Abstract
Animal behaviour may change with age, when young individuals experience different environments from adults. However, the role of ontogeny in personality traits is still open to debate and it is not clear whether individual differences in behaviour are maintained across important ontogenetic changes such as metamorphosis. Here, we repeatedly quantified personality in rural and urban populations of the speckled wood butterfly (Pararge aegeria) at the larval, pupal and adult stages. We detected significant repeatability at all life stages, showing that personality is already present at the immature stages. We found no evidence for landscape-related differences in personality traits, but adult males were bolder and more active than adult females. Adults also became bolder with trial sequence, suggesting habitation to the experimental procedure. Urbanisation, together with sex, affected relationships among larval and adult personality traits. More active larvae with short latencies resulted in more explorative adults. However, this was the case in males of urban origin only, and we detected no such correlation in females or in rural males. We suggest that harsh conditions prevailing in cities may lead to stronger trait integration across metamorphosis but also that these urbanisation-related selective pressures may act differently on males and females.
Significance statement
In many taxa, metamorphosis marks the transition between the juvenile and the adult stages. During this crucial developmental step, morphology and physiology are deeply remodelled, which may have important consequences for the behaviour of individuals. Although personality has emerged as a hot topic in behavioural ecology, little is known about the consequences of metamorphosis for personality stability. We studied rural and urban speckled wood butterflies at the immature and adult stages to examine whether insect personality is retained over metamorphosis. Sexes differed regarding boldness and activity, but rural and urban butterflies behaved similarly. Nevertheless, urbanisation affected relationships among larval and adult personality traits. Some larval and adult traits correlated in urban males, whereas this was not the case in females or in rural males. This suggests that urbanisation may alter trait combinations across metamorphosis, but this in a sex-specific manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal personality refers to individual behavioural differences that are consistent over time and/or across situations (Réale et al. 2007). Importantly, personality trait values are not necessarily fixed during an individual’s lifespan. Instead, differences between individuals are expected to be largely maintained (Réale et al. 2007; Stamps and Groothuis 2010). Such personality traits have been documented in a wide range of organisms, including both vertebrate and invertebrate taxa (Kralj-Fišer and Schuett 2014; Vonk et al. 2017). Additionally, personality is known to be present already in the early life stages (e.g. Wuerz and Krüger 2015; Carter et al. 2016) and its consistency over a large amount of time has been demonstrated in several species (e.g. Gyuris et al. 2012; Debeffe et al. 2015). This suggests that personality traits may be robust and resilient to important ontogenetic processes such as sexual maturation.
In some taxa, internal changes occurring during ontogeny are coupled with abrupt morphological changes. This is especially the case for most amphibians and holometabolous insects, as their complete metamorphosis involves a deep remodelling of the neural and motoric system (Consoulas et al. 2000). In these groups, weak correlations between larval and adult personality traits are expected because personality is hypothesised to be linked to internal factors which undergo strong changes during metamorphosis (Amat et al. 2018). Additionally, some species experience a drastic habitat shift during ontogeny. For example, dragonfly larvae are aquatic and bee larvae live inside closed cells while adults are terrestrial in both cases. For such species, adults and larvae occupy distinct niches, use different habitat resources and face different predators. Consequently, selection pressures are likely to be very different for juveniles and adults and personality is expected to be uncoupled between these life stages (Sih et al. 2004). However, these general predictions are only partly supported by the few empirical studies available (Amat et al. 2018). For example, activity and exploration were correlated across metamorphosis in the lake frog (Rana ridibunda) (Wilson and Krause 2012) and larval behavioural type relates to adult activity and boldness in the damselfly Lestes congener (Brodin 2009), which are both species with habitat-associated niche shifts. In species with both terrestrial larvae and adults, Rodrigues et al. (2016) detected correlations between larval and adult behavioural traits in the lady beetle Eriopis connexa. Contrastingly, personality variation was not consistent across metamorphosis in several beetle species (Müller and Müller 2015; Wexler et al. 2016; Monceau et al. 2017), despite larvae and adults using similar habitats and feeding on identical resources, nor in Drosophila fruit flies (Anderson et al. 2016). This suggests that there may not be a single pattern applying to all species undergoing complete metamorphosis but instead that personality retention could be influenced by ecological factors other than the juvenile to adult niche shift only.
Apart from ontogeny-related changes, personality traits per se and behavioural syndromes (i.e. suites of correlated behavioural traits—Sih et al. 2004) are likely to be influenced by intrinsic and extrinsic factors. Males and females, for example, differ in repeatability (Bell et al. 2009) and often show behavioural differences because both sexes have distinct roles and demands. In invertebrates, males typically spend a significant proportion of their time looking for mating opportunities, whereas females do so looking for suitable oviposition sites. As such, intersexual differences in personality traits (e.g. Wexler et al. 2016; Stanley et al. 2017) and in their repeatability (e.g. Hedrick and Kortet 2012; Wexler et al. 2016) are known to occur in this group. Landscape context is another potential source of variation in personality. For example, Australian desert gobies (Chlamydogobius eremius) inhabiting groundwater spring rivers were less active and bolder than conspecifics from riverine waterholes (Moran et al. 2016), and populations from the two habitat types also differed in behavioural correlations (Moran et al. 2017). Similarly, colonisation of a novel habitat by an aquatic isopod leads to changes in its personality and behavioural syndrome structure compared to isopods from the ancestral habitat (Karlsson Green et al. 2016). In both cases, the shifts are supposed to be caused by altered predation risk (see also Bell and Sih 2007; Dingemanse et al. 2007) and/or by changing intensity of intraspecific competition. These examples show that different landscapes, as a result of diverging selective pressures, not only select for specific behavioural types but also reshape relationships among traits. This is of particular interest for urban ecosystems that often show altered biotic and abiotic selective pressures compared to more natural, rural settings (Alberti 2015; Alberti et al. 2017) and thus require behavioural adjustments for a successful city life (Sol et al. 2013). This includes changes in mean trait value (Lapiedra et al. 2017; Charmantier et al. 2017; Senar et al. 2017) as well as behavioural consistency (Miranda et al. 2013; Hardman and Dalesman 2018) and trait integration (Scales et al. 2011; Carrete and Tella 2017). However, studies investigating urban-rural differences in behaviour are still largely biased toward vertebrates (Miranda et al. 2013), and only a handful of studies have looked at such differences in invertebrates (but see Tüzün et al. 2015; Kralj-Fišer et al. 2017; Schuett et al. 2018).
