Abstract
In invasive species, geographically variable evolutionary and ecological pressures can cause the rapid evolution of divergent behavioural phenotypes. Studies on invasive cane toads (Rhinella marina) in tropical Australia have revealed strong (and heritable) shifts in physiological traits related to dispersal rate. Behavioural phenotypes may have evolved in similar ways. We used standardised arena trials to test field-collected adult female toads from three populations: a range-core area in Queensland (ca. 76 years post-colonisation), a range-front population in Western Australia (<5 years post-colonisation) and an intermediate Northern Territory population (11 years post-colonisation). As predicted, toads from the range-front population were more exploratory and more likely to take risks in a novel arena environment than were conspecifics from the range-core population. We suggest that differential selection on behavioural responses to novel conditions in range-core versus range-front populations has produced a distinctive behavioural phenotype at the range-front that retains a high propensity for exploration and risk-taking (enhancing the ability of range-front toads to locate food and shelter) even when faced with novel environments. In contrast, at the range core where the locations of resources are known, a decrease in exploration and risk-taking in response to a novel environment may be favoured as it assists toads in evading threats.
Significance statement
Ongoing biological invasions provide an ideal opportunity to examine which phenotypic traits drive establishment, range-expansion and invasion success. Furthermore, ongoing invasions allow us to investigate if variation in evolutionary and ecological pressures across an invasion range leads to geographical divergence in phenotypic traits. Dispersal ability is a key factor in invasion success. Behavioural traits such as exploration and a propensity to take risks enhance dispersal as individuals with these traits rapidly move out of their existing range and exploit new habitats and resources. We studied geographic divergence of dispersal-related behavioural traits across the Australian invasion range of cane toads (Rhinella marina) using standardised laboratory trials. We found that range-front toads were more exploratory and more likely to take risks than were conspecifics from range-core areas. Our results suggest that dispersal-enhancing behavioural traits may be important drivers of invasion success in cane toads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions can be catastrophic ecologically (Kolar and Lodge 2001), stimulating extensive research on traits that influence the successful establishment of invasive species (Fogarty et al. 2011; Chapple et al. 2012). Dispersal ability is a strong predictor of invasion success (Sih et al. 2004; Myles-Gonzalez et al. 2015) and behavioural traits such as boldness, sociability and aggression have been associated with behaviour-dependent dispersal and invasion success in fishes (Cote et al. 2010b, 2011; Chapman et al. 2011; Groen et al. 2012), birds (Verbeek et al. 1994; Dingemanse et al. 2003; Duckworth 2006; Duckworth and Badyaev 2007; Duckworth 2008; Liebl and Martin 2012, 2014), reptiles (Aragon et al. 2006a, 2006b; Cote and Clobert 2007; Meylan et al. 2009; Chapple et al. 2012) and invertebrates (Brodin and Drotz 2014; Monceau et al. 2015). Exploration and risk-taking are dispersal-related behavioural traits forming a part of a shy-bold continuum, a key axis of behavioural variation in many taxa (Wilson et al. 1994; Riesch et al. 2009). Boldness can be defined as the propensity of an individual to take risks and explore in a novel environment (Wilson et al. 1993, 1994; Riesch et al. 2009). Individuals with bold behaviour (such as high levels of exploration and risk-taking) are predicted to be more common in vanguard populations as they are more likely to disperse beyond their home range, and accept the risks inherent in dispersal into novel environments, than are shyer conspecifics (Rehage and Sih 2004).
In investigating the role of dispersal-enhancing behavioural traits in invasion success, we need to consider the relative costs and benefits of different behavioural traits in range-front versus range-core populations. If there are differential benefits of certain behavioural types across the invasion range, this could lead to variation in the frequency (prevalence) of these traits in range-front versus range-core populations. Different behavioural types may be favoured at different stages of an invasion (Duckworth and Badyaev 2007; reviewed in Cote et al. 2010a; Chapple et al. 2012). For example, costs associated with range expansion include the risk of novel predators, the unknown availability or location of resources and a potential decrease in reproductive opportunities as the likelihood of encountering a mate may be reduced (Simmons and Thomas 2004; Myles-Gonzalez et al. 2015). Therefore, it may be beneficial for these ‘pioneers’ to be exploratory and willing to take risks in order to seek out and use resources such as food, water and shelter (Verbeek et al. 1994). Conversely, individuals from long-colonised populations are familiar with the location of resources in their environment and, hence, may lower their risk of predation or parasite infection by reducing their activity and risk-taking behaviour.
