Abstract
The geographical variation in animals’ social acoustical signals can reduce recognition across populations and may thus promote assortative mating, reproductive isolation and speciation. However, the social consequences of geographical variation in acoustical signals serving an ecological function are poorly known. Bat echolocation calls are considered to have a dual function; they are used not only for orientation and foraging but also for communication. In this study, we studied the behavioural response of Rhinolophus ferrumequinum to geographical variation in echolocation calls. Using habituation-dishabituation playback experiments, we found that all tested bats from northeast China exhibited obvious responses after switching playback from their own population to those from central east China and southwest China. Using two-choice playback experiments, we showed that the bats from northeast China responded more strongly to echolocation calls from their own population than to those from central east China. Our results demonstrate that Rhinolophus ferrumequinum is able to discriminate between echolocation calls of its local population and a foreign one and that local echolocation calls are preferred over foreign ones in a two-choice context. This study supports the communicative potential of bat echolocation calls and provides insight into the discrimination ability and behavioural preference of bats with respect to geographical variation in echolocation calls.
Significance statement
This study provides behavioural evidence for the communicative role in echolocation calls and indicates that a dual function of echolocation calls exists more commonly in bats. Moreover, this study provides insight into the discrimination ability and preference of bats with respect to frequency variation in echolocation calls and contributes to a better understanding of how geographical evolution in ecological acoustical signals may affect the ability of recognition between populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustical signals serve important social and ecological functions in many animals; evolutionary divergence in acoustical signals may thus have significant social and ecological consequences (Fenton 1974; Wilkins et al. 2013). Acoustical signals exhibit large degrees of spatial variation in a wide range of vertebrate taxa, such as insects (Zuk et al. 2001), frogs (Velásquez 2014), birds (Krebs and Kroodsma 1980), and mammals (Lameira et al. 2010). One known consequence of acoustical geographical variation is that it reduces the ability of populations to recognize each other, which can lead to assortative mating, reproductive isolation and speciation (Baker and Cunningham 1985; Slabbekoorn and Smith 2002; Price 2008; Wilkins et al. 2013). This effect has been frequently tested and observed for signals during social interactions, such as birdsongs used for territorial defence and mate attraction (Baker and Mewaldt 1978; Irwin et al. 2001; Lachlan and Servedio 2004; Gordinho et al. 2015).
Bats, dolphins and some other animals use acoustical signals for important ecological functions like orientation and foraging (Schevill 1956; Griffin 1958; Gould et al. 1964). Their acoustical signals vary geographically as well (Jones and Sayigh 2002; Jiang et al. 2015). However, the consequences of geographical variation in these types of signals remain largely unknown. If the acoustical geographical variation is adaptive, it may be advantageous for an animal to mate with individuals with similar acoustical signals but not those with different types of signals. Therefore, geographical variation in ecological acoustical signals may also have crucial consequences for patterns of dispersal and gene flow.
Of fundamental importance in determining the consequences of geographical variation in acoustical signals are the questions of whether the animals themselves can distinguish amongst the differences and whether the animals prefer local signals (Milligan and Verner 1971). One approach to these questions is to perform playback experiments to observe the behavioural responses of the individuals of one population to the recorded sounds of their own and of foreign populations. Using playback experiments, most studies have found that individuals respond more strongly to local than to foreign acoustical stimuli (Baker et al. 1982; Searcy et al. 2002; Gray 2005; Boul et al. 2007; Podos 2007; Uy et al. 2009; Bradley et al. 2013; Mortega et al. 2014), although some studies have observed opposite results (Baker 1982; Balaban 1988) and some have found asymmetric discrimination amongst populations (Colbeck et al. 2010; Dingle et al. 2010). Nevertheless, research has been largely limited to social acoustical signals. The ability of animals to discriminate amongst geographical differences in ecological acoustical signals remains poorly understood.
Echolocating bats represent an ideal model taxon to study the social consequences of geographical variation in ecological acoustical signals. They produce echolocation calls for environmental perception and prey detection. The features of echolocation calls are related to their ecological niche and are commonly considered to have been shaped by natural selection (Schnitzler et al. 2003; Jones and Holderied 2007). Recently, a growing body of evidence shows that bat echolocation calls play a role in communication and social recognition (e.g. Jones and Siemers 2011; Schuchmann et al. 2012; Bastian and Jacobs 2015; Grilliot et al. 2015), as echolocation pulses do in fact offer sufficient information to allow bats to identify individuals within and between species (Parsons and Jones 2000; Yovel et al. 2009). Some bats can discriminate between echolocation calls of their own species and those of sympatric congeneric species (Schuchmann and Siemers 2010; Li et al. 2014), and some are able to locate conspecifics and heterospecifics by eavesdropping on echolocation pulses (e.g. Barclay 1982; Ruczyński et al. 2007). Echolocation calls clearly serve both ecological and social functions in some bat species.
Two case studies on Rhinolophus mehelyi and Eptesicus fuscus suggest that echolocation calls may be important in a mating context (Puechmaille et al. 2014; Grilliot et al. 2015). Echolocation calls are considered magic traits that have a pleiotropic effect on reproductive isolation via assortative mating (Kingston and Rossiter 2004; Wilkins et al. 2013). It seems reasonable to expect that geographical variation in echolocation calls may lead to assortative mating if the variation affects recognition between acoustically divergent populations. To date, a rapidly mounting number of studies have shown that geographical variation in bat echolocation calls is common, with a mean variation of peak frequency of 5–10 kHz (Lameira et al. 2010; Jiang et al. 2015; Lin et al. 2015). The patterns and causes of the variation have been frequently studied, but the consequences of the evolutionary process remain poorly understood (Puechmaille et al. 2011). Specifically, it is largely unknown whether bats can discriminate between population differences in echolocation calls (Bastian and Jacobs 2015) and whether they prefer local over non-local calls.
In this study, we tested echolocation-call discrimination by the greater horseshoe bat Rhinolophus ferrumequinum (Schreber, 1774). This species typically produce long constant-frequency (CF) echolocation pulses initiated by an upwards frequency modulation (FM) component and followed by a downwards FM component, with the most energy normally being present in the CF component of the second harmonic of the pulses. In China, this species is distributed widely, from the northeast to the southwest. There is significant divergence in both phylogeny and echolocation vocalizations amongst populations in northeast China, central east China, and southwest China (Flanders et al. 2011; Sun et al. 2013). Peak frequencies of echolocation calls vary significantly across populations, with the maximum variation of about 8 kHz, which probably resulted mainly from adaptation to local temperature and from cultural drift (Sun et al. 2013). In the present study, we tested whether the greater horseshoe bats could discriminate between echolocation calls of their own population and those of a foreign population and whether the bats would respond differentially to local and foreign echolocation calls.
