Abstract
A variety of studies on food sharing elucidate both its ultimate and proximate functions in non-human primates, especially in Pan. For chimpanzees, food sharing serves as a means to strengthen social relationships. In contrast, little is known about food sharing in orangutans, since their semi-solitary lifestyle barely provides an opportunity to share food outside of the mother-offspring context. However, recent long-term studies suggest that social bonding might play a more important role for orangutans than previously assumed. In zoos, orangutans are often kept in groups and seem to cope with group living quite well. If captive orangutans use food sharing as a social tool, they are expected to share food frequently and selectively with close social partners and to engage frequently in active transfers. We provided three orangutan groups with monopolizable food and recorded all dyadic food-related interactions. For each dyad, we determined the relationship quality and tested whether it predicts food sharing. We found that, in support of our predictions, almost two thirds of interactions involving food resulted in sharing and that the probability for an individual to share food with a particular partner increased with the strength of their relationship. Exceeding our expectations, food sharing occurred even between individuals from two neighboring groups. Finally, a comparison with studies on captive chimpanzees revealed a significantly higher proportion of active transfers for orangutans suggesting species-specific sharing psychologies.
Significance statement
Sharing of food is a universal prosocial behavior in humans. Recent research aims to elucidate its adaptive functions and proximate mechanisms by comparison with other species, especially non-human primates, in natural and captive settings. For bonobos and chimpanzees, our closest relatives, the quality of social relationships was revealed to be important for food sharing. In contrast, there is very limited knowledge on food sharing in orangutans, our most distant and semi-solitary living hominid relatives. This study provides the first systematic investigation of food-sharing patterns and the role of relationship quality in captive orangutans. The results demonstrate that group-living orangutans share frequently and selectively with close associates and even more actively than found for chimpanzees. These findings add further evidence supporting the hypothesis that social bonding played a role in the evolution of human prosocial behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosocial behavior comprises “actions intended to benefit one or more people other than oneself […]” (Batson and Powell 2003, p. 653), such as sharing of resources, comforting, cooperating, helping, or altruistic punishment. Across human cultures, prosocial behaviors play a central role in regulating social relationships and are fundamental for maintaining social as well as moral norms (Levine et al. 2001; Goetz et al. 2010). While social psychologists have investigated prosocial behavior already for nearly a century (Dovidio et al. 2006; Eisenberg et al. 2007), only more recently comparative psychologists and biologists developed a growing interest in this topic often aiming to elucidate the evolutionary origins of human prosocial behavior (e.g., Gintis et al. 2003; de Waal 2008; Clutton-Brock 2009; Cheney 2011; Silk and House 2011; Tomasello and Vaish 2013).
A particular kind of sharing of resources is food sharing, which has been defined as the tolerated “transfer of a defensible food item from one food-motivated individual to another” (Feistner and McGrew 1989, p. 22). In humans, sharing is not restricted to kin or group members and emerges early in ontogeny (Zahn-Waxler et al. 1992; Gurven and Jaeggi 2015). However, food sharing is not limited to Homo sapiens, but has been found in many non-human primates as well as non-primate species (e.g., de Kort et al. 2003; Carter and Wilkinson 2013; Jaeggi and Gurven 2013a). In 38 non-human primate species, adults share food with immature offspring, and in less than half of these species, sharing also occurs among unrelated adults. Since there is no primate species in which sharing occurs among adults, but not between adults and their offspring, Jaeggi and van Schaik (2011) suggested that sharing with offspring has been an evolutionary predecessor for sharing in other contexts.
In terms of its ultimate function, food sharing with offspring potentially increases inclusive fitness (Hamilton 1964) due to nutritional and/or informational benefits (Brown et al. 2004). Kin selection (Maynard Smith 1964) is therefore a widely accepted explanation for its evolution (Jaeggi and van Schaik 2011). Sharing with non-kin, on the other hand, could have evolved through different mechanisms, e.g., avoiding costs of harassment (Stevens and Stephens 2002), reciprocity (reciprocal altruism: Trivers 1971) or costly signaling (Zahavi 1995). Thus, food sharing may increase the possessor’s fitness immediately or in the long run (Jaeggi and Gurven 2013b), and the “currency” of these benefits (e.g., food, grooming, coalitionary support, or access to mates) may vary between and within species (Stevens and Gilby 2004; Mitani 2006; Jaeggi and van Schaik 2011; Silk et al. 2013). Since recent research has accumulated evidence for a correlation between enduring, close relationships (social bonds) and long-term reciprocity and cooperation in primates (Carter 2014; Freidin et al. 2015), the interaction between social bonds and food sharing is of particular interest.
Strong social bonds are particularly evident among both kin and reciprocating individuals (Silk and House 2011). The potential individual or inclusive fitness benefits, which are mediated by social bonds, include enhanced access to food or mating partners, grooming, support during agonistic encounters, or in acquiring dominance rank (Silk 2007). Moreover, stable and long-term bonds, especially between individuals of different generations, correlate with opportunities for social learning and thereby for cultural transmission of information, such as ecological knowledge and foraging skills (van Schaik and Burkart 2011). Schino and Aureli (2009) suggest that close and long-term relationships develop by emotional bookkeeping and therefore do not require sophisticated cognitive skills. The value of a social partner “encoded in the brain as a compressed score of the relation history” (Jaeggi and Gurven 2013a, p. 193), including exchanged benefits, adjusts the species-specific sharing psychology, which results in more frequently positive responses toward solicitations of more valuable partners. Accordingly, the influence of social bonds and food sharing is mutual: On the one hand, relationship quality should be a useful predictor for the probability of sharing with a particular partner, because the value of this partner is based on previously received benefits and influences the decision to share or not. An act of food sharing, on the other hand, adds another received benefit to the emotional record of the partner and might thereby strengthen their social bond.