The cane toad (Rhinella marina) is a highly successful invasive species in Australia. Intensive research has documented acceleration in dispersal rate and the rapid evolution of dispersal-enhancing traits during its Australian invasion. For example, invasion-front toads grow faster, have longer legs and move more often and for longer periods than do conspecifics from long-colonised areas (Phillips et al. 2006, 2007; Phillips 2009; Brown et al. 2013; LindstrÖm et al. 2013). Their locomotor endurance may also be higher (Llewellyn et al. 2010), and genes associated with metabolism and cellular repair are upregulated at the invasion front (Rollins et al. 2015). Evolutionary theory suggests that natural selection and spatial sorting for enhanced rates of dispersal should favour shifts in any phenotypic traits that enable a toad to disperse more rapidly (e.g., Shine et al. 2011). Hence, behavioural traits that enhance dispersal may well be at least as important as evolved shifts in morphology and physiology in this respect (in cane toads as in other species).
We tested for variation in exploratory and risk-taking behaviour during the cane toad invasion by running standardised laboratory-based trials on wild-caught adult toads from three locations across the invasion range with different times since colonisation (ca. 76 years, 11 years and <5 years post-colonisation). We conducted two separate behavioural trials: an exploration trial in which we measured time spent moving and rate of movement in a novel environment, and a risk-taking trial in which we recorded whether or not an individual emerged from a shelter into a novel environment, and its latency to emerge. We predicted that toads from the range front would exhibit higher levels of exploration and risk-taking behaviour (i.e., would be bolder) than conspecifics from intermediate and range-core populations.
Materials and methods
Study animals and maintenance
In 2014, we collected a total of 48 adult female cane toads (Rhinella marina) comprising 16 toads from each of three locations (toads were collected from three sample sites within each location) across their invasion range in Australia: Cairns, Queensland (17° 56′ S, 145° 56′ E; 76 years post-colonisation; mean annual rainfall 1999.7 mm; mean annual maximum temperature 29.0 °C); Middle Point, Northern Territory (12° 34′ S, 131° 18′ E; 11 years post-colonisation; mean annual rainfall 1421.7 mm; mean annual maximum temperature 33.1 °C); and Purnululu, Western Australia (17° 27′ S, 128° 33′ E; <5 years post-colonisation; mean annual rainfall 760.8 mm; mean annual maximum temperature 34.7 °C) (Australian Government Bureau of Meteorology [www.bom.gov.au] 2016). ‘Years post-colonisation’ for each population represents a mean calculated from the ‘years post-colonisation’ of each of the three sample sites within each population across the invasion range. Years since colonisation for all sub-sample sites ranged from 76 to 80 years at the range core, 10 to 11 years for the intermediate populations, and 4 to 5 years for the range-front populations. Toads were weighed to the nearest 0.1 g on a digital scale, measured (snout-urostyle length [SUL]) to the nearest 0.01 mm using digital callipers and transported to animal holding facilities at Macquarie University (Sydney: 33° 46′ S, 151° 06′ E), where they were housed in a temperature-controlled room (27–30 °C). Because adult toads are most active at night (Zug and Zug 1979; Lever 2001), room lights were set to a reverse day-night cycle to allow behavioural trials to be carried out during the day (dark phase). Two to three toads were housed as groups in large (100 L) plastic tubs with mesh lids. Toads were fed crickets dusted with calcium and multi-vitamin powders three times per week and water was provided ad libitum. Toads were weighed and measured before and after each trial block to monitor their health and to detect any negative impact of experimental procedures. None of the toads showed any signs of illness or weight loss throughout their time in captivity. Toads were given 3 weeks to adjust to the reverse day-night cycle before trials commenced.