Methods
Collection and husbandry of bats
Adult Rhinolophus ferrumequinum were collected in a cave in Panshi County, Changchun (CC) City, Jilin Province, northeast China, on June 15, 2015 (Fig. 1a). We captured bats at the entrance of the cave with a mist net after sunset and determined their sex and reproductive status based on external morphological characteristics (Racey 2009). A total of 25 adults (14 males and 11 females) were collected for sound recording, and 16 (8 males and 8 non-pregnant females) of them were used for playback experiments.
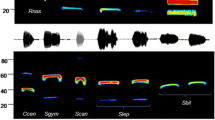
Map of sampling localities (a) and frequency distribution of the constant frequency (CF) components of echolocation calls (b) of Rhinolophus ferrumequinum from Changchun (CC), Jian (JA), Jinan (JN) and Tianshui (TS) in China. The grey parts in the map depict three acoustically divergent groups of Rhinolophus ferrumequinum in China (referred to Sun et al. 2013). The dashed lines in a represent Qinling Mountains. The inset in b is the spectrogram of an echolocation pulse of R. ferrumequinum
The bats captured for playback experiments were transferred to and housed in a husbandry room (6.5 m long × 5.5 m wide × 2.1 m high). A 12-h light/dark cycle (light: 0600–1800 h; dark: 1800–0600 h) was maintained using an astronomical light timer. Temperature and relative humidity were controlled around 23 °C and 60 %, respectively. We decorated the room with numerous artificial plants randomly hung from the ceiling or attached to the walls to enrich the environment. Bats were maintained in a cage (1.5 m long × 1.0 m wide × 0.8 m high) in the room for the first week. During this period, bats were artificially fed with water and mealworms until they could feed themselves. The bats were then released from the cage and maintained in the room where they could fly freely. They were given ad libitum access to water and mealworms, and their diet was enriched with vitamin and mineral supplements.
Sound recording and generation of playback files
We recorded echolocation calls emitted in the resting state of Rhinolophus ferrumequinum from CC (N = 25), Jian (JA; N = 42), Jinan (JN; N = 17) and Tianshui (TS; N = 20) (Fig. 1a). Bats were taken into a temporary laboratory (5 m long × 4 m wide × 2.5 m high) near the roosts where they were captured. Resting echolocation calls for each individual were recorded using a condenser ultrasound microphone (UltraSoundGate CM16/CMPA, Avisoft Bioacoustics, Berlin, Germany), which was positioned at a distance of about 1 m from the bat. The microphone was connected to an ultrasound recording system (UltraSoundGate 116, Avisoft Bioacoustics, Berlin, Germany), with a sample rate of 375 kHz at 16 bits/sample. We obtained several hundred high-quality calls (signal-to-noise rate > 30 dB) for each individual.
We created playback files by following the methods of Schuchmann and Siemers (2010) and Li et al. (2014). We assembled five playback files, each 30 s long, for each population using Avisoft-SASLab Pro 5.2. The files were created by randomly mixing calls from six different individuals (three males and three females) belonging to the same population; at least three of the six individuals were different between any two files. For each bat, call series were divided into shorter segments with durations ranging from 100 to 1000 ms, which were then randomly selected to combine with those of other individuals to create a playback file. Each file contained at least 20 echolocation calls for each of the 6 individuals. The ratio of the number of male and female calls in each playback file is approximately 1 to 1 (Table S1, Online Resource 1). These methods induce bats to classify the playback files by population, not by individual or sex. We did not standardize the length of the intervals between echolocation calls. The call rate in each playback file was approximately eight calls per second. We normalized the files so that the peak amplitude of the weakest call was around −30 dB. Each playback file was resampled to 375 kHz and high-passed at 30 kHz.
Choice of playback methods
Habituation-dishabituation and two-choice playback experiments are both widely used to examine acoustical discrimination in animals (Murphy and Gerhardt 2000; Schuchmann and Siemers 2010; Li et al. 2014; Puechmaille et al. 2014; Bastian and Jacobs 2015; Höbel 2015). Based on prior experiments in this study, we found that the two-choice method is more appropriate for examining the response differences of a bat to two different stimuli. However, when two-choice experiments are repeated more than twice for an individual, behavioural responses in subsequent experiments often decrease, probably because of habituation to playback conditions (playback stimuli and/or ambience). These decreased responses may lead to a biased conclusion of response differences to different stimuli. By comparison, the habituation-dishabituation method is more suitable to determine whether a bat can distinguish between two different stimuli when each individual must be tested many times. If an individual resumes any behavioural activity after switching from a habituation stimulus to a dishabituation stimulus, it is considered evidence of discrimination between the two stimuli. Therefore, even if the tested individual shows a decreased response in subsequent experiments, discrimination can still be concluded as long as the individual responds after switching signals.
In this study, we performed habituation-dishabituation playback experiments to test the prediction that the greater horseshoe bats are able to distinguish between differences in echolocation calls from different populations. We examined whether bats from CC could distinguish echolocation calls of their own population from those of JA, JN and TS. We carried out two-choice playback experiments to address the question of whether the bats respond differentially to local and foreign echolocation calls. We examined whether bats from CC would respond differentially to echolocation calls of their own population and those of JN. We did not present playback files of CC-JA and CC-TS combinations. One reason is that our purpose was not to examine variation in acoustical discrimination across populations. Another is that the bats normally decrease responses when tested more than twice, even if the interval between the two tests is more than a week. We first performed the two-choice experiments with each bat only once to make sure that the reactions of the bats were not affected by habituation to playback conditions (see the “Two-choice experiments” section for more details). To minimize observer bias, a blinded method was used so that the person who counted the bats’ behavioural responses had no knowledge of their treatment or identity.
Two-choice experiments
Two-choice experiments were conducted in an iron wire mesh cage with a mesh size of 1.0 cm2 (Fig. 2a). The cage was sufficient for the greater horseshoe bat to fly in given its high manoeuvrability. The cage was lined outside with sound-absorbing foam. A foam board was placed in the middle of the cage, dividing the cage into three compartments (A, B and C). Speakers (Ultrasonic Dynamic Speaker Vifa, Avisoft Bioacoustics, Berlin, Germany) were positioned on the walls of compartments A and B, and each speaker was connected to an ultrasound playback interface (UltraSoundGate player 116, Avisoft Bioacoustics, Berlin, Germany). Each tested bat was placed hanging at an end of the ceiling of compartment C and was monitored via two infrared cameras (Sony HDR PJ760E, Sony, Tokyo, Japan). Camera 1 could monitor the overall behaviour of the bat, and camera 2 could accurately determine the time when the bat flew into and out of compartments A and B.