Here, we investigated whether personality is conserved across the larval and adult stages in the speckled wood butterfly (Pararge aegeria). Despite important differences in food resources among stages, both are terrestrial and there is some evidence that butterfly larvae and adults share at least part of their predators—notably birds (Feltwell 1982). As such, we predict that this species does not experience a strong metamorphosis-associated habitat shift. Additionally, Blackiston et al. (2008) showed that learned behaviours can be retained through metamorphosis, suggesting that at least some regions of the brain are conserved during this important ontogenetic step. Hence, we expect behavioural trait correlation across metamorphosis (i.e. a behavioural syndrome across metamorphosis) in butterflies. Additionally, we expect adult urban butterflies to be bolder, more explorative and more active compared to non-urban butterflies, due to lower predation pressure from invertebrate predators and parasitoids in urbanised landscapes (e.g. Denys and Schmidt 1998; Shochat et al. 2004; Lagucki et al. 2017). We also expect intersexual differences but only at the adult stage. Speckled wood males are known to use active mate-locating strategies, whereas females make short flight bouts close to the vegetation, stopping frequently to lay eggs and bask (Shreeve 1986). We therefore predict adult males to be bolder and more active than adult females.
Methods
Study species and sampling sites
The Speckled wood (P. aegeria L.) is a satyrine butterfly common throughout Europe. This multivoltine species primarily occurs in woodlands but also in wooded parks in densely built-up areas (Bergerot et al. 2012). Females lay eggs singly on the leaves of various grass species upon which the larvae feed (Shreeve 1986). For the cohort following the direct developmental pathway, estimated timespans of the larval, pupal and adult stages are 24, 15 and 18 days, respectively (Bink 1992), while Davies (1978) calculated a mean life expectancy of about 7 days for adult males based on capture-recapture data and mean residence times. In September 2014, we captured gravid females at four sites in central Belgium. Rural sites (i.e. Bois de Lauzelle and Meerdaalwoud—Fig. S1) consist of large deciduous woodlands located in landscapes dominated by woodlands; urban sites (i.e. ULB Campus ‘La Plaine’ and Parc Schuman—Fig. S2) are parks (46 and 13 ha, respectively) of the Brussels agglomeration surrounded by a dense urban matrix (i.e. buildings and motorways) with a high prevalence of impervious surfaces. Overall, urban sites appear to be located in more fragmented landscapes. In line with recent studies on urbanisation in the same region (Kaiser et al. 2016; Merckx et al. 2018), weather probes placed at two sites show warmer temperature (+ 1.5 °C in night-time temperature) and lower relative humidity (− 3% in night-time relative humidity) in urban areas. Landscape characteristics of each site and climatic data are available at Table S1 of the Supplementary material. At each site, we collected three female butterflies and brought them to the laboratory for oviposition on the grass Poa trivialis in individual cages, which hence resulted in having 12 families in total.
Host plant and butterfly rearing
Host plants (P. trivialis) were grown from seeds on a standardised soil mixture and under standard conditions in a climate room (16 L:8D; 25:16 °C). We randomly selected seven eggs of each female and transferred them attached to their grass leave to individually labelled Petri dishes. We provided a piece of wet cotton to increase ambient humidity and checked every day for hatching. Larvae were fed in their Petri dish for 2 weeks. Leaves were changed every day and we removed excrement pellets to avoid bacterial contamination. After this period, they were transferred individually to a labelled potted plant, enclosed in nylon netting. Next, pupae were placed individually in labelled plastic cups and checked twice a day for emergence.
Behavioural tests
A single observer (AK) conducted all behavioural observations. We initially aimed to test all individuals twice in each of the tests, but this was not always feasible because some individuals did not survive till adulthood and some larvae were missed due to their small size and camouflage colours during the time spent looking for them in their potted plant. Individuals that died before adult emergence (i.e. for which sex was unknown) and adult butterflies with wing deformation were discarded from the analyses.
Larval latency and activity
Three-week-old larvae (N = 67) were submitted to an open-field test which took place in a 25 °C climate room under constant light conditions. Larvae were removed from their host plant and placed on a sheet of white paper, in the centre of a 25-mm-diameter circle, covered with a glass Petri dish (diam. 15 cm). At that moment, all larvae were in a defensive posture (i.e. curled), typically adopted when disturbed. We video-recorded activity during 10 min after an initial 30-s acclimation period. From the videos, we retrieved (1) latency (in s) to resume activity (i.e. leaving the defensive posture) and (2) net displacement (in cm) (i.e. distance to the centre of the arena at the end of the test). We assume the latter behaviour reflects activity. Individuals that did not resume activity within the observation period were given the maximal value (i.e. 600 s—occurring in 27.6% (37/134) of the trials) and an activity score of zero. For each individual, we repeated the test after 64 ± 25 min (mean ± SD) on the same day.
Pupal anti-predator behaviour
We conducted an anti-predator test on pupae (N = 73) 4 days after pupation. Although butterfly pupae show no mobility once attached to the pupation substrate, they are nonetheless able to move abdominal segments in the form of quick movements of short duration (‘jerks’) to scare predators and parasitoids (Cole 1959; Brakefield et al. 1992). In order to simulate an encounter with a predator, individual pupae were repeatedly touched with a wooden stick. Some pupae reacted to this stimulation within a few seconds. We then recorded the amount of individual jerks. When individuals did not respond during the 30 s of the trial, we stopped and gave a value of zero to these individuals. Each pupa was tested twice; tests were conducted in early morning and late afternoon of the same day.