Several studies provide evidence for such a correlation between social bonds and sharing in non-human primates. While most research focuses on chimpanzees (Pan troglodytes) and bonobos (Pan paniscus), both in the wild (e.g., Gomes and Boesch 2009; Wittig et al. 2014; Yamamoto 2015) and in captivity (e.g., Jaeggi et al. 2013; Silk et al. 2013; Calcutt et al. 2014), very little is known about the two Asian great ape species, Bornean (Pongo pygmaeus) and Sumatran orangutans (Pongo abelii). The difficulties in observing these arboreal apes in their natural habitat might only partly account for this lack of knowledge. More importantly, their social organization differs from that of other great apes and seems to offer little opportunities for developing strong social bonds outside the mother-offspring context. Orangutans are characterized by an individual-based fission-fusion sociality with a mean party size of less than two individuals (van Schaik 1999). The only stable and long-term “groups” are mothers with their one or two offspring, which are characterized by extraordinarily strong and long-lasting bonds (van Noordwijk et al. 2009). Within these close social units, food transfer occurs regularly from mothers to dependent offspring and conduces most likely to social learning of diet and foraging skills (Jaeggi et al. 2008; van Noordwijk et al. 2009). However, recent studies on orangutans suggest that social bonds might also be relevant for associations among females and male-female consortships (Mitra Setia et al. 2009). For example, related females with dependent offspring, who form clusters with overlapping home ranges, often tolerate or enable social play among their offspring, sometimes feed in proximity, and occasionally even share food (van Schaik 1999; Singleton et al. 2009; van Noordwijk et al. 2009, 2012). Conceivable functions of female-female bonds might consist in safe opportunities for their offspring for social play with peers and social learning from other role models than their mother (van Noordwijk et al. 2009, 2012). Males and sexually active females engage in temporary consortships, which have been suggested to be a female strategy to reduce sexual harassment (Fox 2002). Females show preferences to associate with particular males and often heavily resist mating attempts by non-preferred males (Utami Atmoko et al. 2009a). Within these consortships, food sharing has been observed on some occasions, predominantly from males to females and probably as a means of female partner choice (van Noordwijk and van Schaik 2009). Given these hints of a possible correlation between social bonds and food sharing in wild orangutans, one objective of the present study is to systematically investigate sharing patterns and the effect of relationship quality on food sharing in captivity. Although social behavior in captivity is not necessarily representative for natural conditions, we expect, if orangutans have evolved tendencies to form social bonds and share food, these tendencies to turn out particularly in long-term groups, where orangutans have more opportunities to interact with conspecifics.
Considering proximate aspects of food sharing, primate species differ with regard to the extent of the possessor’s active contribution (Jaeggi and Gurven 2013a). In passive sharing, which is the most frequent sharing type in non-human primates, a non-possessor is allowed to take food without resistance or assistance by the possessor. With regard to its socio-cognitive preconditions, some inhibitory control should be sufficient. In contrast, active sharing involves an action by the possessor that facilitates or performs the transfer and can either be reactive, i.e., upon request, or proactive, i.e., possessor initiated (Jaeggi et al. 2010a). Active sharing is much less common in non-human primates. It occurs mostly upon request and to adults, with the exception of some callitrichids, who frequently proactively offer food to infants (Feistner and McGrew 1989; Jaeggi et al. 2010a). Active sharing requires the abilities to recognize and respond to the needs of others (Jaeggi and Gurven 2013a). Unlike in passive sharing, the actively sharing possessor has maximum control over the recipient’s identity, the amount and the quality of the food she is going to transfer. Control over the distribution of food has been suggested as a precondition for selective sharing (Jaeggi and Gurven 2013b), which in turn is necessary for using food sharing as a social tool.
In chimpanzees and bonobos, but not in orangutans, food sharing among adults preferentially involves meat following a hunt (e.g., Hohmann and Fruth 2008; Gomes and Boesch 2009) and large fruits or cultivated plant food (Hockings et al. 2007; Yamamoto 2015). These are compact, monopolizable, and high-valued food items, which seem to elicit food sharing in primates (Jaeggi and Gurven 2013a). In contrast, all recorded instances of sharing between adult orangutans involved food that had been readily obtainable for both partners (van Noordwijk and van Schaik 2009), while for the very rare consumptions of vertebrate meat, no sharing among adults has been reported to date (Sugardjito and Nurhuda 1981; Utami and van Hooff 1997; Hardus et al. 2012). These differences between species probably reflect different selection pressures, which might have led to species-specific sharing psychologies (Jaeggi and Gurven 2013a).
Food sharing in captive chimpanzees and bonobos has been systematically studied by providing monopolizable food items to induce food-related interactions (e.g., de Waal 1997a; Crick et al. 2013; Jaeggi et al. 2013; Silk et al. 2013). Up to date, no such study has been conducted for orangutans. If there is a correlation between social bonds and food sharing, orangutans are of particular interest: Despite their semi-solitary lifestyle in the wild, they are often kept in zoos in permanent groups, where they cope—with some variability between the two species (Weingrill et al. 2011)—with group life quite well (Jantschke 1972; Edwards and Snowdon 1980; Poole 1987), which reflects a remarkable flexibility in their social behavior.