All procedures were approved under animal ethics project numbers 2013/5805 and ARA2013/035. Toads were captured at night by hand and placed in moist calico bags (no more than two toads per bag). Toads were classified as adults based on body size, and their sex was determined to be female based on the absence of nuptial pads on the forelimbs and lack of a ‘release call’ when held (only males are able to make release calls in this species: Bowcock et al. 2008). During transportation to the laboratory, toads were held in moist calico bags inside plastic boxes with air holes, inside insulated boxes. Toads were released into their housing tubs and were provided with water as soon as they arrived at the laboratory. The housing tubs were kept within an air-conditioned room, maintained at temperatures (27–30 °C) well within the usual activity range for this species (McCann et al. 2014). Toads were housed in groups of two to three per tub, and identified by toe-clipping. Only the very tips of the toes were clipped and the animals exhibited no overt signs of discomfort during the procedure. Toe-clipping does not elevate plasma corticosterone levels of this species above those induced by handling alone (Fisher et al. 2013). Handling of toads was kept to a minimum throughout their time in captivity and toads were transferred to and from trial arenas in dark plastic tubs to reduce stress. Toads were left undisturbed during non-trial periods to keep the stress of captivity to a minimum. Toads ate well and showed no signs of illness, and all animals maintained or increased mass during their time in captivity. Because wildlife permits do not allow this invasive species to be released into the wild, all toads were humanely euthanased by injection of sodium brevital at the conclusion of the trials.
General methods
Trials began after toads had spent 4 weeks in captivity. We conducted behavioural trials between 0800 and 1700 h (the dark phase of the reverse day-night cycle). Trials were split over 2 days, with half of the animals from each population tested on each day. Individuals from each population were randomly allocated to a trial time and arena within each trial day and we ran 24 toads (eight toads from each population) each day. All toads experienced trial types in the same order, that is, a risk-taking trial followed by an exploratory trial with one rest day in between. Trials took place in rectangular opaque plastic arenas (115 × 71 × 40 cm). We covered the floor of each arena with plain paper, which was changed between every trial to eliminate scent cues from previously tested toads. We also measured the arena substrate temperature before the commencement of each trial (arena substrate temperatures ranged from 26–30 °C). All trials were recorded using CCTV cameras and we scored videos using Ethovision XT10 behavioural analysis software. Ethovision scored all videos in a standardised way without information on population of origin (to ensure blind scoring). The investigator left the room during trials to avoid interfering with toad behaviour.
Risk-taking (emergence behaviour) trial
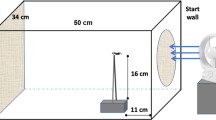
The arena contained two artificial rocks (each rock was ca. 10 cm in diameter, and the toads could not crawl beneath them to seek shelter) and fly-screen material hanging from the walls to provide visual novelty. We also placed a shelter at one end of the arena (Fig. 1a). At the commencement of trials, we placed a toad in the shelter and allowed 2 min for the animal to settle down. We then gently lifted an outer cover allowing the toad the option of leaving the shelter through an exit hole. We filmed trials for 30 min. Our scores for risk-taking behaviour were based on whether or not a toad emerged from the shelter during a trial, and the time it took a toad to emerge from the shelter (latency to emerge in seconds) during the 30-min trial. We classed toads as having emerged from the shelter only when their entire body was visible. We allocated a score of 1800 s to toads that did not emerge.
Exploratory behaviour trial
The same test arenas were re-used for the exploratory trials, but the wall-hanging material and rocks were removed and replaced with four equally-spaced shelters (one along each side of the arena), allowing toads the option of seeking refuge (Fig. 1b). Thus, high activity levels in this trial were associated with exploration, not shelter-seeking. To begin a trial, we placed a toad under a rest shelter in the centre of the arena for 2 min. The rest shelter was then removed and the toad was filmed for the next 30 min. We measured the total distance a toad moved (cm) and the total time a toad spent moving (s) for the duration of the trial.