Adult boldness, exploration and activity
Behavioural tests on adult butterflies (N = 66) started on the day following emergence, i.e. at day 1 (but 11 butterflies—2 rural and 2 urban females, 3 rural and 4 urban males—were tested at day 2 and two butterflies—1 rural and 1 urban male—at day 3). After the last test, butterflies were killed and stored by freezing (− 21 °C).
We designed a boldness test (i.e. activity in stressful conditions) inspired by docility tests conducted on vertebrates (e.g. Martin and Réale 2008; Brommer and Kluen 2012). A butterfly was placed in a semi-transparent glassine bag (63 × 97 mm envelope), which allowed to see the butterfly’s movements. We positioned the butterfly (with closed wings) in the centre of the bag and maintained it in this position by gently pressing two of the corners of the envelope. We started a stopwatch and counted the number of struggles during 1 min. Here, we define struggles as series of leg, head or wing movements interrupted by pauses of inactivity. The test took place in a 25 °C room under constant light conditions. The elapsed time between the first and the second trial was around 2 h.
After boldness tests, we quantified exploration and activity for each individual. Prior to each test, butterflies were placed at 30 °C in an incubator for 5 min in order to raise body temperature close to the optimum to initiate voluntary flight (30–34 °C; Van Dyck and Matthysen 1998). Each butterfly was then released at the extremity of a greenhouse tunnel (9 m × 4 m × 2 m; installed in a larger greenhouse maintained at a temperature of 21 °C) whose floor was taped to delineate 12 squares of 1.5 m × 2 m. During the test, the observer stood still at the entrance of the tunnel and moved as little as possible. Each butterfly was allowed to move freely in the tunnel during 4 min and the observer recorded (1) the number of squares visited at least once (hereafter adult exploration) and (2) time elapsed before the first landing (hereafter adult activity). For each individual, time between the two tests was 154 ± 58 min (mean ± SD).
Statistical analysis
Effects of fixed variables on behavioural traits
In order to test for differences among landscapes, sexes and trials, we ran several univariate (generalised) linear mixed-effects models (lme4 and MASS packages). Each of the measured behaviours was treated as the response variable. Landscape of origin (rural versus urban), sex, test number (sequence; first versus second trial) and their interactions were included as fixed effects in all models. We additionally added larval body surface (in cm2—retrieved from the video when larvae were in the defensive posture) and adult forewing area (in cm2—standardised within each sex) as proxies for larval and adult body size, respectively. For adult exploration and activity (i.e. traits measured in the greenhouse), ambient temperature was included as an additional covariate. All models included individual identity (each individual was given a single identifier), family and sampling site of the mother as random effects.
Activity at the larval and adult stages was log-transformed, then analysed with a linear mixed model. Pupal anti-predator strategy was modelled with a Poisson distribution error, whereas adult boldness and exploration were modelled with a quasi-Poisson distribution error with the square-root link function, since equivalent generalised linear models with Poisson distribution errors showed signs of overdispersion. Larval latency time was right-censored, as larvae still immobile at the end of the test were treated as censored data. Therefore, it was modelled with a Cox proportional hazards model allowing to include random effects (coxme package).
Repeatability of behavioural traits
Based on the final model retained in the previous step, we used the rptR package (Stoffel et al. 2017) to calculate an adjusted repeatability (i.e. accounting for fixed effects) for all behavioural traits. In the case of larval latency, data were log-transformed then treated with a Gaussian error distribution. Note that we report repeatability on the link-scale for non-Gaussian data as advised by Nakagawa and Schielzeth (2010).
Within- and among-stage syndromes
In individuals for which all behavioural traits were known (N = 12 for rural females; 15 for rural males; 11 for urban females; and 19 for urban males), we tested for within- and between-stage behavioural associations. We first averaged each behavioural trait over the two successive measurements. Then, we conducted a series of linear mixed models with the mean score at one test as response variable and the mean score at another test (together with landscape of origin, sex and their interactions with the behavioural score) as explanatory variables. Family and sampling site of the mother were included as random effects. When the behavioural score included as an explanatory variable was involved in a significant interaction with sex and/or landscape of origin, we further tested the slope for significance of each group separately.
Because we expect larval traits to predict behaviour at a subsequent developmental stage, larval behavioural traits were treated as explanatory variables when adult behavioural traits were included as the response variable. When considered as the response variable, larval activity and adult boldness were log(x + 1) transformed and adult activity was log-transformed to improve residual distribution, but note that this did not alter the shape of the relationship.
Since only 14% (8/57) of individuals showed a mean pupal anti-predator score greater than zero, we did not test for relationships between this trait and other larval or adult behaviour traits.
We used type II Wald tests to obtain P values for the fixed effects. Non-significant interactions (P value > 0.10) were removed from the model, starting with the interaction with the highest P value.
All statistical analyses were performed with R 3.4.2 (R Core Team 2018).
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Results
Repeatability of behavioural traits
We found significant (adjusted) repeatability for all behavioural traits (Table 1). Repeatability estimates range from 0.23 for the two larval traits to 0.98 for the pupal anti-predator behaviour.
Effects of fixed variables on behavioural traits
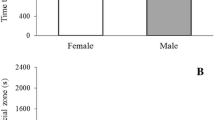
For adult boldness, sex (χ21 = 9.630; P = 0.002) and the sequence (χ21 = 13.226; P < 0.001) sorted effects: males behaved bolder than females (males = 5.80 ± 0.54; females = 3.55 ± 0.51; mean ± SE), and adults scored higher during the second trial (first trial = 4.57 ± 0.59; second trial = 5.19 ± 0.52; mean ± SE). We also found an effect of sex on adult activity (F1, 55.5 = 8.799; P = 0.004): the duration of the first flight bout was on average 20 s longer in males than in females (males = 47.18 ± 5.99 s; females = 27.69 ± 5.25 s; mean ± SE). For adult exploration, larger individuals tended to explore more (forewing area = χ21 = 3.53; P = 0.060), but there was no such effect of body size for the other adult traits (boldness = χ21 = 0.712; P = 0.398; activity: F1, 53.2 = 1.065; P = 0.306). Neither landscape of origin as a main effect nor its interaction with sex or sequence had an effect on adult personality traits (all P values > 0.10). Contrastingly, none of the tested fixed effects (sex, landscape of origin, sequence—and their interactions—and larval body size) had an influence on larval and pupal traits (all P values > 0.10). Mean values (± SE) for all measured behaviours and associated sample sizes are available in the Supplementary material (Tables S2–7).