We conducted the first systematic investigation of food-sharing behavior in socially housed Sumatran orangutans using monopolizable food items. Based on (a) the flexibility in social behavior demonstrated by orangutans in captivity, (b) the presence of socio-cognitive preconditions necessary for selective sharing, (c) the proposed relationship between control over food and active and selective sharing, and (d) the previously suggested potential functions of grouping and social bonds for wild orangutans, we expect the following: (1) food sharing to occur frequently and in various forms, (2) relationship quality to be a predictor for the occurrence of food sharing, (3) active sharing to occur frequently and especially between closely associated adults, and (4) potential differences regarding active sharing compared to studies on chimpanzees and bonobos, due to different species-specific sharing psychologies.
Methods
Study groups and housing conditions
We tested three groups of Sumatran orangutans in their indoor enclosures in the zoological gardens of Berlin and Dortmund, Germany. The Berlin group consisted of four individuals: one adult male, one adult female, their independent immature daughter, and a second, unrelated adult female (Table 1). Depending on the weather, they had access to two connected indoor enclosures (59 m2 each) and an outdoor enclosure (490 m2). The two groups at Zoo Dortmund consisted of three and four individuals, respectively; group A comprised one adult male, one adult female, and their dependent daughter, while group B consisted of two unrelated adult females, one of them with a 3-month-old infant that was not regarded as participating in the study, and an unrelated independent immature, who was the older daughter of the adults of group A (Table 1). During the summer, both groups usually join each other in the outdoor enclosure (1515 m2), while in the winter—during the observation period – they are kept in adjacent indoor enclosures (48 and 65 m2), each with additional sleeping boxes and alternating temporary access to a third compound (140 m2). Since these indoor enclosures were separated by flexible mesh, the two groups had the opportunity to interact with each other.
The enclosures in both zoos were equipped with resting and climbing structures, nesting material, objects to manipulate, devices for behavioral enrichment, and permanent access to water. The indoor enclosures in Dortmund and the outdoor enclosure in Berlin were covered with steel mesh, which provided additional climbing opportunities. The main diet in both zoos consisted of a mixture of vegetables and fruits, but included also leaves, grain, yogurt, cooked eggs, occasionally cooked meat, and special food items for behavioral enrichment, usually distributed throughout the compounds or given directly to each animal. No changes to the daily routine—such as alterations regarding feeding procedures or times—were required for testing.
Experimental setting and procedure
To elicit food-related interactions (hereafter: food interactions), we used monopolizable food items. Unlike other studies with chimpanzees and bonobos that used bundles of twigs and leaves or paper bags filled with leaves, fruits, or vegetables (e.g., de Waal 1989; Jaeggi et al. 2010c), we chose single large fruits or vegetables with hard peels, because (i) they could be monopolized and defended more easily, and (ii) it would take more time to fully consume the food.
For each group, we conducted 11 trials on separate days. During the first six trials, each group was provided with a moderately preferred vegetable they were not very familiar with (Hokkaido pumpkin), followed by five trials with a familiar, highly preferred fruit (melon in Berlin, pineapple in Dortmund). The trials were conducted in the indoor enclosures with all group members present in February and March 2013. Because of visibility problems in Berlin, three trials had to be repeated in June 2013. Each group was tested only once per day. In Dortmund, the two groups were tested consecutively in a daily alternating test order. During the tests, both groups were present in their separate, neighboring enclosures.
The orangutans had unlimited access to water and at no time they were deprived from food. Before testing started, the procedure and the food items to be used were thoroughly discussed with the curators and keepers. Each test session was conducted with the assistance of at least one zookeeper. During the test periods, the keepers monitored the general social behavior of the orangutans to be aware of any signals indicating stress that might have been caused by the study. In this case, all testing would have been canceled immediately; however, this was not necessary.
All trials were continuously video-recorded by KSK using the digital camcorder CANON Legria FS200. A session started by placing the food in the enclosure and then providing access by opening a sliding door. A session ended when the provided food item had been either finished or abandoned for at least 5 min.
Data coding and definitions
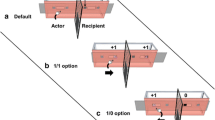
We used all occurrences sampling as the sampling rule (Altmann 1974; Martin and Bateson 2007) and applied a coding scheme (Fig. 1) to code all food interactions. For each event, we additionally recorded the respective dyad, food type, on- and offset, requesting, and resistance behavior (cf. online resource 1, ST1–3 for operational definitions).
We defined a food interaction as a food-related interaction between a possessor of food and a non-possessing, but food-interested solicitor (online resource 1, ST1). Only interactions where the possessor had the opportunity to defend his possession were considered as food interactions. Therefore, contrary to Jaeggi et al. (2010c), instances of stealing, which indicated a sudden snatching of food that could not have been prevented by the possessor, were not included. Following de Waal (1989), we included collect near as a form of tolerated transfer, since in some cases possessors defended leftovers within their reach.
A food interaction started when the solicitor began to show interest in the food (or in the very rare case of proactive transfer by the possessor-initiated transfer) and ended when a piece of food was transferred or when the solicitor did not show interest for at least 5 s. It usually comprised a request, a resistance behavior and/or a food transfer. Request behaviors included visual and tactile gestures (Liebal et al. 2006) as well as actions (online resource 1, ST1). In a strict sense, only active behaviors were considered as resistance, with one exception: when a possessor had shown no reaction until the solicitor stopped showing interest, this passive “ignoring” was also considered as resistance. We distinguished between five degrees of resistance: no reaction, slight resistance (e.g., turn away), moderate resistance (e.g., shield food), strong resistance (e.g., move away or struggle), and threat (e.g., threat face or bite). When different resistance behaviors were combined, we categorized them with regard to the strongest behavioral element (online resource 1, ST1).