To ensure that the reactions of the bats were spontaneous and to avoid pseudoreplication, we only presented playback files of CC-JN combinations (including 25 unique combinations) and each bat was only tested once with a random selected unique combination. Two bats were tested twice because they exhibited low-frequency response in the first trial (see the “Result” section). The assignment of CC or JN file to the speaker in compartment A or B was randomized prior to each individual’s testing. After a bat was placed hanging in the listening area in compartment C, we simultaneously played CC and JN signals with an amplitude of around 60 dB SPL (minimum 51.74 dB; maximum 65.55 dB; 95 % confidence interval for mean 59.80–60.02 dB) at the site where the bat was hanging. We recorded and counted the behavioural responses in each playback compartment for each bat until the bat showed no interest to the stimuli. We defined a bat showing no interest to the stimuli when the bat kept on grooming or kept silent for more than 45 s. This time boundary was established by pooling the frequency distributions of intervals between any two consecutive flights regardless of individuals. For each individual, we pooled all intervals between any two consecutive flights observed before the bat kept on grooming or kept silent for more than 2 min. A total of 423 intervals were pooled. The numbers of observations decreased obviously for intervals with duration of >45 s (Fig. S1, Online Resource 1). We used four response variables to measure the behaviour of the bats towards either local or foreign echolocation calls: number of flights (occurrence, count data), time spent in flight (duration), number of landings (occurrence, count data) and time spent in detection (duration) in each playback compartment (A or B). The time spent in detection refers to the time a bat spent in suspension and/or movement on the wire mesh in compartment A or B.
Habituation-dishabituation experiments
Habituation-dishabituation experiments were conducted in a smaller iron wire mesh cage (Fig. 2b) in a sound recording room (5 m long × 2 m wide × 2.7 m high). The walls and ceiling of the recording room were covered with sound-absorbing foam. Each bat was positioned hanging in the middle of the cage and was monitored via an infrared camera positioned on a shorter wall of the cage. An ultrasonic loudspeaker (Ultrasonic Dynamic Speaker Vifa) and an ultrasonic microphone (UltraSoundGate CM16/CMPA) were placed 0.60 m from the longer wall of the cage and directed towards the tested bat. The speaker was connected to an ultrasound playback interface (UltraSoundGate player 116), and the microphone was connected to an ultrasound recording system (UltraSoundGate 116).
Once a bat was placed in the cage, we began to playback an assembled file of its own population (CC), with an amplitude of around 60 dB SPL (minimum 51.74 dB; maximum 65.55 dB; 95 % confidence interval for mean 59.64–59.96 dB) at the site where the bat was hanging, in an infinite loop until the bat habituated. We defined habituation as the bat becomes motionless, with no body or head movements, no crawling activity, no stretching of wings or legs, and no echolocation for 30 s. After the bat remained habituated for 30 s, we switched the habituation file to another file of CC which differs from the habituation file (from CC to CC’) or to a file of other populations (from CC to JA, JN or TS). Each bat was presented with four different types of combinations of habituation and test file (CC-CC’, CC-JA, CC-JN and CC-TS) and was tested randomly with one combination per day. The CC-CC’ combination was used as a control (including 20 unique combinations) and other combinations (CC-JA, CC-JN and CC-TS; each including 25 unique combinations) were used to test discrimination of non-local calls. To avoid pseudoreplication, each individual was tested with a unique combination for each of the four types. A total of 64 unique combinations of habituation and test signals were used for the 16 tested bats.
Generally, the tested bats responded to the signals within 5 s and the responses decreased dramatically for periods longer than 60 s after signal changed. We scored the bats’ behaviour for 60 s after changing signals. If a bat showed any behavioural activity (body or leg movement, nodding, wing stretching, or echolocation emitting), we took this as evidence that the bat had discriminated between the two playback signals. If a bat remained habituated, we considered this as indication that the bat regarded the changing signal as belonging to the same class as the habituation signal (Schuchmann and Siemers 2010; Li et al. 2014). We defined the behaviour of bats as those described by Li et al. (2014) except that each movement of the head and the body was counted as one behavioural response. For each playback combination, less than four individuals (<25 %) showed wing stretching or leg movement. Therefore, we removed these two behaviours in the final analysis to minimize the effects of individual variation.
We did not use a control stimulus at the end of the test stimulus to control for false negatives, i.e. for failure of a bat to react to a test stimulus because of sensory/experimental fatigue. In prior trials, we played habituation stimulus to three bats for 15, 20 and 25 min, respectively, and then switched to test stimuli. All bats reacted to the test stimuli. These results suggest that a playback with duration of about 20 min should not lead to sensory/experimental fatigue in this species. In fact, the duration of playback in most of the trials with negative response is shorter than 20 min (Table S2, Online Resource 1) and is not significantly different from that of playback with positive response (Mean 15.73 versus 17.76 min; Mann-Whitney U test: U = 378, N 1 = 26, N 2 = 38, P = 0.112). We are convinced that this lack of motivational control at the end of the test stimulus does not change our conclusion.
Statistical analysis
For two-choice experiments, all response measures were x + 1 transformed before statistical analysis because some of the measures had values of zero. We first examined the differences in each of the four behavioural responses between the two playback treatments (CC and JN) using Mann-Whitney U tests. We then performed a principal component analysis that included the four response parameters and used the Mann-Whitney U test to evaluate differences in principal component (PC) scores between playback treatments (CC and JN) and between playback compartments (compartments A and B). We also performed a paired samples Student’s t test to determine whether the average difference between PC scores for local (CC) and foreign (JN) echolocation calls is significantly different from zero for the bat group. We considered a significant difference as an indication of acoustical discrimination. Differences significantly greater than zero indicated a stronger response to local calls, whereas differences significantly less than zero indicated a stronger response to foreign calls.
For habituation-dishabituation experiments, we used Pearson chi-square tests to determine whether the proportion of bats showing any response after switching signals differed significantly amongst the four different types of playback combinations (CC-CC’, CC- JA, CC-JN and CC-TS). We used the Fisher exact test for post hoc pairwise comparisons if significant differences were found. Moreover, we used ANOVA tests to compare the counts of body movement and head nodding amongst different types of test combinations and the Kruskal-Wallis H test to compare the counts of echolocation emitting.