Within-stage correlations
We found significant within-stage correlations among behavioural traits (Table 2). Larval latency and activity were related, but the relationship between these two traits was affected by the interaction between sex and the landscape of origin. Larval latency and activity were negatively related in all groups, but the relationship was weaker in males of urban origin. Post hoc comparisons revealed that rural and urban females had similar slopes (rural females versus urban females: P = 0.860) while slopes differ between rural and urban males (rural males versus urban males: P = 0.004). At the adult stage, exploration and boldness were positively related as well as activity and exploration.
Among-stage associations
We detected significant associations between larval and adult traits (Table 3). Adult boldness and larval activity were negatively related (Fig. 1). The relationships between adult exploration and larval latency and between adult exploration and larval activity both depended on the interaction between sex and the landscape of origin. In urban males, larval latency and activity tend to be related to adult exploration, whereas this was not the case in females nor in males of rural origin (Figs. 2 and 3). Post hoc comparisons showed that in both cases, slopes of the relationship were similar in rural and urban females (adult exploration and larval latency: rural females versus urban females: P = 0.357; adult exploration and larval activity: rural females versus urban females: P = 0.661) but differed between rural and urban males (adult exploration and larval latency: rural males versus urban males: P = 0.027; adult exploration and larval activity: rural males versus urban males: P = 0.012).
Discussion
We studied the stability of individual behavioural variation in the speckled wood butterfly (P. aegeria), an insect species that undergoes a complete metamorphosis. All behavioural traits were repeatable, suggesting that personality is not restricted to the adult stage in this species. While males were more active and bolder than females, mean values of the measured personality traits did not differ among individuals from rural and urban populations. Nevertheless, the consistency of personality across metamorphosis tended to depend on complex sex by landscape of origin interactions in some cases, with only urban males displaying correlations between larval and adult personality traits.
Repeatability of behavioural traits in P. aegeria
Personality traits have been documented in several butterfly species, including our study species (Ducatez et al. 2012, 2014; Ducatez and Baguette 2016). However, these studies have focused on adults only, without considering the immature stages. Here, we detected significant repeatability for all behavioural trait, spread across the three life stages of P. aegeria. This shows that not only adult butterflies behave consistently between the two successive trials but that larvae too exhibited consistent individual differences in behaviour. This adds to a growing body of literature describing the existence of personality traits in the larval stage of several insect species (e.g. Alcalay et al. 2014; Wexler et al. 2016; Monceau et al. 2017; Stanley et al. 2017). Additionally, we show that pupal anti-predator behaviour was repeatable, which is, to our knowledge, the first evidence of a personality trait at this life stage. Although the pupa may be viewed as a largely immobile stage, we propose that it should not be ignored in behavioural studies. For example, pupal movements are known to deter parasitoids (Cole 1959) in some Lepidoptera, and they may thus be linked to survival. In some butterfly species, pupae are able to produce sounds and vibrations used not only in defensive contexts (Downey and Allyn 1973; Álvarez et al. 2014) but also to elicit mutualistic interactions with ants (Travassos and Pierce 2000). This behaviour has not been studied in the context of personality, but based on our results, it would be of interest to investigate inter-individual variation and consistency in pupal sound production and potential effects on ant guarding.
As a consequence of our experimental design, these estimates should be seen as short-term repeatability. Indeed, estimates were obtained from tests conducted on the same day, usually only a few hours apart. Repeatability is expected to decrease with increasing intervals between consecutive behavioural tests (Bell et al. 2009). This may at least partly explain why our repeatability estimates are relatively high (ranging from 0.23 to 0.98). Nevertheless, we expect this relatively short time interval between tests to be ecologically relevant for our study species. As already mentioned by Ducatez et al. (2012), most butterfly species are short-lived animals—the expected adult lifespan is less than 2 weeks for temperate-zone species without adult diapause (Scott 1974)—for which a couple of hours hence represent a significant part of their lifespan.
Sex and test sequence, but not landscape of origin, influence personality
While we did not detect any effect of the landscape of origin on the mean value of personality traits, we found strong effects of sex in two out of three adult behavioural traits. Flight bouts before the first landing were longer in males, and they also had higher boldness scores compared to females. We argue that these intersexual differences reflect the respective strategies of each sex, i.e. the type of activities they devote their time to. Under natural conditions, males either defend a small territory against conspecifics where they await females (‘perching’ strategy—involving frequent fast flights to intercept and chase intruders) or engage in extensive mate location flights ( ‘patrolling’ strategy) (Shreeve 1984; Merckx and Van Dyck 2005). In contrast, females usually only make relatively short flight bouts, frequently interrupted to oviposit or hide in the vegetation (Shreeve 1986). We believe higher levels of activity and boldness are advantageous for males in order to locate mates or to gain access to high-quality territories by deterring rivals (see for example, Rudin and Briffa 2012; Lane and Briffa 2017), whereas such investment is not required for females. Interestingly, sex-related differences were not observed in larvae or pupae of the speckled wood, suggesting that intersexual differences in personality traits arise late in development in this species (Chippindale et al. 2001). Although differences among sexes in feeding and anti-predator behaviours, for example, are known to occur in the larval stage of several insects (e.g. Baker et al. 1992; Karl and Fischer 2008; Wormington and Juliano 2014), Monceau et al. (2017) found no sex-related differences in personality traits in the flour beetle Tenebrio molitor, neither at the larval nor at the adult stage. Thus, the developmental stage at which sex-related difference in behaviour arises is likely to be trait-specific, depending on sex- and stage-specific needs and constraints.