A food interaction was coded as either no food sharing or food sharing. Since failed transfer occurred rarely and neither of the two categories could be ascribed reliably, those events were excluded from the analysis, although they were reported as a distinct category (Fig. 1). On- and offset of a food interaction was generally defined from the perspective of the solicitor, therefore a food interaction that initially included some form of resistance, but finally led to unresisted transfer, was coded as food sharing and referred to as “initial resistance” (online resource 1, ST2).
Inter-rater reliability
All coding was done by KSK. To assess inter-rater reliability, 22 % of the total coded events were coded again by a second person with focus on tolerance, requesting, and resistance behavior, while an additional 9 % of all events were coded by a third person with focus on transfer types; both raters were unfamiliar with the hypotheses. We calculated Cohen’s kappa coefficient of agreement (Cohen 1960) for both comparisons, using function kappa2 from the R package irr (Gamer et al. 2012). Both raters showed good agreement with KSK (second rater: κ = 0.736, n = 210, p < 0.001; third rater: κ = 0.605, n = 85, p < 0.001). It was not possible to record or code data blindly with regard to the identity of the individuals, because our study required the focus on interactions involving the particular individual who was in possession of the food.
Statistical analysis
Descriptive statistics
In a first step, we analyzed the data with regard to characteristics of food possession, dynamics, and types of food interactions on a group and dyadic level using descriptive statistics.
Analysis of active sharing in comparison to chimpanzees and bonobos
To test our prediction that the frequency of active food transfer differs between the great ape species, we compared our data to the data of chimpanzees and bonobos retrieved from a meta-analysis on food sharing in primates by Jaeggi et al. (2010a), which comprised data from both the wild and captivity, as well as three more recent studies (Crick et al. 2013; Silk et al. 2013; Yamamoto 2015). To ensure comparability, we had to subset our data as follows: we excluded all cases of tolerated transfer for which a categorization as passive or active was impossible, since the meta-analysis by Jaeggi et al. (2010a) did not consider indeterminable cases. Likewise, we excluded all cases with immatures as possessors. Since there were only marginal occurrences of proactive sharing across the species, we subsumed both re- and proactive transfers as active sharing. Furthermore, while Jaeggi et al. (2010a) included all non-human great apes into one category, we computed the percentage of active sharing with regard to all food-sharing events separately for chimpanzees, bonobos, and Bornean and Sumatran orangutans. Following Jaeggi et al. (2010a), we counted the percentage of active sharing from each study or independent population within a study as data points. However, contrary to their original paper, we computed medians, not means, due to the small number of data points. In their meta-analysis, Jaeggi et al. (2010a) distinguished two conditions: sharing with infants and sharing among adults. Since two of the three immatures in our study were already independent (Table 1; the distinction between age classes follows van Noordwijk and van Schaik 2005), we grouped those food interactions where an immature had been the recipient into a broader third class sharing with immatures regardless whether it was already weaned or not. We conducted a Mann-Whitney’s U tests for comparing genera using R function wilcox.exact from R package exactRankTests and assessed statistical significance at the α level of 0.05.
To avoid a possible influence of the setting (wild or captive), we conducted a second comparison that was restricted to data from captivity. Due to small sample sizes, we restricted the statistical analysis to the comparison of chimpanzees and orangutans for the condition sharing among adults, by conducting Mann-Whitney’s U test with an assessed statistical significance at the α level of 0.05.
Test of potentially influencing factors on the probability of food sharing
We conducted a generalized linear mixed model (GLMM, Baayen 2008) to test the influence of different factors on food sharing (Table 2), both to control for non-independence of observations from the same dyad—and therefore to avoid pseudo-replication—and to account for random effects within particular dyads or possessors.
-
a)
Test predictors and control variables
Social relation was included as the test predictor. We established a sociality index as a measure for relationship quality generally following Silk et al. (2006) with some important alterations which account for the possessor’s perspective and for some characteristics of the study groups. As proposed by Silk et al. (2006), we coded the frequencies of contact and proximity for each dyad. Using randomly chosen videos from an observational study on prosocial behavior conducted in 2012 and in the summer of 2013 (KSK and KL unpublished data), we carried out group scans (Altmann 1974) on 8 days in Berlin and 10 days in Dortmund for approximately 1 h per day which cover the daily main activity period. We coded the relative positions of all group members as contact, proximity and out of reach, respectively, on every 15 s. This resulted in a total of 2147 scans per group. We did not include grooming as a measure, because this behavior occurred only very rarely in orangutans. To account both for the possessor’s perspective and the considerable variation of general gregariousness among individuals, we used the mean frequencies of contact and proximity of all dyads that included the particular possessor x (Contact mean(x) and Proxi mean(x) , respectively) as reference values, instead of referring to groups’ means. We then computed the possessor-centered sociality index for each dyad xy (PSIxy) as follows:
$$ {\mathrm{PSI}}_{xy}=\frac{\left(\frac{{\mathrm{Contact}}_{xy}}{{\mathrm{Contact}}_{mean(x)}}+\frac{{\mathrm{Proxi}}_{xy}}{{\mathrm{Proxi}}_{mean(x)}}\right)}{2} $$In this equation, the terms Contact xy and Proxi xy , refer to the frequencies of contact and proximity, respectively, for a directed dyad xy. The PSIxy measures the relationship quality of this dyad from the possessor’s perspective and relative to the general sociability of the possessor x. The value of the PSI is a rational number with a minimum at zero, while a value around one indicates a middle relationship quality. The higher the PSI value, the stronger is the social relationship with regard to all social relationships the possessor is involved in.