All statistical analyses were performed using SPSS v22.0 (IBM SPSS Statistics for Windows, IBM Corporation, Armonk, NY, USA). The normality of the data distribution was tested using the Kolmogorov-Smirnov (K-S) test, and the homogeneity of the data was tested using Levene homogeneity of variance test prior to statistical analysis. The distribution of differences between paired PC scores is normal (K-S Z = 0.42; P = 0.994). The counts of body movement and head nodding are normally distributed within each compared group (all K-S Z > 0.40; all P > 0.630), and there was homogeneity of variance amongst the compared groups (both P > 0.610).
Results
The peak frequencies of echolocation pulses of CC (mean ± standard deviation 68.96 ± 0.33 kHz, N = 25), JA (68.78 ± 0.38 kHz, N = 42), JN (76.89 ± 0.38 kHz, N = 17) and TS (73.52 ± 0.73 kHz, N = 20) displayed a trimodal distribution (Fig. 1b). There were significant differences in the peak frequencies amongst the four populations (Kruskal-Wallis test: H 3 = 75.84, P < 0.001). Results of pairwise comparisons showed a non-significant difference between CC and JA (Mann-Whitney U test: U = 376, N 1 = 25, N 2 = 42, P = 0.053) but a significant difference for all of the remaining comparisons (CC vs JN, CC vs TS, JA vs JN, JA vs TS, JN vs TS; Mann-Whitney U test: all P < 0.001).
Two-choice experiments
When echolocation stimuli were played, the tested bats immediately emitted echolocation calls and flew towards the speakers on average 3 min later (mean ± standard deviation 185 ± 179 s, ranging from 1 to 503 s, N = 16). Five individuals flew towards the speakers less than 3 s after the initiation of playback. One bat (bat D) flew quickly towards and landed in the local call compartment and never flew out. Another bat (bat B) flew towards the two compartments (A and B) each once and then finally landed in the local call compartment. Similar results with fewer than three flights in each playback compartment were also found in the repeated trails for these two bats. We used the results of the first trial for the statistical analyses. The other bats generally flew and landed back and forth between compartments A and B in the earlier stage. Most of them then flew and landed in the local call compartment more often (Online Resource 2). These bats sometimes flew over the speaker broadcasting local calls and landed on and crawled around the wire mesh near the speaker.
Thirteen bats spent more time in flight in local call compartments compared to foreign call compartments, 12 of which flew and landed more often and spent more time in suspension and/or movement in the local than in the foreign call compartments (Fig. 3; Table S3, Online Resource 1). Overall, there was a significant difference in number of landings (Mann-Whitney U test: U = 75.50, N 1 = N 2 = 16, P = 0.046), time spent in flight (Mann-Whitney U test: U = 75.50, N 1 = N 2 = 16, P = 0.048) and time spent in detection (Mann-Whitney U test: U = 51.50, N 1 = N 2 = 16, P = 0.004), but there was a non-significant difference in number of flights (Mann-Whitney U test: U = 83.00, N 1 = N 2 = 16, P = 0.089) between local and foreign call compartments (Table 1).
Results of two-choice experiments for Rhinolophus ferrumequinum. Number of flights (a), time spent in flight (b; unit: second), number of landings (c) and time spent in detection (d; unit: second) are shown for each bat in local (CC) and foreign (JN) call compartments during playback. Each circle indicates a bat with a capital letter as bat label. Scatterplots in lower right depict the overall distribution of behaviours in the two compartments. Lines connect the paired data points from the same individual. Statistically significant levels based on Mann-Whitney U test: * P < 0.05; ** P < 0.01
The first principal component explains 80.99 % of the variance in the data for the four response variables. All response variables were positive for PC1 (Table 2). Higher PC1 scores indicate higher levels of response to a playback type. The PC1 scores did not differ significantly between compartments A and B (Mann-Whitney U test: U = 110.00, N 1 = N 2 = 16, P = 0.498), indicating that the side of the cage playing local calls did not have an effect on the results of the tests. In contrast, there were statistically significant differences in the PC1 scores between playback treatments (Mann-Whitney U test: U = 57.00, N 1 = N 2 = 16, P = 0.007), suggesting that the bats respond differentially to playback of local and foreign echolocation calls. The results of the paired samples Student’s t tests suggested that bats responded more strongly to local than to foreign echolocation calls (t 15 = 4.56, P < 0.001).
Habituation-dishabituation experiments
All bats showed a response to the habituation onset. In control trials, 15 of 16 bats remained habituated after changing the playback files from CC to CC’. Five of the 16 bats resumed body and nodding movement and echolocation activity when tested with JA files. All 16 bats exhibited obvious responses after switching playback from CC to JN and from CC to TS (Fig. 4a; Online Resource 3). Amongst the four different types of combinations, the proportion of responding bats differed significantly (Pearson chi-square test: χ 2 = 45.86, df = 3, P < 0.001). The proportion of responding bats did not differ significantly between CC-CC’ and CC-JA combinations (Fisher exact test: P = 0.172). In comparison, the proportion of responding bats in both CC-CC’ and CC-JA combinations differed significantly from that in CC-TS and that in CC-JN combinations (Fisher exact test: all P < 0.001). We thus concluded that the greater horseshoe bats from CC could distinguish echolocation calls of TS and JN from those of their own population, and that a small portion of the bats could distinguish echolocation calls of JA from those of their own population. There was no significant difference in the numbers of either nodding (ANOVA: F 2, 34 = 0.35, P = 0.709; Fig. 4b), body movement (ANOVA: F 2, 34 = 0.27, P = 0.765; Fig. 4c), or echolocation calls (Kruskal-Wallis test: H 2 = 1.40, P = 0.496; Fig. 4d) amongst the CC-JA, CC-JN and CC-TS combinations.
Results of habituation-dishabituation experiments for Rhinolophus ferrumequinum. a Number and percentage of responding bats for each playback combination. b–d Boxplots of number of nodding, body movement, and echolocation calls of responding bats for each playback combination. Plots include the median, interquartile range and full range. Abbreviations for playback stimuli: CC Changchun, JA Jian, TS Tianshui, JN Jinan
Discussion
In this study, we found that all tested Rhinolophus ferrumequinum exhibited obvious responses after switching playback from their own population to those from allopatric populations in the habituation-dishabituation experiments. Moreover, we found that Rhinolophus ferrumequinum responded more strongly to local than to foreign echolocation calls in the two-choice experiments. These results suggest that Rhinolophus ferrumequinum is able to discriminate between echolocation calls of its local population and a foreign one and that local echolocation calls are preferred over foreign ones in a two-choice context.