In addition to the sexual dimorphism in adult behavioural traits, we also observed a sequence effect on adult boldness with both males and females having higher scores during the second trial. This increase in boldness is likely a result of individual butterflies becoming more familiar with the test. Organisms usually show stress responses (i.e. ‘freezing’ or trying to escape) when confronted with unfamiliar conditions, but progressively overcome initial fear when repeatedly exposed to these conditions. This may take the form of an increase or decrease in the focal trait with test order, depending on the system and the trait of interest (Dingemanse et al. 2012), indicating habituation sensu lato. For example, activity decreased with trial order in eastern chipmunks (Martin and Réale 2008), whereas exploration increased in great tits when birds are tested repeatedly (Dingemanse et al. 2012). The here observed increase in boldness suggests that similar processes are at work in invertebrates too (see also Kralj-Fišer et al. 2017; Müller and Müller 2017).
Larval and adult personalities are linked by an urbanisation × sex interaction
The existence of ontogenetic consistency of behavioural traits in insects is still a matter of debate, and evidence of such consistency remains scarce. Amat et al. (2018) found only six studies investigating the link between larval and adult personality traits in holometabolous insects. Interestingly, five out of these six studies showed personality uncoupling across metamorphosis, with all five conducted on Coleopterans, suggesting that our current knowledge on the relationship between personality consistency and metamorphosis may be taxonomically biased. Here, we observed that butterfly larvae that were more active developed into shy adults. In the small white butterfly (Pieris rapae), larval and adult life-history traits are genetically associated (Tanaka 1989). Our results suggest that behavioural traits at different life stages also share a genetic basis or are linked to intrinsic variables (i.e. hormone levels and metabolic rates—see Niemelä and Dingemanse 2018) in P. aegeria. These relationships appear robust to metamorphosis despite the profound internal changes occurring during this ontogenetic step.
However, in some cases, relationships among larval and adult behavioural traits also depended on both the landscape of origin and sex. Larval latency and activity were related to adult exploration in males of urban origin but not in females nor in rural males. Behavioural syndromes may differ among populations, and those differences can sometimes be linked to diverging ecological conditions such as differences in predation risk (Bell 2005; Dingemanse et al. 2007) or in pesticide exposure (Royauté et al. 2014). Our results suggest that the same could apply to behavioural syndromes across metamorphosis too and that larval-adult trait relationships can be shaped by both environmental conditions and intrinsic factors. In our study, two of the three detected across-stage correlations were only present in urban males. Although care must be taken regarding the relatively small number of tested families, these results offer novel perspectives on rural-urban differences in behavioural syndromes. Urbanisation has repeatedly been shown to alter behavioural syndrome structures in birds (Evans et al. 2010; Bókony et al. 2012). Similarly, Tüzün et al. (2017) detected an activity-boldness syndrome in urban but not in rural damselfly larvae in a pesticide-free behavioural trial. The authors suggest that cities provide relatively harsher conditions compared to more natural settings and that they hence favour tighter behavioural correlations as a result of stronger selection pressures. We believe this may be the case in our system too. P. aegeria is primarily a drought-sensitive (Oliver et al. 2015) woodland species whose juvenile survival tends to be negatively affected by warm and dry conditions (Schweiger et al. 2006). Elevated temperature and low humidity in urban areas (Kaiser et al. 2016; Merckx et al. 2018) are expected to impose strong selective pressures on this species through direct (i.e. egg and/or larval mortality) and indirect (i.e. changes in host plant quality) effects (see Talloen et al. 2004). Such unfavourable developmental conditions would favour stronger trait integration and promote personality stability across metamorphosis in urban areas. Although this provide an interesting framework for explaining urban-rural differences in male behavioural syndromes in our study, a detailed evaluation of this hypothesis would be needed, especially because urbanisation typically involves a cocktail of co-occurring environmental changes and its effects are likely to depend on the species’ ecology. The absence of equivalent behavioural correlations in urban females probably reflects sex-specific selective pressures of urbanisation (Bonier et al. 2007) due to intersexual differences in resource acquisition for growth and daily activities (i.e. mainly mate location for males versus oviposition and feeding for females).
Summary and perspectives
Our results show that personality traits are not adult-specific but are also present in juvenile stages of a species with a complex life cycle. Additionally, our study suggests that urbanisation, together with sex, plays a role in shaping relationships among behavioural traits. Detailed knowledge about the fitness consequences of personality is currently lacking in our study system. Such information remains scarce for most invertebrates (but see Goulet et al. 2016). As such, we believe unravelling this link should be the next step to fully understand the ecological relevance of personality traits.