Food value was included into the model as a control variable, with pumpkin coded as medium-preferred food, and melon and pineapple coded as highly preferred food, since the preference for the particular food might influence the willingness for the possessor to share.
Latency with regard to monopolization by the possessor was included as a control variable, since it probably reflected the degree of repletion, which might affect the motivation of the possessor to share or not.
-
b)
Statistical analysis
We used a GLMM with a binomial error structure and logit link function. Into the full model, we included social relation, food value, and latency with regard to monopolization as fixed effects and dyad and possessor as random effects (Table 2). Furthermore, we included random slopes components (social relation within possessor) and the respective correlation between random slope and intercept. We compared the full model with a null model that lacked the test predictor but contained the control variables as well as the random effects of the full model, using a likelihood ratio test (R function anova with argument test set to “Chisq”).
Before fitting the model, we log-transformed the data of the predictor variable latency with regard to monopolization due to their right skewed distribution. Moreover, the data were unbalanced with regard to the frequency of interactions per dyad, but due to the already relatively small sample size, we dropped only one dyad level (with only one observed interaction) to minimize unwanted effects due to unbalanced data and to allow for the inclusion of random slopes components. Since no interaction took place during the fourth trial in the Dortmund group A, only ten trials were included into the model for this group. This resulted in a total sample size of 954 observations (727 of them were determinable food interactions) of ten possessors and 23 dyads.
To rule out multicollinearity, we computed variance inflation factors (VIF) for a standard linear model excluding the random effects using the R package car version 2.0–26 (Fox and Weisberg 2011). The VIF of 1.0 for social relation and 1.1 for latency with regard to monopolization as well as for food value indicated that there was no collinearity problem. Over-dispersion was revealed not to be an issue either (dispersion parameter 0.98). We used the functions influence and dfbetas provided by the R package influence.ME (Nieuwenhuis et al. 2012) to detect influential data. According to the calculated values for DFBETAS, we determined some cases on possessor and dyadic level as probably influential. Nonetheless, performing the sigtest diagnostic, the deletion of none of these data changed the level of significance of the social relation variable. In contrast, for the control predictor food value, we detected an influential case on each level of random effects that changed the level of significance. Given the small sample size, we did not exclude the influential cases from the model. As elaborately discussed by Nieuwenhuis et al. (2012), influential cases are not necessarily outliers. An expansion of the sample size (not possible for this study) might reveal that a previously influential observation is no longer influential. However, the outcome of the model for the effect of food value should be extremely cautiously interpreted. (online resource 1, ST4–ST9).
The model was implemented in the R 3.2.2 (R Core Team 2015) using the function glmer of the R package lme4 1.1–10 (Bates et al. 2015). To derive predictions for the particular fixed effects, we conducted likelihood ratio tests using the R function drop1. Statistical significance was assessed at the α level of 0.05. Confidence intervals for predictions were computed using the function confint.merMod.
It was not possible to include kin relation as a separate factor with three levels (parent-offspring, offspring-parent, and non-kin), as originally intended, because of its collinearity with social relation (rpb = −0.61). When we restricted kin relation to a factor with two levels (mother-offspring vs. all other combinations), collinearity became even stronger (rpb = −0.75). However, when we included kin relation despite the collinearity issue, this did not lead to an improvement of the model (online resource 1, ST10).
Results
First we describe the dynamics and patterns of food interactions on group and dyadic level in the three orangutan groups, including those interactions between the two Dortmund groups. Then we compare sharing patterns with regard to the active involvement to those published in studies on other great apes. Finally, we specifically analyze factors potentially influencing food sharing to test whether stronger social relationships are indicative of higher proportions of food sharing.
Description of food interactions
Across trials and groups, food was monopolized immediately after provisioning. On average, a trial lasted 16.6 min (SD = 4.8) for melons and pineapples and 39.5 min (SD = 22.5) for pumpkins (online resource 2, SF1). Although each individual was at least twice in food possession over all trials, adult and dominant individuals were the main possessors and monopolized the food on average for 14.7 min (SD = 15.9). Immature and subordinate individuals monopolized the food less frequently and mostly for only short periods (online resource 2, SF2). Food possessors varied in their rates of food interactions, i.e., the number of food interactions per minute while they monopolized the food (online resource 2, SF3). In all groups, the number and the order of food-possessing individuals as well as the identities of the main possessors varied considerably across the trials (online resource 2, SF4).
In total, we observed 1018 food interactions. Almost all food interactions were initiated by a non-possessing individual, except five instances of proactive food sharing (for an example cf. online resource 3, SV1). Only 21 % (n = 216) of all food interactions were direct attempts to take food, while most food interactions (68 %, n = 693) included at least one, but often more elements of request behavior like extended peering, manual and/or tactile gestures, or actions. In another 64 cases, the possessor acted on the mere approach of the solicitor or tolerated collecting food. For 40 food interactions, the request behavior could not be determined.
A variety of resistance behavior has been observed in response to request. In 25 % of all instances of resistance, the possessor showed no reaction until the solicitor stopped the request, while slight resistance occurred in 3 %, moderate resistance in 42 %, and strong resistance, which mainly consisted in move away, in 26 %. Threat occurred only rarely (4 %).
The vast majority (94 %) of food transfers was tolerated; either without any resistance (79 %) or with initial slight resistance, which was ultimately abandoned (15 %). Tolerated transfers occurred in several forms. In case of passive transfer, tolerated taking was the most frequent transfer type (80 %). For active transfers, active giving was the most common behavior (67 %), though facilitated taking was with 26 % quite frequent.