Statistical analyses
We used a general linear model in JMP 11.0 (SAS Institute, Cary, NC) to analyse the effects of years post-colonisation (range front = 5 years, intermediate = 11 years, range core = 76 years) on behavioural traits. Years post-colonisation were calculated as the mean of the time since colonisation of each of three sub-sample sites within each population and modelled as a categorical variable in all analyses. Potentially confounding factors such as day of trial, time of trial, arena number, arena temperature and toad mass (g) were included in all initial models with behavioural traits as the dependent variables. We ran separate models for exploratory and risk-taking trials. We analysed the following measures: latency to emerge and emergence (binomial, whether individuals emerged during trials) for risk-taking trials and time spent moving and rate of movement (as quantified by the residual scores from a general linear regression of total distance moved against total time spent moving) for exploratory trials. We used Tukey’s post hoc tests to run pairwise comparisons between populations. A generalised linear model with a binomial logit link function (GENMOD function in SAS 9.3–SAS Institute, Cary, NC) was used to compare populations with respect to whether or not toads emerged during trials. We used top-down stepwise model selection, starting with a full model including all factors, covariates and their interactions and sequentially deleting non-significant terms. Only those factors and interactions with P < 0.05 were retained and the final model was selected using the AIC comparison method. Arena temperature, arena number, time of trial and toad mass had non-significant effects on behavioural traits and thus were excluded from the final models. All data were checked for normality and homoscedasticity and log-transformed to meet these assumptions as required.
Results
Risk-taking (emergence) behaviour
Emergence
Years post-colonisation had a significant effect on the likelihood that a toad would emerge from its shelter during trials (z = 2.27, n = 48, P = 0.023). Range-front toads were more likely to emerge from the shelter than were toads from range-core (z = 2.83, n = 32, P = 0.012; Fig. 2a) or intermediate populations (but the difference did not attain statistical significance: z = 2.19, n = 32, P = 0.072; Fig. 2a).
a Proportion of toads to emerge from a shelter during a risk-taking behavioural trial. b The latency to emerge from a shelter of cane toads from range-front, intermediate and range-core populations from across their Australian invasion range during a risk-taking behavioural trial. Different letters above columns indicate significant differences (P < 0.05) among populations after Tukey’s post hoc tests. Bars indicate ± standard errors
Latency to emerge
Years post-colonisation had a significant effect on the latency of toads to exit a shelter during emergence trials (F 2,36 = 6.00, P = 0.001). Toads from the range-edge population were quicker to emerge from the shelter than were conspecifics from the range-core (P = 0.011; Fig. 2b) or intermediate populations (P = 0.017; Fig. 2b). The day that toads experienced the trial also had an effect on a toad’s latency to emerge from a shelter (toads tested on day 2 were quicker to emerge than those that were trialled on day 1: F 2,36 = 9.14, P = 0.005). There was no significant interaction effect between years post-colonisation and day of trial on the latency of emergence (F 2,41 = 0.68, P = 0.51).
Exploratory behaviour
Time spent moving
The time a toad spent moving during exploration trials varied with years post-colonisation (F 2,36 = 8.26, P = 0.001). Toads from the range-front and the intermediate populations spent more time moving than did toads from the range-core population (range front vs. range core P = 0.002, intermediate vs. range core P = 0.011; Fig. 3a). Range-front and intermediate populations did not differ significantly in this respect (P = 0.90; Fig. 3a).
a Total time spent moving. b Rate of movement of cane toads from range-front, intermediate and range-core populations across their Australian invasion range during exploratory trials (total time spent moving data were log-transformed for analyses and have been back-transformed for graphs for visualisation purposes. Rate of movement measures were calculated from residuals of time spent moving and total distance moved during exploratory trials). Different letters above columns indicate significant differences (P < 0.05) among populations after Tukey’s post hoc tests. Bars indicate ± standard errors
Rate of movement
Years post-colonisation had a significant effect on the rate of toad movement during exploration trials (F 2,36 5.34, P = 0.009). Range-edge toads had a higher rate of movement than did range-core toads (P = 0.002; Fig. 3b). Toads from the intermediate population did not differ significantly from either the range-core or range-front populations in their rate of movement (range edge vs. intermediate P = 0.15, intermediate vs. range core P = 0.54; Fig. 3b).
Discussion
As predicted, cane toads from the range front were more exploratory and more willing to take risks (exhibited a bolder behavioural phenotype) in a novel environment than were conspecifics from range-core populations. The divergence we have documented in behavioural traits across the invasion range in cane toads may be due to either adaptive or non-adaptive processes, and may be either inherited or environmentally induced. The mechanisms underlying these processes are poorly understood. Traits such as high exploration and propensity to take risks might enable an individual either to disperse more rapidly (and hence be present at the expanding range front for that reason) or to thrive in the novel conditions encountered at the range front (Chapple et al. 2011). If lowered density of conspecifics reduces intraspecific competition for food at the range front and hence enhances feeding opportunities for fast-dispersing individuals [as occurs in cane toads (Brown et al.2013)], natural selection may favour such traits (Myles-Gonzalez et al. 2015). Conversely, more sedentary risk-averse behaviour may enhance individual fitness in range-core areas, where the location of resources is known and predators and parasites are more common (due to higher invader population densities; Wright et al. 2010). These scenarios suggest that behavioural divergence between range-core and range-front populations may be driven by natural selection, because different traits optimise fitness in the two situations.