The communicative role in echolocation calls
Bat echolocation calls are suggested to serve a dual function; they are used not only for orientation and object detection but also for communication (Fenton 1985, 2003; Jones and Siemers 2011). Bats can recognize the individual identity (Kazial et al. 2008; Yovel et al. 2009), gender (Kazial and Masters 2004; Schuchmann et al. 2012), group membership (Voigt-Heucke et al. 2010) and species identity (Schuchmann and Siemers 2010; Li et al. 2014; Bastian and Jacobs 2015) of the calling bat from echolocation calls. The social functions of echolocation calls have been reported in some bat species, such as Eptesicus fuscus (Kazial and Masters 2004; Grilliot et al. 2014, 2015), Noctilio albiventris (Voigt-Heucke et al. 2010), Nyctalus noctula (Ruczyński et al. 2007), Saccopteryx bilineata (Knörnschild et al. 2012) and several species of Myotis (Ruczyński et al. 2009; Yovel et al. 2009) and Rhinolophus (Schuchmann and Siemers 2010; Schuchmann et al. 2012; Li et al. 2014; Puechmaille et al. 2014; Bastian and Jacobs 2015), but remain far unexplored compared with the rich biodiversity of bats. In the present study, we provide behavioural evidence for the communicative potential of echolocation calls in Rhinolophus ferrumequinum. Our data indicate that echolocation calls may commonly serve a dual function in bats, especially in Rhinolophidae, a family probably capable of vocal production learning (Knörnschild 2014).
Bat echolocation calls often exhibit geographical variation (Lameira et al. 2010; Jiang et al. 2015; Lin et al. 2015), but it had been poorly known whether bats could discriminate and would respond differently to these call differences. A recent study showed that a horseshoe bat, Rhinolophus capensis, echolocating at 75 kHz in peak frequency is able to discriminate different phonetic populations echolocating at 85 and 86 kHz in habituation-dishabituation experiments (Bastian and Jacobs 2015). Our results confirmed that another horseshoe bat, Rhinolophus ferrumequinum, is capable of discriminating differences in peak frequency of echolocation calls from different populations, though it does not necessarily mean this species can differentiate the geographical origin of the individuals. Moreover, our study showed that Rhinolophus ferrumequinum reacted more strongly to local than to foreign echolocation calls in a two-choice paradigm. In the lesser bulldog bats, Noctilio albiventris, echolocation calls from unfamiliar social groups induced stronger behavioural responses compared to calls from familiar group (<20 km amongst groups) (Voigt-Heucke et al. 2010). This earlier finding together with our results suggest that echolocation calls of unfamiliar bats can elicit a greater response than echolocation calls of familiar bats and, on a population level, that local unfamiliar calls can elicit a greater response than foreign unfamiliar calls.
Preference for local echolocation calls
We found that Rhinolophus ferrumequinum responded more strongly to echolocation calls from their own population than to those from the foreign population in a two-choice context. This finding is in accord with those of most studies on discrimination between local and non-local acoustical signals (Baker et al. 1982; Searcy et al. 2002; Podos 2007; Bradley et al. 2013; Mortega et al. 2014). Generally, there are at least two possible explanations for stronger reactions to local than to foreign signals: (1) individuals preferentially select as mates the ones who produce local signals, which are considered to be adapted to local environmental conditions (Baker and Cunningham 1985), or, conversely, (2) individuals with local signals may represent a greater relative threat to resources to elicit a stronger response from signal receivers (McArthur 1986; Rothstein and Fleischer 1987).
The first explanation seems more likely to explain the observations in our study. Firstly, in the present study, Rhinolophus ferrumequinum often crawled around the speakers playing local stimuli, displaying a friendly behaviour but differing from the aggressive behaviours (e.g. a succession of displays and aggressive vocalizations) usually observed in bats (Bohn et al. 2008; Fernandez et al. 2014). Secondly, it has been reported that male Rhinolophus ferrumequinum produce echolocation calls before copulation, indicating that the calls may serve for contacting females (Liu et al. 2013). Similarly, female Rhinolophus mehelyi select males based on echolocation calls during the mating season (Puechmaille et al. 2014), and mate selection of male Eptesicus fuscus is influenced by echolocation calls of females (Grilliot et al. 2015). These data suggest that echolocation calls may be important in a mating context. Echolocation calls are considered magic traits insofar as divergence in the traits can result in assortative mating and speciation (Kingston and Rossiter 2004; Wilkins et al. 2013). Taken together, stronger reactions to local than to foreign echolocation calls observed in Rhinolophus ferrumequinum probably indicate preferential responses to local echolocation calls. Nevertheless, the preference to local calls may not necessarily regard mate choice. The Rhinolophus ferrumequinum are social animals therefore they could prefer the signals of their own population, possibly as they simply like to stay close to their known roost members. Our results cannot exclude the possibility that the stronger reactions may indicate a greater relative threat of local calls, but if that was the case, Rhinolophus ferrumequinum may always exhibit aggressive responses to each other since they are highly gregarious and frequently emit echolocation calls for orientation.
Sensory bias may be another possible explanation for the stronger reactions to local than to foreign signals in Rhinolophus ferrumequinum. It is well known that CF bats (rhinolophids, hipposiderids and moustached bats) have an acoustical fovea in the cochlea (Suga and Jen 1976; Schuller and Pollak 1979; Bruns and Schmieszek 1980). The bat’s hearing is most sensitive within a narrow frequency range, such as 1.5 kHz (83.0–84.5 kHz) in Rhinolophus ferrumequinum that have resting frequency ranging from 83.5 to 83.8 kHz (Schuller and Pollak 1979). Hence, they can hear best and may respond preferentially to individuals with similar peak frequency to their own echolocation pulses. However, this explanation needs to be further examined. Firstly, the resting frequency in each CF bat can change. For example, in Hipposideros terasensis, the resting frequency (approximately 70 kHz) has been found to vary up to 4.81 kHz over a 4-year observation period (Hiryu et al. 2006). In Rhinolophus ferrumequinum, echolocation calls can change over a lifetime as well (Jones and Ransome 1993). The auditory system of CF bats seems to have the appropriate adaptations to accommodate the frequency changes. Thus, it remains to be determined if the Rhinolophus ferrumequinum studied here can quickly adapt to the frequency differences of 4.5–8.0 kHz between playback stimuli. Secondly, it needs to be determined whether sensory bias is necessary to lead to differences in behavioural response. Li and colleagues have shown that, amongst the four CF bat species (three Rhinolophus and one Asellisus), call design has only a minor effect on the behavioural responses to heterospecific echolocation calls with frequency differences of 10–70 kHz. The responses may instead be related to the degree of interspecific food competition (Li et al. 2014). This suggests that sensory bias with respect to frequency difference in echolocation calls may not necessarily have a significant impact on behavioural response.