In most cases, the amount of shared food was small, usually a mouthful or less, and often of lower quality. However, there were considerable exceptions: in some cases, a possessor divided the food and handed over the larger piece, or the food had been broken into large pieces which were subsequently shared, or a solicitor was permitted to take several bites from the pulp in a row (online resource 3, SV2–SV3). Moreover, all parts of the pumpkins and melons including peels and seeds were eatable; even the pineapple peels were totally consumed. For active sharing, most instances (72 %, n = 108) included pieces of good quality, i.e., pulp, flesh, or seeds; only 19 % (n = 28) of the cases regarded low quality food, i.e., peel. For the remaining 14 instances, the quality was not determinable.
Frequencies and patterns of food interactions within and between the groups
Sharing within groups
In total, we observed 955 food interactions within the groups of which 728 cases were identifiable with regard to tolerance. The number of food interactions as well as the total proportion of food sharing, i.e., the food-getting success (de Waal 1997a), and the proportion of active sharing varied both across groups (Table 3) and dyads (Fig. 2, also online resource 2, SF5). While most interactions took place within the two mother-daughter dyads (Berlin 125, Dortmund 112), the food-getting success differed considerably between them (Berlin 51.2 %, Dortmund 99.1 %). However, there were two dyads of unrelated adults with both a high number of interactions and a high proportion of sharing: the male-female dyad Enche-Djasinga in Berlin (68 food interactions, 91.2 % sharing) and the female-female dyad Suma-Djamuna of the Dortmund group B (51 food interactions, 70.8 % sharing). On the other hand, although a large number of food interactions took place in the female-female dyad Njamuk-Djasinga in Berlin, the proportion of sharing was very low (80 food interactions, 7.5 % sharing).
Absolute frequencies of determinable food interactions (n = 786) with regard to the transfer type for each dyad of the three study groups and for interactions between the two Dortmund groups. Each bar represents a particular dyad; black (at the bottom) indicates the proportion of interactions that did not resulted in food transfer due to resistance by the possessor, dark gray indicates non-tolerated transfer despite the possessors’ resistance, while all other shades indicate food sharing, differentiated with regard to the active involvement of the possessor. The particular age-sex class for an individual is referred to as follows: f adult female, m adult male, i immature. Mother-daughter dyads are indicated with asterisks
Sharing between groups
The setting in Dortmund was exceptional because the adjacent indoor enclosures enabled food interactions between groups, while simultaneously the mesh prevented harassment by members of the other group. In total, we observed 63 food interactions of which 58 cases were determinable with regard to tolerance (Table 3). Half of these interactions took place between Toba and her older daughter Tao, of which 75 % ended in food sharing and all but one were active. In some cases, Toba moved toward her requesting daughter and held the whole pineapple in position to enable Tao to bite off from the fruit, even at the very beginning of a session and before Toba had had a proper bite for herself. The other food interactions occurred mostly between the adult females, including two proactive transfers, and within the female-male dyad Djamuna-Walter (Fig. 2, also online resource 3, SV4–SV7).
Comparison of active sharing in orangutans, chimpanzees, and bonobos
For this analysis, we compared a subset of our data (cf. section “Methods”) with the data from Jaeggi et al. (2010a) and three more recent studies (Crick et al. 2013; Silk et al. 2013; Yamamoto 2015). The data subset consisted of a total 368 food sharing events, of which were 210 cases sharing with immatures (Berlin 75, Dortmund A 102, Dortmund B 33) and 158 cases sharing among adults (Berlin 91, Dortmund A 16, Dortmund B 51). Comparing the proportions of active sharing between adults across species, Sumatran orangutans were revealed to share more frequently actively (median = 40.84) than both bonobos (median = 0.0), chimpanzees (median = 0.2) and Bornean orangutans (only one field study 0.0) (for details cf. online resource 2, SF6). A comparison of the genera Pongo vs. Pan, however, did not indicate a significant difference (Mann-Whitney’s U test: U = 33, exact p = 0.23).
In a second analysis, which comprised only studies in captivity to eliminate potential effects of the setting, we compared the proportion of active transfer for chimpanzees, bonobos, and Sumatran orangutans (Fig. 3) and found a clear difference between the species; while there were no active transfers among adults in both bonobo studies, chimpanzees shared considerably less actively than Sumatran orangutans (sharing among adults: medianchimp = 8.0 vs. medianorang = 48.4; sharing with infants/immatures: medianchimp = 3.41 vs. medianorang = 15.15). For the condition sharing among adults, we found a significant difference between chimpanzees and orangutans (Mann-Whitney’s U test: U = 0, exact p = 0.0357).