Alternatively, behaviours that increase an individual’s rate of dispersal may be more common at the leading edge of an expanding population through non-adaptive processes such as spatial sorting (Shine et al. 2011). That is, only the fastest dispersers reach the expanding frontline of the invasion, where (inevitably) they interbreed with each other. Some of their progeny inherit genes for fast dispersal from both parents and thus disperse even more rapidly than their parents. This cumulative process can generate highly dispersive phenotypes at the range front, even in the absence of any fitness benefit to rapid dispersal (Phillips et al. 2006, 2008; Brown et al. 2007; Shine et al. 2011). Although studies on cane toads to date have emphasised the role of morphological and physiological traits in enhancing rates of dispersal (Phillips et al. 2006, 2007; Brown et al. 2013; LindstrÖm et al. 2013), behavioural phenotypes such as high exploration and risk-taking may be just as important in this respect (e.g. González-Bernal et al. 2014).
Whether or not the geographic variation in behavioural traits is adaptive, the mechanistic basis of that variation is also of interest. At one extreme, behavioural traits may be heritable (encoded by genes or epigenes: Drent et al. 2003; van Oers et al. 2004). At the other extreme, the variation may result from phenotypically plastic responses to environmental conditions in different parts of that range (Dall et al. 2004; Réale et al. 2010). For example, thermal and hydric regimes vary considerably from Queensland to Western Australia (see “Materials and methods”). Even at a proximate level, spatial variation in factors such as temperature, moisture, water quality or conspecific densities can substantially modify developmental trajectories of anuran amphibians (Indermaur et al. 2010; Ducatez et al. 2016). Variation in behavioural plasticity across the invasion-range warrants future research.
In summary, cane toads in Australia have rapidly evolved traits that enhance their rates of dispersal. Previous research has shown that toads at the range edge grow faster, have longer legs and more gracile bodies, move more often and for more prolonged periods than do toads from long-colonised areas; and these geographic divergences are also seen in progeny that have been raised in standardised conditions (Phillips et al. 2006, 2007, 2010; Brown et al. 2013, 2014; Hudson et al. 2016a). Here, we have shown that range-front toads also exhibit bolder, more dispersal-enhancing behavioural traits than do toads from range-core areas. To investigate if the behavioural variation found in this study is due to genetic divergence or to environmentally-induced (plastic) responses, future research could usefully repeat our studies on common-garden-raised offspring from across the invasion range (as has been used to calculate the heritability of physiological and morphological traits in cane toads: Llewellyn et al. 2010; Brown et al. 2013; Hudson et al. 2016b).