Implications for social interaction
In many species, such as songbirds, acoustical signals play an important role in maintaining reproductive cohesion amongst populations of the same species (Brown and Farabaugh 1991; Ptacek 2000; Marler 2004). Geographical variation in acoustical signals may challenge the ability of individuals to recognize sounds from different populations (Irwin et al. 2001; Searcy et al. 2002; Podos 2007; Colbeck et al. 2010). Acoustical discrimination amongst populations revealed in playback experiments has thus been cited as evidence of reproductive isolation (Balakrishnan and Sorenson 2006; Danner et al. 2011).
In the present study, we found that Rhinolophus ferrumequinum preferred local over foreign echolocation calls in a two-choice context. It is uncertain whether this finding implies social discrimination and assortative mating between these acoustically divergent populations because their distribution ranges do not overlap anyway. Nevertheless, our finding may provide insight into the social interaction between populations across acoustical boundaries.
The contemporary geographical distribution patterns of Rhinolophus ferrumequinum in China are considered to be a result of postglacial expansion from multiple glacial refugia at least 15–23 thousand years ago (Flanders et al. 2009, 2011). Flanders and colleagues used mitochondrial sequences to show that the populations originating in central east China and southwest China have come into contact in two localities (E110° 25′, N32° 58′; E110° 09′, N 32° 25′) on the acoustical boundary (Flanders et al. 2011). Interestingly, analysis of nuclear microsatellite loci has shown that within each colony, individuals of different origins are genetically divergent. Similar results have been found in Rhinolophus ferrumequinum from two geographically proximal caves on the acoustical boundary (about 33 km apart; E109° 10′, N 33° 35′; E 109° 19′, N 33° 19′) in our unpublished work. The bats from these two caves exhibit obvious differences in echolocation calls with mean peak frequencies of 72.91 kHz (N = 8) and 75.63 kHz (N = 12), respectively. These two colonies originate from southwest China and central east China, respectively, as inferred from mitochondrial control region marker; they also exhibit genetic differentiation revealed by nuclear microsatellite loci (Liu et al. unpublished data). These findings probably suggest reproductive isolation between populations from central east China and southwest China. The behavioural evidence in the present study implies that social discrimination due to divergent echolocation calls may be a possible reason for the reproductive isolation. This explanation is in accord with the suggestions that echolocation calls play an important role in mate choice (Schuchmann and Puechmaille 2012; Grilliot et al. 2014, 2015; Puechmaille et al. 2014) and that divergence in echolocation calls may promote reproductive isolation (Kingston and Rossiter 2004; Wilkins et al. 2013). If this is in fact the case, this is an interesting association between sensory ecology and genetic divergence in Rhinolophus ferrumequinum. Genetic differentiation due to geographical isolation during glacial periods created the conditions that favour the evolution of echolocation differences via local adaptation (Flanders et al. 2011; Sun et al. 2013), and then echolocation divergence later promotes genetic differentiation between acoustically divergent populations when they come into secondary contact. This may also imply that the evolution of echolocation vocalization for ecological function may affect other evolutionary processes associated with the social function of the vocalization.
However, this explanation is challenged by the possibility that the potential reproductive isolation between the populations originating in central east China and southwest China arose due to mechanisms other than echolocation divergence and by the suggestion that Rhinolophus ferrumequinum is capable of vocal produce learning (Jones and Ransome 1993; Sun et al. 2013). The greater horseshoe bats have a diversity of vocalizations for social interaction (Matsumura 1979; Andrews et al. 2006; Ma et al. 2006). Geographical variation in communication calls used for mate choice or individual recognition may promote social discrimination and restrict gene flow between populations. Alternatively, Rhinolophus ferrumequinum individuals dispersed from a neighbouring area may be able to learn the echolocation calls of local population. The homogenization of the vocalizations may eliminate social discrimination and promote gene flow between local and non-local populations in secondary contact zone as suggested in some birds (e.g., Wright et al. 2005; Leader et al. 2008). At least in some CF bats, individuals can change their peak frequency to ‘match’ those of the neighbouring conspecifics (Hiryu et al. 2006).
In conclusion, this study demonstrates that Rhinolophus ferrumequinum is able to discriminate between echolocation calls from its own population and from a foreign one and prefers local over foreign echolocation calls. These findings support the communicative potential of bat echolocation calls and provide insight into the discrimination ability and preference of CF bats with respect to frequency variation in echolocation calls. Future work will improve the present study by performing reciprocal playback experiments and genetic analysis as well as by investigating the ability of vocal learning for acoustically divergent populations in secondary contact zone, which may uncover the effects of population divergence in echolocation calls on mate choice and gene flow.