References
Alberti M (2015) Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol Evol 30:114–126. https://doi.org/10.1016/j.tree.2014.11.007
Alberti M, Marzluff J, Hunt VM (2017) Urban driven phenotypic changes: empirical observations and theoretical implications for eco-evolutionary feedback. Philos Trans R Soc B Biol Sci 372:20160029. https://doi.org/10.1098/rstb.2016.0029
Alcalay Y, Barkae ED, Ovadia O, Scharf I (2014) Consequences of the instar stage for behavior in a pit-building antlion. Behav Process 103:105–111. https://doi.org/10.1016/j.beproc.2013.11.009
Álvarez M, Munguira ML, Martínez-Ibáñez MD (2014) Comparative study of the morphology of stridulatory organs of the Iberian lycaenid butterfly pupae (Lepidoptera). J Morphol 275:414–430. https://doi.org/10.1002/jmor.20224
Amat I, Desouhant E, Gomes E, Moreau J, Monceau K (2018) Insect personality: what can we learn from metamorphosis? Curr Opin Insect Sci 27:46–51. https://doi.org/10.1016/j.cois.2018.02.014
Anderson BB, Scott A, Dukas R (2016) Social behavior and activity are decoupled in larval and adult fruit flies. Behav Ecol 27:820–828. https://doi.org/10.1093/beheco/arv225
Baker RL, Forbes MRL, Proctor HC (1992) Sexual differences in development and behaviour of larval Ischnura verticalis (Odonata: Coenagrionidae). Can J Zool 70:1161–1165. https://doi.org/10.1139/z92-162
Bell AM (2005) Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473. https://doi.org/10.1111/j.1420-9101.2004.00817.x
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834. https://doi.org/10.1111/j.1461-0248.2007.01081.x
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783. https://doi.org/10.1016/j.anbehav.2008.12.022
Bergerot B, Merckx T, Van Dyck H, Baguette M (2012) Habitat fragmentation impacts mobility in a common and widespread woodland butterfly: do sexes respond differently? BMC Ecol 12:5. https://doi.org/10.1186/1472-6785-12-5
Bink FA (1992) Atlas van de Dagvlinders van Noordwest-Europa. Schuyt & Co, Haarlem, NL
Blackiston DJ, Silva Casey E, Weiss MR (2008) Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS One 3:e1736. https://doi.org/10.1371/journal.pone.0001736
Bókony V, Kulcsár A, Tóth Z, Liker A (2012) Personality traits and behavioral syndromes in differently urbanized populations of house sparrows (Passer domesticus). PLoS One 7:e36639. https://doi.org/10.1371/journal.pone.0036639
Bonier F, Martin PR, Sheldon KS, Jensen JP, Foltz SL, Wingfield JC (2007) Sex-specific consequences of life in the city. Behav Ecol 18:121–129. https://doi.org/10.1093/beheco/arl050
Brakefield PM, Shreeve TG, Thomas JA (1992) Avoidance, concealment, and defence. In: Dennis RLH (ed) The ecology of butterflies in Britain. Oxford University Press, Oxford, UK, pp 93–119
Brodin T (2009) Behavioral syndrome over the boundaries of life—carryovers from larvae to adult damselfly. Behav Ecol 20:30–37. https://doi.org/10.1093/beheco/arn111
Brommer JE, Kluen E (2012) Exploring the genetics of nestling personality traits in a wild passerine bird: testing the phenotypic gambit. Ecol Evol 2:3032–3044. https://doi.org/10.1002/ece3.412
Carrete M, Tella JL (2017) Behavioral correlations associated with fear of humans differ between rural and urban burrowing owls. Front Ecol Evol 5:54. https://doi.org/10.3389/fevo.2017.00054
Carter AW, Paitz RT, McGhee KE, Bowden RM (2016) Turtle hatchlings show behavioral types that are robust to developmental manipulations. Physiol Behav 155:46–55. https://doi.org/10.1016/j.physbeh.2015.11.034
Charmantier A, Demeyrier V, Lambrechts M, Perret S, Grégoire A (2017) Urbanization is associated with divergence in pace-of-life in great tits. Front Ecol Evol 5:1–13. https://doi.org/10.3389/fevo.2017.00053
Chippindale AK, Gibson JR, Rice WR (2001) Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc Natl Acad Sci 98:1671–1675. https://doi.org/10.1073/pnas.98.4.1671
Cole LR (1959) On the defences of lepidopterous pupae in relation to the oviposition behaviour of certain Ichneumonidae. J Lepid Soc 13:1–10
Consoulas C, Duch C, Bayline RJ, Levine RB (2000) Behavioral transformations during metamorphosis: remodeling of neural and motor systems. Brain Res Bull 53:571–583. https://doi.org/10.1016/S0361-9230(00)00391-9
Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria URL: https://www.R-project.org/
Debeffe L, Lemaître JF, Bergvall UA, et al (2015) Short- and long-term repeatability of docility in the roe deer: sex and age matter. Anim Behav 109:53–63. https://doi.org/10.1016/j.anbehav.2015.08.003
Davies NB (1978) Territorial defence in the speckled wood butterfly (Pararge aegeria): the resident always wins. Anim Behav 26:138–147. https://doi.org/10.1016/0003-3472(78)90013-1
Denys C, Schmidt H (1998) Insect communities on experimental mugwort (Artemisia vulgaris L.) plots along an urban gradient. Oecologia 113:269–277. https://doi.org/10.1007/s004420050378
Dingemanse NJ, Wright J, Kazem AJN et al (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138. https://doi.org/10.1111/j.1365-2656.2007.01284.x
Dingemanse NJ, Bouwman KM, van de Pol M, van Overveld T, Patrick SC, Matthysen E, Quinn JL (2012) Variation in personality and behavioural plasticity across four populations of the great tit Parus major. J Anim Ecol 81:116–126. https://doi.org/10.1111/j.1365-2656.2011.01877.x
Downey JC, Allyn AC (1973) Butterfly ultrastructure. I. Sound production and associated abdominal structures in pupae of Lycaenidae and Riodinidae. Bull Allyn Mus 14:1–47
Ducatez S, Baguette M (2016) Inter-individual variation in shivering behaviour in the migratory painted lady Vanessa cardui. Ecol Entomol 41:131–137. https://doi.org/10.1111/een.12283