Percentage of active sharing in tolerated transfers for bonobos, chimpanzees and Sumatran orangutans in studies with a captive setting. The additional “inf.” following the species name means sharing with infants, “im.” means sharing with immatures and “ad.” means sharing among adults. Black triangles show data points, each referring to a study or independent group within a study. Boxplots summarize these data for the particular species and condition. Horizontal black lines indicate medians, colored boxes indicate interquartile ranges, whiskers indicate minima and maxima, and outliers are plotted as single data points. The numbers below the species names indicate the number of data points. The brace with asterisk indicates a significant difference. The data of the following Pan studies have been retrieved from a meta-analysis by Jaeggi et al. (2010a), Electronic supplementary information, Table S2): de Waal (1992) and Jaeggi et al. (2010c) for bonobos; de Waal (1989); de Waal (1997a) and Jaeggi et al. (2010c) for chimpanzees. These data have been supplemented with results from Crick et al. (2013) and Silk et al. (2013) for chimpanzees and from the present study for Sumatran orangutans
Factors that influence food sharing
We tested the impact of different factors on the probability of food sharing by conducting a generalized linear mixed model. The comparison of the full model with the test predictor social relation (measure: PSI) excluded, but with all control variables and random effects included, and the null model with those factors excluded revealed a clear effect of social relation on the probability of food sharing (likelihood ratio test: χ2 = 9.069, df = 1, p = 0.003; Table 4). More precisely, the individual probability to share food with another conspecific increased with the strength of their social relationship, as indicated by their corresponding PSI. The estimated effect of PSI as relative odds ratio shows that if the PSI increases by one unit, the predicted probability to share increases by factor 7.96, with all other factors constant. Furthermore, the model revealed a significant effect of food value, which was a fixed effect we controlled for (Table 4). Medium-preferred food increased the probability of sharing compared to highly preferred food. However, since we determined influential cases on both levels of random effects, which could not be excluded, this result has to be treated with caution. The latency between food monopolization by the possessor and the actual food interaction (latency with regard to monopolization) as a measure of the degree of repletion had no obvious impact on the probability of food sharing. Overall, the predictions derived by the model support our hypothesis on the effect of social relation on food sharing (Table 4).
Due to its collinearity with social relation, we could not include kin relation as a separate predictor into the model (cf. section “Methods”). However, when we ran a respective expanded model while ignoring the collinearity, neither did this alter the level of significance of social relation (likelihood ratio test: χ2 = 9.041, p = 0.0026), nor was kin relation a significant predictor itself (p = 0.719). The comparison of the expanded model with the original full model revealed no significant improvement due to the inclusion of kin relation (p = 0.712, for more details cf. online resource 1, ST10).
Discussion
The current study demonstrated that provisioning socially housed Sumatran orangutans with a monopolizable food item induced a large number and variety of food interactions among individuals of all age and sex classes, which frequently resulted in tolerated, often active transfers. Although the unequal possession of food created a potential conflict situation, both forced transfers despite the possessor’s resistance and aggressive responses to requests were uncommon. Request behavior very often involved persistent periods of peering and begging gestures awaiting a reaction, even by males and dominant females. This reflects inhibitory control in the presence of food by the solicitor and tolerance by the possessor which is consistent with more general observations that captive orangutan rarely engage in severe agonistic interactions (Jantschke 1972; Edwards and Snowdon 1980; Poole 1987) and show a moderate social tolerance (Amici et al. 2012), despite their unusual group structure in the zoo.
Social bonds and selective food sharing
While food sharing was frequent across groups, the probability for an individual to share food with another one increased with their relationship quality. Food sharing was not restricted to mother-offspring dyads which are naturally closely bonded (van Noordwijk and van Schaik 2005), but occurred frequently also among particular unrelated adults, both of the same sex and different sexes. Moreover, in Dortmund, food sharing took place even between groups, i.e., among individuals who were separately housed during the winter, but joined each other during the summer. Although the steel mesh between the groups restricted their opportunities to interact, it apparently did not prevent long-term associates from maintaining their relationships.
Especially the found selective sharing among adults concurs with the approach by Jaeggi and Gurven (2013a). They suggested that the actual readiness to share food depends on the value of the partner and therefore on their relationship history consisting of a succession of social interactions, including given, received, and denied benefits, which modulates the sharing psychology. In the wild, adult orangutans do not form coalitions, neither males nor females, and are barely involved in affiliative interaction (Utami Atmoko et al. 2009b; van Noordwijk et al. 2012). In captivity, on the other hand, orangutans regularly engage in affiliative interactions, e.g., social play (Jantschke 1972; Zucker et al. 1978, 1986), contact sitting and—though less frequently—allogrooming (Edwards and Snowdon 1980; Maple 1980), and even third-party intervention to cease conflicts (Tajima and Kurotori 2010; KSK and KL unpublished data). We therefore suggest that food sharing alongside other affiliative behaviors might serve as a means to strengthen social bonds in captive orangutans as in other primate species.
Mother-offspring sharing
We found high proportions of food sharing within the mother-daughter dyads, even when they were housed in neighboring groups, which concurs with observations in the wild, where sharing with offspring provides nutritional, but mainly informational benefits (Jaeggi et al. 2010b). Mothers, who are—at least until weaning—the only role models for young orangutans to acquire essential skills as to identify and process eatable food, frequently comply to begging infants and even facilitate the access to food (van Noordwijk et al. 2009). After weaning, mothers and their offspring spent increasingly less time in association and mothers become less tolerant toward their older offspring, especially when a new sibling has been born (van Noordwijk and van Schaik 2005; Jaeggi et al. 2008). However, there seems to be individual and—probably—species variation with regard to closeness and duration of those post-weaning relationships in orangutans (van Noordwijk et al. 2009). In our study, the mother in the Dortmund group A complied with the majority of requests by her independent immature daughter in the neighbored enclosure indicating still strong bonds between them. Our finding that nearly all solicitations by the dependent immature in Dortmund resulted in food transfer, while only half of the solicitations by the independent one in Berlin were successful, might reflect the decrease of food sharing with age observed under natural conditions (van Noordwijk and van Schaik 2005; Jaeggi et al. 2008), though, given the small sample size, these data are not representative.
Sharing between sexes
Both males were largely tolerant when they were in possession of food. While the male in Dortmund monopolized the food only on a few occasions, the male in Berlin particularly monopolized high-valued food and shared preferentially with the female with whom he was most closely associated. The male in Dortmund even received food from a female of the neighboring group. These findings concur with reports on female partner choice and male-female food sharing within consortships and the hypothesis that male generosity and tolerance in the presence of food might have been favored by sexual selection (Utami Atmoko et al. 2009a; van Noordwijk and van Schaik 2009). But again, the sample size hardly allows for an extrapolation.