References
Aragon P, Clobert J, Massot M (2006a) Individual dispersal status influences space use of conspecific residents in the common lizard, Lacerta vivipara. Behav Ecol Sociobiol 60:430–438
Aragon P, Meylan S, Clobert J (2006b) Dispersal status-dependent response to the social environment in the common lizard, Lacerta vivipara. Funct Ecol 20:900–907
Bowcock H, Brown GP, Shine R (2008) Sexual communication in cane toads (Bufo marinus): what cues influence the duration of amplexus? Anim Behav 75:1571–1579
Brodin T, Drotz MK (2014) Individual variation in dispersal associated behavioural traits of the invasive Chinese mitten crab (Eriocheir sinensis, H. Milne Edwards, 1854) during initial invasion of Lake Vanern, Sweden. Curr Zool 60:410–416
Brown GP, Shilton C, Phillips BL, Shine R (2007) Invasion, stress, and spinal arthritis in cane toads. P Natl Acad Sci USA 104:698–700
Brown GP, Kelehear C, Shine R (2013) The early toad gets the worm: cane toads at an invasion front benefit from higher prey availability. J Anim Ecol 82:854–862
Brown GP, Phillips BL, Shine R (2014) The straight and narrow path: the evolution of straight-line dispersal at a cane-toad invasion front. Proc R Soc B 281:20141385
Chapman BB, Hulthén K, Blomqvist DR, Hansson L-A, Nilsson JÅ, Brodersen J, Nilsson PA, Skov C, Brönmark C (2011) To boldly go: individual differences in boldness influence migratory tendency in a cyprinid fish. Ecol Lett 14:871–876
Chapple DG, Simmonds SM, Wong BB (2011) Know when to run, know when to hide: can behavioural differences explain the divergent invasion success of two sympatric lizards? Ecol Evol 1:278–289
Chapple DG, Simmonds SM, Wong BBM (2012) Can behavioural and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64
Cote J, Clobert J (2007) Social personalities influence natal dispersal in a lizard. Proc R Soc Lond B 274:383–390
Cote J, Clobert J, Brodin T, Fogarty S, Sih A (2010a) Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos T Roy Soc B 365:4065–4076
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010b) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc R Soc Lond B 277:1571–1579
Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A (2011) Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proc R Soc Lond B 278:1670–1678
Dall SRX, Houston AI, McNamara JM (2004) The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett 7:734–739
Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ (2003) Natal dispersal and personalities in great tits (Parus major). Proc R Soc Lond B 270:741–747
Drent PJ, van Oers K, van Noordwijk AJ (2003) Realised heritability of personalities in the great tit (Parus major). Proc R Soc Lond B 270:45–51
Ducatez S, Crossland M, Shine R (2016) Differences in developmental strategies between long-settled and invasion-front populations of the cane toad in Australia. J Evol Biol 29:335–343
Duckworth RA (2006) Aggressive behaviour affects selection on morphology by influencing settlement patterns in a passerine bird. Proc R Soc Lond B 273:1789–1795
Duckworth RA (2008) Adaptive dispersal strategies and the dynamics of a range expansion. Am Nat 172:4–17
Duckworth RA, Badyaev AV (2007) Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. P Natl Acad Sci USA 104:15017–15022
Fisher KJ, Guilfoyle KJ, Hatch KA (2013) Stress induced by toe-clipping in cane toads (Rhinella marina). Copeia 2013:539–542
Fogarty S, Cote J, Sih A (2011) Social personality polymorphism and the spread of invasive species: a model. Am Nat 177:273–287
González-Bernal E, Brown GP, Shine R (2014) Invasive cane toads: social facilitation depends upon an individual’s personality. PLoS One 9:e102880
Groen M, Sopinka NM, Marentette JR, Fox MG, Reddon AR, Marsh-Rollo SE, Balshine S, Brownscombe JW (2012) Is there a role for aggression in round goby invasion fronts? Behaviour 149:685–703
Hudson CM, McCurry MR, Lundgren P, McHenry CR, Shine R (2016a) Constructing an invasion machine: the rapid evolution of a dispersal-enhancing phenotype during the cane toad invasion of Australia. PLoS One 19:e0156950
Hudson CM, Brown GP, Shine R (2016b) It’s lonely at the front: contrasting evolutionary trajectories in male and female invaders. Roy Soc open sci 3:160687
Indermaur L, Schmidt BR, Tockner K, Schaub M (2010) Spatial variation in abiotic and biotic factors in a floodplain determine anuran body size and growth rate at metamorphosis. Oecologia 163:637–649
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Lever C (2001) The cane toad. The history and ecology of a successful colonist. Westbury Academic and Scientific Publishing, Otley, West Yorkshire
Liebl AL, Martin LB (2012) Exploratory behaviour and stressor hyper-responsiveness facilitate range expansion of an introduced songbird. Proc R Soc Lond B 279:4375–4381