References
Andrews MM, Andrews PT, Wills DF, Bevis SM (2006) Ultrasound social calls of greater horseshoe bats (Rhinolophus ferrumequinum) in a hibernaculum. Acta Chiropterol 8:197–212
Baker MC (1982) Vocal dialect recognition and population genetic consequences. Am Zool 22:561–569
Baker MC, Cunningham MA (1985) The biology of bird-song dialects. Behav Brain Sci 8:85–133
Baker MC, Mewaldt LR (1978) Song dialects as barriers to dispersal in white-crowned sparrows, Zonotrichia leucophrys nuttalli. Evolution 32:712–722
Baker MC, Spitler-Nabors KJ, Bradley DC (1982) The response of female mountain white-crowned sparrows to songs from their natal dialect and an alien dialect. Behav Ecol Sociobiol 10:175–179
Balaban E (1988) Cultural and genetic variation in swamp sparrows (Melospiza georgiana): behavioral salience of geographic song variants. Behaviour 105:292–322
Balakrishnan CN, Sorenson MD (2006) Song discrimination suggests premating isolation among sympatric indigobird species and host races. Behav Ecol 17:473–478
Barclay RMR (1982) Interindividual use of echolocation calls: eavesdropping by bats. Behav Ecol Sociobiol 10:271–275
Bastian A, Jacobs DS (2015) Listening carefully: increased perceptual acuity for species discrimination in multispecies signalling assemblages. Anim Behav 101:141–154
Bohn KM, Schmidt-French B, Ma ST, Pollak GD (2008) Syllable acoustics, temporal patterns, and call composition vary with behavioral context in Mexican free-tailed bats. J Acoust Soc Am 124:1838–1848
Boul KE, Funk WC, Darst CR, Cannatella DC, Ryan MJ (2007) Sexual selection drives speciation in an Amazonian frog. Proc R Soc Lond B 274:399–406
Bradley DW, Molles LE, Waas JR (2013) Local–foreign dialect discrimination and responses to mixed-dialect duets in the North Island kōkako. Behav Ecol 24:570–578
Brown ED, Farabaugh SM (1991) Song sharing in a group-living songbird, the Australian magpie, Gymnorhina tibicen part III sex specificity and individual specificity of vocal parts in communal chorus and duet songs. Behaviour 118:244–274
Bruns V, Schmieszek E (1980) Cochlear innervation in the greater horseshoe bat: demonstration of an acoustic fovea. Hear Res 3:27–43
Colbeck GJ, Sillett TS, Webster MS (2010) Asymmetric discrimination of geographical variation in song in a migratory passerine. Anim Behav 80:311–318
Danner JE, Danner RM, Bonier F, Martin PR, Small TW, Moore IT (2011) Female, but not male, tropical sparrows respond more strongly to the local song dialect: implications for population divergence. Am Nat 178:53–63
Dingle C, Poelstra JW, Halfwerk W, Brinkhuizen DM, Slabbekoorn H (2010) Asymmetric response patterns to subspecies-specific song differences in allopatry and parapatry in the gray-breasted wood-wren. Evolution 64:3537–3548
Fenton MB (1974) The role of echolocation in the evolution of bats. Am Nat 108:386–388
Fenton MB (1985) Communication in the Chiroptera. Indiana University Press, Bloomington
Fenton MB (2003) Eavesdropping on the echolocation and social calls of bats. Mammal Rev 33:193–204
Fernandez AA, Fasel N, Knörnschild M, Richner H (2014) When bats are boxing: aggressive behaviour and communication in male Seba’s short-tailed fruit bat. Anim Behav 98:149–156
Flanders J, Jones G, Benda P, Dietz C, Zhang S, Li G, Sharifi M, Rossiter SJ (2009) Phylogeography of the greater horseshoe bat, Rhinolophus ferrumequinum: contrasting results from mitochondrial and microsatellite data. Mol Ecol 18:306–318
Flanders J, Wei L, Rossiter SJ, Zhang S (2011) Identifying the effects of the Pleistocene on the greater horseshoe bat, Rhinolophus ferrumequinum, in East Asia using ecological niche modelling and phylogenetic analyses. J Biogeogr 38:439–452
Gordinho LDO, Matheu E, Hasselquist D, Neto JM (2015) Song divergence between subspecies of reed bunting is more pronounced in singing styles under sexual selection. Anim Behav 107:221–231
Gould E, Negus NC, Novick A (1964) Evidence for echolocation in shrews. J Exp Zool 156:19–37
Gray DA (2005) Does courtship behavior contribute to species-level reproductive isolation in field crickets? Behav Ecol 16:201–206
Griffin DR (1958) Listening in the dark. Yale University Press, New Haven
Grilliot ME, Burnett SC, Mendonça MT (2014) Sex and season differences in the echolocation pulses of big brown bats (Eptesicus fuscus) and their relation to mating activity. Acta Chiropterol 16:379–386
Grilliot ME, Burnett SC, Mendonça MT (2015) Choice experiments demonstrate that male big brown bats (Eptesicus fuscus) prefer echolocation calls of high copulatory females. Acta Chiropterol 17:411–417
Hiryu S, Katsura K, Nagato T, Yamazaki H, Lin LK, Watanabe Y, Riquimaroux H (2006) Intra-individual variation in the vocalized frequency of the Taiwanese leaf-nosed bat, Hipposideros terasensis, influenced by conspecific colony members. J Comp Physiol A 192:807–815
Höbel G (2015) Sexual differences in responses to cross-species call interference in the green treefrog (Hyla cinerea). Behav Ecol Sociobiol 69:695–705
Irwin DE, Bensch S, Price TD (2001) Speciation in a ring. Nature 409:333–337
Jiang T, Wu H, Feng J (2015) The patterns and causes of geographic variation in bat echolocation pulses. Integr Zool 10:241–256
Jones G, Holderied MW (2007) Bat echolocation calls: adaptation and convergent evolution. Proc R Soc Lond B 274:905–912
Jones G, Ransome RD (1993) Echolocation calls of bats are influenced by maternal effects and change over a lifetime. Proc R Soc Lond B 252:125–128
Jones G, Siemers BM (2011) The communicative potential of bat echolocation pulses. J Comp Physiol A 197:447–457
Jones GJ, Sayigh LS (2002) Geographic variation in rates of vocal production of free-ranging bottlenose dolphins. Mar Mammal Sci 18:374–393
Kazial KA, Masters WM (2004) Female big brown bats, Eptesicus fuscus, recognize sex from a caller’s echolocation signals. Anim Behav 67:855–863
Kazial KA, Pacheco S, Zielinski KN (2008) Information content of sonar calls of little brown bats (Myotis lucifugus): potential for communication. J Mammal 89:25–33
Kingston T, Rossiter SJ (2004) Harmonic-hopping in Wallacea’s bats. Nature 429:654–657
Knörnschild M (2014) Vocal production learning in bats. Curr Opin Neurobiol 28:80–85
Knörnschild M, Jung K, Nagy M, Metz M, Kalko E (2012) Bat echolocation calls facilitate social communication. Proc R Soc Lond B 279:4827–4835
Krebs JR, Kroodsma DE (1980) Repertoires and geographical variation in bird song. Adv Study Behav 11:143–177
Lachlan RF, Servedio MR (2004) Song learning accelerates allopatric speciation. Evolution 58:2049–2063
Lameira AR, Delgado RA, Wich SA (2010) Review of geographic variation in terrestrial mammalian acoustic signals: human speech variation in a comparative perspective. J Evol Psychol 8:309–332
Leader N, Geffen E, Mokady O, Yom-Tov Y (2008) Song dialects do not restrict gene flow in an urban population of the orange-tufted sunbird, Nectarinia osea. Behav Ecol Sociobiol 62:1299–1305