Ducatez S, Legrand D, Chaput-Bardy A et al (2012) Inter-individual variation in movement: is there a mobility syndrome in the large white butterfly Pieris brassicae? Ecol Entomol 37:377–385. https://doi.org/10.1111/j.1365-2311.2012.01375.x
Ducatez S, Humeau A, Congretel M, Fréville H, Baguette M (2014) Butterfly species differing in mobility show different structures of dispersal-related syndromes in the same fragmented landscape. Ecography (Cop) 37:378–389. https://doi.org/10.1111/j.1600-0587.2013.00365.x
Evans J, Boudreau K, Hyman J (2010) Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116:588–595. https://doi.org/10.1111/j.1439-0310.2010.01771.x
Feltwell J (1982) Large white butterfly: the biology, biochemistry and physiology of Pieris brassicae (Linnaeus). Dr W. Junk Publishers, The Hague
Goulet CT, Ingley SJ, Scharf I, Pruitt JN (2016) Thermal effects on survival and reproductive performance vary according to personality type. Behav Ecol 27:1635–1641. https://doi.org/10.1093/beheco/arw084
Gyuris E, Feró O, Barta Z (2012) Personality traits across ontogeny in firebugs, Pyrrhocoris apterus. Anim Behav 84:103–109. https://doi.org/10.1016/j.anbehav.2012.04.014
Hardman SI, Dalesman S (2018) Repeatability and degree of territorial aggression differs among urban and rural great tits (Parus major). Sci Rep 8:5042. https://doi.org/10.1038/s41598-018-23463-7
Hedrick AV, Kortet R (2012) Sex differences in the repeatability of boldness over metamorphosis. Behav Ecol Sociobiol 66:407–412. https://doi.org/10.1007/s00265-011-1286-z
Kaiser A, Merckx T, Van Dyck H (2016) The urban heat island and its spatial scale dependent impact on survival and development in butterflies of different thermal sensitivity. Ecol Evol 6:4129–4140. https://doi.org/10.1002/ece3.2166
Karl I, Fischer K (2008) Why get big in the cold? Towards a solution to a life-history puzzle. Oecologia 155:215–225. https://doi.org/10.1007/s00442-007-0902-0
Karlsson Green K, Eroukhmanoff F, Harris S, Pettersson LB, Svensson EI (2016) Rapid changes in genetic architecture of behavioural syndromes following colonization of a novel environment. J Evol Biol 29:144–152. https://doi.org/10.1111/jeb.12769
Kralj-Fišer S, Schuett W (2014) Studying personality variation in invertebrates: why bother? Anim Behav 91:41–52. https://doi.org/10.1016/j.anbehav.2014.02.016
Kralj-Fišer S, Hebets EA, Kuntner M (2017) Different patterns of behavioral variation across and within species of spiders with differing degrees of urbanization. Behav Ecol Sociobiol 71:125. https://doi.org/10.1007/s00265-017-2353-x
Lagucki E, Burdine JD, McCluney KE (2017) Urbanization alters communities of flying arthropods in parks and gardens of a medium-sized city. PeerJ 5:e3620. https://doi.org/10.7717/peerj.3620
Lane SM, Briffa M (2017) Boldness is for rookies: prefight boldness and fighting success in a sea anemone. Anim Behav 132:13–20. https://doi.org/10.1016/j.anbehav.2017.07.012
Lapiedra O, Chejanovski Z, Kolbe JJ (2017) Urbanization and biological invasion shape animal personalities. Glob Chang Biol 23:592–603. https://doi.org/10.1111/gcb.13395
Martin JGA, Réale D (2008) Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim Behav 75:309–318. https://doi.org/10.1016/j.anbehav.2007.05.026
Merckx T, Van Dyck H (2005) Mate location behaviour of the butterfly Pararge aegeria in woodland and fragmented landscapes. Anim Behav 70:411–416. https://doi.org/10.1016/j.anbehav.2004.12.005
Merckx T, Souffreau C, Kaiser A, Baardsen LF, Backeljau T, Bonte D, Brans KI, Cours M, Dahirel M, Debortoli N, de Wolf K, Engelen JMT, Fontaneto D, Gianuca AT, Govaert L, Hendrickx F, Higuti J, Lens L, Martens K, Matheve H, Matthysen E, Piano E, Sablon R, Schön I, van Doninck K, de Meester L, van Dyck H (2018) Body-size shifts in aquatic and terrestrial urban communities. Nature 558:113–116. https://doi.org/10.1038/s41586-018-0140-0
Miranda AC, Schielzeth H, Sonntag T, Partecke J (2013) Urbanization and its effects on personality traits: a result of microevolution or phenotypic plasticity? Glob Chang Biol 19:2634–2644. https://doi.org/10.1111/gcb.12258
Monceau K, Moreau J, Richet J, Motreuil S, Moret Y, Dechaume-Moncharmont FX (2017) Larval personality does not predict adult personality in a holometabolous insect. Biol J Linn Soc 120:869–878. https://doi.org/10.1093/biolinnean/blw015
Moran NP, Mossop KD, Thompson RM, Wong BBM (2016) Boldness in extreme environments: temperament divergence in a desert-dwelling fish. Anim Behav 122:125–133. https://doi.org/10.1016/j.anbehav.2016.09.024
Moran NP, Mossop KD, Thompson RM, Chapple DG, Wong BBM (2017) Rapid divergence of animal personality and syndrome structure across an arid-aquatic habitat matrix. Oecologia 185:55–67. https://doi.org/10.1007/s00442-017-3924-2
Müller T, Müller C (2015) Behavioural phenotypes over the lifetime of a holometabolous insect. Front Zool 12:S8. https://doi.org/10.1186/1742-9994-12-S1-S8
Müller T, Müller C (2017) Phenotype of a leaf beetle larva depends on host plant quality and previous test experience. Behav Process 142:40–45. https://doi.org/10.1016/j.beproc.2017.05.017
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Niemelä PT, Dingemanse NJ (2018) Meta-analysis reveals weak associations between intrinsic state and personality. Proc R Soc B Biol Sci 285:20172823. https://doi.org/10.1098/rspb.2017.2823
Oliver TH, Marshall HH, Morecroft MD, Brereton T, Prudhomme C, Huntingford C (2015) Interacting effects of climate change and habitat fragmentation on drought-sensitive butterflies. Nat Clim Chang 5:941–945. https://doi.org/10.1038/nclimate2746
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Rodrigues AS, Botina L, Nascimento CP, Gontijo LM, Torres JB, Guedes RNC (2016) Ontogenic behavioral consistency, individual variation and fitness consequences among lady beetles. Behav Process 131:32–39. https://doi.org/10.1016/j.beproc.2016.08.003