Liebl AL, Martin LB (2014) Living on the edge: range edge birds consume novel foods faster than established ones. Behav Ecol 25:1089–1096
Lindström T, Brown GP, Sisson SA, Phillips BL, Shine R (2013) Rapid shifts in dispersal behaviour on an expanding range edge. P Natl Acad Sci USA 110:13452–13456
Llewellyn J, Phillips BL, Alford RA, Schwarzkopf L, Shine R (2010) Locomotor performance in an invasive species: cane toads from the invasion front have greater endurance, but not speed, compared to conspecifics from a long-colonised area. Oecologia 162:343–348
McCann S, Greenlees MJ, Newell D, Shine R (2014) Rapid acclimation to cold allows the cane toad (Rhinella marina) to invade montane areas within its Australian range. Funct Ecol 28:1166–1174
Meylan S, de Fraipont M, Aragon P (2009) Are dispersal-dependent behavioural traits produced by phenotypic plasticity? J Exp Zool A 311:377–388
Monceau K, Moreau J, Poidatz J, Bonnard O, Thiéry D (2015) Behavioural syndrome in a native and an invasive hymenoptera species. Insect Sci 22:541–548
Myles-Gonzalez E, Burness G, Yavno S, Rooke A, Fox MG (2015) To boldly go where no goby has gone before: boldness, dispersal tendency, and metabolism at the invasion front. Behav Ecol 26:1083–1090
Phillips BL (2009) The evolution of growth rates on an expanding range edge. Biol Lett 5:802–804
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803
Phillips BL, Brown GP, Greenlees M, Webb JK, Shine R (2007) Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral Ecol 32:169–176
Phillips BL, Brown GP, Travis JMJ, Shine R (2008) Reid’s paradox revisited: the evolution of dispersal kernels during range expansion. Am Nat 172:34–48
Phillips BL, Brown GP, Shine R (2010) Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. J Evol Biol 23:2595–2601
Réale D, Dingemanse NJ, Kazem AJN, Wright J (2010) Evolutionary and ecological approaches to the study of personality. Philos T Roy Soc B 365:3937–3946
Rehage JS, Sih A (2004) Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol Invasions 6:379–391
Riesch R, Duwe V, Herrmann N, Padur L, Ramm A, Scharnweber K, Schulte M, Schulz-Mirbach ZM, Plath M (2009) Variation along the shy-bold continuum in extremophile fishes (Poecilia Mexicana, Poecilia sulphuraria). Behav Ecol Sociobiol 63:1515–1526
Rollins LA, Richardson MF, Shine R (2015) A genetic perspective on rapid evolution in cane toads (Rhinella marina). Mol Ecol 24:2264–2276
Shine R, Brown GP, Phillips BL (2011) An evolutionary process that assembles phenotypes through space rather than through time. P Natl Acad Sci USA 108:5708–5711
Sih A, Bell A, Johnson JC (2004) Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Simmons AD, Thomas CD (2004) Changes in dispersal during species’ range expansions. Am Nat 164:378–395
van Oers K, Drent PJ, de Jong G, van Noordwijk AJ (2004) Additive and nonadditive genetic variation in avian personality traits. Heredity 93:496–503
Verbeek MEM, Drent PJ, Wiepkema PR (1994) Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav 48:1113–1121
Wilson DS, Coleman K, Clark AB, Biederman L (1993) Shy-bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J Comp Psychol 107:250–260
Wilson DS, Clark AB, Coleman K, Dearstyne T (1994) Shyness and boldness in humans and other animals. Trends Ecol Evol 9:442–445
Wright TF, Eberhard JR, Hobson E, Avery ML, Russello M (2010) Behavioural flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 22:393–404
Zug GR, Zug PB (1979) The marine toad Bufo marinus: a natural history resumé of native populations. Smithson Contrib Zool 284:1–58
Acknowledgements
We thank Cameron Hudson and Damian Holden for their assistance with toad collection, Simon Ducatez and Jayna Devore for help with analyses, and Lorene Chieze for assistance with trials and husbandry. We also thank two anonymous reviewers for their valuable comments. This work was funded by the Australian Research Council (FL120100074).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. Ethical approval for this study was granted by the Macquarie University Animal Ethics Committee under protocol number: ARA2013/035 and the University of Sydney Animal Ethics Committee under animal ethics project number: 2013/5805. All procedures performed in studies involving animals were in accordance with the ethical standards of the institutions under which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Communicated by K. Summers
Rights and permissions
About this article
Cite this article
Gruber, J., Brown, G., Whiting, M.J. et al. Geographic divergence in dispersal-related behaviour in cane toads from range-front versus range-core populations in Australia. Behav Ecol Sociobiol 71, 38 (2017). https://doi.org/10.1007/s00265-017-2266-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2266-8