the barn owl (Tyto alba). J Neurophysiol 96:730–745
Wignall AE, Jackson RR, Wilcox RS, Taylor PW (2011) Exploitation of environmental noise by an araneophagic assassin bug. Anim Behav 82:1037–1042
Yoshizawa M, Gori S, Soares D, Jeffery WR (2010) Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr Biol 20:1631–1636
Zeil J, Hemmi JM (2005) The visual ecology of fiddler crabs. J Comp Physiol A 192:1–25
Acknowledgements
We thank Osa Conservation staff and the Laidre lab for assistance in the field. This study was supported by a EEES/GAANN fellowship to ES and Dartmouth College startup funds to ML. For helpful feedback, we thank Mike Webster and an anonymous reviewer as well as the audience of a conference talk on these experiments at the summer 2018 International Society for Behavioral Ecology in Minneapolis.
Author information
Authors and Affiliations
Contributions
ML conceived the study and designed the experiments; both authors then worked jointly to collect the data in the field, analyze the data, and write the manuscript together.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by T. Breithaupt
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4318 kb)
Rights and permissions
About this article
Cite this article
Steele, E.P., Laidre, M.E. Leaf me alone: visual constraints on the ecology of social group formation. Behav Ecol Sociobiol 73, 53 (2019). https://doi.org/10.1007/s00265-019-2662-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2662-3