Royauté R, Buddle CM, Vincent C (2014) Interpopulation variations in behavioral syndromes of a jumping spider from insecticide-treated and insecticide-free orchards. Ethology 120:127–139. https://doi.org/10.1111/eth.12185
Rudin FS, Briffa M (2012) Is boldness a resource-holding potential trait? Fighting prowess and changes in startle response in the sea anemone, Actinia equina. Proc R Soc B Biol Sci 279:1904–1910. https://doi.org/10.1098/rspb.2011.2418
Scales J, Hyman J, Hughes M (2011) Behavioral syndromes break down in urban song sparrow populations. Ethology 117:887–895. https://doi.org/10.1111/j.1439-0310.2011.01943.x
Schuett W, Delfs B, Haller R, Kruber S, Roolfs S, Timm D, Willmann M, Drees C (2018) Ground beetles in city forests: does urbanization predict a personality trait? PeerJ 6:e4360. https://doi.org/10.7717/peerj.4360
Schweiger O, Dormann CF, Bailey D, Frenzel M (2006) Occurrence pattern of Pararge aegeria (Lepidoptera: Nymphalidae) with respect to local habitat suitability, climate and landscape structure. Landsc Ecol 21:989–1001. https://doi.org/10.1007/s10980-005-6057-7
Scott JA (1974) Lifespan of butterflies. J Res Lepid 12:225–230
Senar JC, Garamszegi LZ, Tilgar V, Biard C, Moreno-Rueda G, Salmón P, Rivas JM, Sprau P, Dingemanse NJ, Charmantier A, Demeyrier V, Navalpotro H, Isaksson C (2017) Urban great tits (Parus major) show higher distress calling and pecking rates than rural birds across Europe. Front Ecol Evol 5:163. https://doi.org/10.3389/fevo.2017.00163
Shochat E, Stefanov WL, Whitehouse MEA, Faeth SH (2004) Urbanization and spider diversity: influences of human modification of habitat structure and productivity. Ecol Appl 14:268–280. https://doi.org/10.1890/02-5341
Shreeve TG (1984) Habitat selection, mate location, and microclimatic constraints on the activity of the speckled wood butterfly Pararge aegeria. Oikos 42:371–377. https://doi.org/10.2307/3544407
Shreeve TG (1986) Egg-laying by the speckled wood butterfly (Pararge aegeria): the role of female behaviour, host plant abundance and temperature. Ecol Entomol 11:229–236. https://doi.org/10.1111/j.1365-2311.1986.tb00298.x
Sih A, Bell AM, Johnson JC, Ziemba RE (2004) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277. https://doi.org/10.1086/422893
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. https://doi.org/10.1016/j.anbehav.2013.01.023
Stamps J, Groothuis TGG (2010) The development of animal personality: relevance, concepts and perspectives. Biol Rev 85:301–325. https://doi.org/10.1111/j.1469-185X.2009.00103.x
Stanley CR, Mettke-Hofmann C, Preziosi RF (2017) Personality in the cockroach Diploptera punctata: evidence for stability across developmental stages despite age effects on boldness. PLoS One 12:e0176564. https://doi.org/10.1371/journal.pone.0176564
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Talloen W, Van Dyck H, Lens L (2004) The cost of melanization: butterfly wing coloration under environmental stress. Evolution (N Y) 58:360–366. https://doi.org/10.1111/j.0014-3820.2004.tb01651.x
Tanaka Y (1989) Genetic variance and covariance patterns of larval development in the small white butterfly Pieris rapae crucivora Boisduval. Res Popul Ecol (Kyoto) 31:311–324. https://doi.org/10.1007/BF02513208
Travassos MA, Pierce NE (2000) Acoustics, context and function of vibrational signalling in a lycaenid butterfly–ant mutualism. Anim Behav 60:13–26. https://doi.org/10.1006/anbe.1999.1364
Tüzün N, Debecker S, Op de Beeck L, Stoks R (2015) Urbanisation shapes behavioural responses to a pesticide. Aquat Toxicol 163:81–88. https://doi.org/10.1016/j.aquatox.2015.04.002
Tüzün N, Müller S, Koch K, Stoks R (2017) Pesticide-induced changes in personality depend on the urbanization level. Anim Behav 134:45–55. https://doi.org/10.1016/j.anbehav.2017.10.007
Van Dyck H, Matthysen E (1998) Thermoregulatory differences between phenotypes in the speckled wood butterfly: hot perchers and cold patrollers? Oecologia 114:326–334. https://doi.org/10.1007/s004420050454
Vonk J, Weiss A, Kuczaj SA (2017) Personality in nonhuman animals. Springer International Publishing, Cham
Wexler Y, Subach A, Pruitt JN, Scharf I (2016) Behavioral repeatability of flour beetles before and after metamorphosis and throughout aging. Behav Ecol Sociobiol 70:745–753. https://doi.org/10.1007/s00265-016-2098-y
Wilson ADM, Krause J (2012) Personality and metamorphosis: is behavioral variation consistent across ontogenetic niche shifts? Behav Ecol 23:1316–1323. https://doi.org/10.1093/beheco/ars123
Wormington JD, Juliano SA (2014) Hunger-dependent and sex-specific antipredator behaviour of larvae of a size-dimorphic mosquito. Ecol Entomol 39:548–555. https://doi.org/10.1111/een.12129
Wuerz Y, Krüger O (2015) Personality over ontogeny in zebra finches: long-term repeatable traits but unstable behavioural syndromes. Front Zool 12:S9. https://doi.org/10.1186/1742-9994-12-S1-S9
Acknowledgements
AK is a research fellow with the Belgian Fund of Scientific Research F.S.R.-FNRS. The research was supported by ARC grant no. 10/15-031 (UCL) and IAP grant no. P7/04 ‘SPEEDY-project’ of the Belgian Science Policy Office BELSPO and by PDR-grant T.0188.14 of the F.R.S.-FNRS Fund both to HVD. This is publication number BRC 397 of the Biodiversity Research Centre (ELI/ELIB, UCL).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Pruitt
Electronic supplementary material
ESM 1
(DOCX 1343 kb)
Rights and permissions
About this article
Cite this article
Kaiser, A., Merckx, T. & Van Dyck, H. Urbanisation and sex affect the consistency of butterfly personality across metamorphosis. Behav Ecol Sociobiol 72, 188 (2018). https://doi.org/10.1007/s00265-018-2616-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2616-1