Li Y, Wang J, Metzner W, Luo B, Jiang T, Yang S, Shi L, Huang X, Yue X, Feng J (2014) Behavioral responses to echolocation calls from sympatric heterospecific bats: implications for interspecific competition. Behav Ecol Sociobiol 68:657–667
Lin A, Jiang T, Kanwal JS, Lu G, Luo J, Wei X, Luo B, Feng J (2015) Geographical variation in echolocation vocalizations of the Himalayan leaf-nosed bat: contribution of morphological variation and cultural drift. Oikos 124:364–371
Liu Y, Metzner W, Feng J (2013) Vocalization during copulation behavior in greater horseshoe bats, Rhinolophus ferrumequinum. Chin Sci Bull 58:2179–2184
Ma J, Kobayasi K, Zhang S, Metzner W (2006) Vocal communication in adult greater horseshoe bats, Rhinolophus ferrumequinum. J Comp Physiol A 192:535–550
Marler P (2004) Bird calls: their potential for behavioral neurobiology. Ann N Y Acad Sci 1016:31–44
Matsumura S (1979) Mother-infant communication in a horseshoe bat (Rhinolophus ferrumequinum nippon): development of vocalization. J Mammal 60:76–84
McArthur PD (1986) Similarity of playback songs to self song as a determinant of response strength in song sparrows (Melospiza melodia). Anim Behav 34:199–207
Milligan MM, Verner J (1971) Inter-populational song dialect discrimination in the white-crowned sparrow. Condor 73:208–213
Mortega KG, Flinks H, Helm B (2014) Behavioural response of a migratory songbird to geographic variation in song and morphology. Front Zool 11:85
Murphy CG, Gerhardt HC (2000) Mating preference functions of individual female barking treefrogs, Hyla gratiosa, for two properties of male advertisement calls. Evolution 54:660–669
Parsons S, Jones G (2000) Acoustic identification of twelve species of echolocating bat by discriminant function analysis and artificial neural networks. J Exp Biol 203:2641–2656
Podos J (2007) Discrimination of geographical song variants by Darwin’s finches. Anim Behav 73:833–844
Price T (2008) Speciation in birds. Roberts and Company, Greenwood Village
Ptacek MB (2000) The role of mating preferences in shaping interspecific divergence in mating signals in vertebrates. Behav Process 51:111–134
Puechmaille SJ, Borissov IM, Zsebok S, Allegrini B, Hizem M, Kuenzel S, Schuchmann M, Teeling EC, Siemers BM (2014) Female mate choice can drive the evolution of high frequency echolocation in bats: a case study with Rhinolophus mehelyi. PLoS One 9:e103452
Puechmaille SJ, Gouilh MA, Piyapan P, Yokubol M, Mie KM, Bates PJ, Satasook C, Nwe T, Bu SSH, Mackie IJ (2011) The evolution of sensory divergence in the context of limited gene flow in the bumblebee bat. Nat Commun 2:573
Racey PA (2009) Reproductive assessment of bats. In: Kunz TH, Parsons S (eds) Ecological and behavioral methods for the study of bats. The John Hopkins University Press, Baltimore, pp. 249–264
Rothstein SI, Fleischer RC (1987) Vocal dialects and their possible relation to honest status signalling in the brown-headed cowbird. Condor 89:1–23
Ruczyński I, Kalko EKV, Siemers BM (2007) The sensory basis of roost finding in a forest bat, Nyctalus noctula. J Exp Biol 210:3607–3615
Ruczyński I, Kalko EKV, Siemers BM (2009) Calls in the forest: a comparative approach to how bats find tree cavities. Ethology 115:167–177
Schevill W (1956) Evidence for echolocation by cetaceans. Deep Sea Res Pt II 3:153–154
Schnitzler HU, Moss CF, Denzinger A (2003) From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol 18:386–394
Schuchmann M, Siemers BM (2010) Behavioral evidence for community-wide species discrimination from echolocation calls in bats. Am Nat 176:72–82
Schuchmann M, Puechmaille SJ, Siemers BM (2012) Horseshoe bats recognise the sex of conspecifics from their echolocation calls. Acta Chiropterol 14:161–166
Schuller G, Pollak G (1979) Disproportionate frequency representation in the inferior colliculus of doppler-compensating greater horseshoe bats: evidence for an acoustic fovea. J Comp Physiol A 132:47–54
Searcy W, Nowicki S, Hughes M, Peters S (2002) Geographic song discrimination in relation to dispersal distances in song sparrows. Am Nat 159:221–230
Slabbekoorn H, Smith TB (2002) Bird song, ecology and speciation. Philos Trans R Soc B 357:493–503
Suga N, Jen PH (1976) Disproportionate tonotopic representation for processing CF-FM sonar signals in the mustache bat auditory cortex. Science 194:542–544
Sun K, Luo L, Kimball RT, Wei X, Jin L, Jiang T, Li G, Feng J (2013) Geographic variation in the acoustic traits of greater horseshoe bats: testing the importance of drift and ecological selection in evolutionary processes. PLoS One 8:e70368
Uy JAC, Moyle RG, Filardi CE (2009) Plumage and song differences mediate species recognition between incipient flycatcher species of the Solomon Islands. Evolution 63:153–164
Velásquez NA (2014) Geographic variation in acoustic communication in anurans and its neuroethological implications. J Phys Paris 108:167–173
Voigt-Heucke SL, Taborsky M, Dechmann DKN (2010) A dual function of echolocation: bats use echolocation calls to identify familiar and unfamiliar individuals. Anim Behav 80:59–67
Wilkins MR, Seddon N, Safran RJ (2013) Evolutionary divergence in acoustic signals: causes and consequences. Trends Ecol Evol 28:156–166
Wright TF, Rodriguez AM, Fleischer RC (2005) Vocal dialects, sex-biased dispersal, and microsatellite population structure in the parrot Amazona auropalliata. Mol Ecol 14:1197–1205
Yovel Y, Melcon ML, Franz MO, Denzinger A, Schnitzler HU (2009) The voice of bats: how greater mouse-eared bats recognize individuals based on their echolocation calls. PLoS Comput Biol 5:e1000400
Zuk M, Rotenberry JT, Simmons LW (2001) Geographical variation in calling song of the field cricket Teleogryllus oceanicus: the importance of spatial scale. J Evol Biol 14:731–741
Acknowledgments
We thank the two anonymous reviewers for their helpful suggestions on the previous version of the manuscript. This work was supported by the National Natural Science Foundation of China (grant nos. 31500314 and 31500307) and China Postdoctoral Science Foundation (grant no. 2015M581384).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Bat capture and playback experiments in this study were approved by the National Animal Research Authority in Northeast Normal University, China. Our work did not cause any effects on bat mortality. We maintained the bats carefully and released them at the site of capture when all trials were completed.
Additional information
Communicated by C. Voigt
Rights and permissions
About this article
Cite this article
Lin, A., Liu, H., Chang, Y. et al. Behavioural response of the greater horseshoe bat to geographical variation in echolocation calls. Behav Ecol Sociobiol 70, 1765–1776 (2016). https://doi.org/10.1007/s00265-016-2182-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2182-3