Sharing within sex
Food sharing also occurred frequently among unrelated females with close social relationships, which recently has been demonstrated also for captive chimpanzees (Eppley et al. 2013). Long-term studies on wild orangutans provide evidence for female philopatry, which is uncommon in other great apes and most probably has an impact on female association and bonding (van Schaik 1999; Singleton et al. 2009; Arora et al. 2012; van Noordwijk et al. 2012). While mature males disperse, orangutan females tend to stay in their natal area (Mitra Setia et al. 2009). Maternally related females form clusters, show more tolerance and less aggression, and frequently associate among each other. Within these “safe” associations with familiar relatives, females tolerate or even enable social play among their offspring, which probably promotes the development of motor and cognitive skills and behavioral flexibility. Moreover, immatures might improve their ecological competence by social learning from other trustworthy role models than their mothers (van Noordwijk et al. 2012). While affiliative interactions among females themselves are uncommon in Bornean populations; in Sumatra, feeding in proximity and occasional food sharing has been observed (Singleton and van Schaik 2002). Although the question of direct fitness benefits for females is still unsettled, the observed patterns strongly suggest that female philopatry is an effective selection pressure in favor of social tolerance and bonding among maternally related, familiar females (van Noordwijk et al. 2012). The fact that most intergroup transfers in Dortmund took place between a female and her older daughter is in line with this explanation. Contrary to natural female clusters, our study groups also comprised unrelated females. However, although they had all been reared in different zoos, at the time of our study, they had already lived together for 5–8 years and had developed differentially strong long-term relationships, as proxied with the PSI. We suggest that food sharing among unrelated, but familiar females demonstrates the orangutan’s flexibility to use particular behaviors and abilities, which have evolved for other reasons, to meet the challenges of permanent group life.
In summary, informational dependence from the mother, female choice and female philopatry might have been effective selection pressures in favor of tolerant tendencies and social bonding and correspondingly might have shaped the species-specific sharing psychologies (Jaeggi and Gurven 2013a) in orangutans.
Unfortunately, it was not possible to provide a statistical prediction for the effect of relationship quality on the probability for sharing actively. Since sharing with immatures is mostly passive in non-human primates (Jaeggi et al. 2010a) and at the same time the strongest bonds are those between mothers and their immature offspring, we had to exclude immatures as solicitors to avoid this antagonizing effect, which resulted in a sample size too small and with data too unbalanced to build a stable model. In further studies, the effect of relationship quality on the probability of active sharing should be investigated by including more and larger groups with unrelated adults.
Active sharing across species
In the current study, orangutans engaged significantly more frequently in active food transfers than chimpanzees in similar studies. Species-specific sharing psychologies and differences in resulting behaviors are most likely due to different past selection pressures, i.e., differential necessities for sharing or needs for partners (Jaeggi et al. 2010a; Jaeggi and Gurven 2013a). Female philopatry has probably been a relevant selection pressure for orangutans, but not for chimpanzees (van Noordwijk et al. 2012), for whom need for supporters and direct food competition were probably more important. Reactive food transfer, the almost only type of active transfer in great apes, requires certain cognitive abilities in both sharing partners, especially inhibitory control as well as the production and the comprehension of signals of need (Jaeggi and Gurven 2013a). However, while these cognitive preconditions are present in great apes (Pika et al. 2005; Amici et al. 2008), direct food competition might have been a much stronger selective pressure in favor of successful competitive behavior for chimpanzees than for orangutans (Pelé et al. 2009), who adopted dispersion as a strategy to avoid direct food competition. This might have led to differing predispositions for active food sharing. A similar explanation has been suggested by Shumaker et al. (2001), who found that orangutans show better results than chimpanzees in a particular physical cognition task that required inhibitory control when confronted with highly preferred food (but see Vlamings et al. 2006). The fact that actively sharing orangutans in our study transferred more frequently good quality food than low quality food, which is contrary to findings for chimpanzee (Ueno and Matsuzawa 2004), also suggests different sharing psychologies with regard to active sharing.
It might be argued that the found species difference in active sharing may rather be the result of different food types used in tests with chimpanzees and orangutans, respectively. We cannot exclude that the food type has an influence on the sharing type. For example, in contrast to the provided food in our study, leafy branches or paper bags filled with vegetables as used in a part of the Pan studies (de Waal 1989, 1992, 1997a; Jaeggi et al. 2010c; Crick et al. 2013) are less compact and therefore more difficult to monopolize. The effort of defending them could be more costly than tolerating the occasional taking, which might result in a lower proportion of active in favor of passive sharing. However, other studies with chimpanzees included in this comparison used ice blocks containing banana sections or peanuts (Crick et al. 2013; Silk et al. 2013) which were as compact and defensible as pumpkins, melons, and pineapples provided in our study. Crick et al. (2013), who used both branches and ice blocks, found that trials with ice blocks induced more active sharing than those with branches, but the difference was not significant. Moreover, despite the usage of paper bags, the chimpanzees in the study by Jaeggi et al. (2010c) shared more often actively than in the studies using ice blocks (Crick et al. 2013; Silk et al. 2013). To investigate the possible separate effects of species-specific sharing psychologies and food type, food sharing in chimpanzees, bonobos, and Bornean orangutans should be similarly tested with large fruits as used in our study.
Proximate aspects
Psychological and contextual aspects of food sharing and other prosocial behaviors in non-human primates receive increasing attention (e.g., Penner et al. 2005; Schino and Aureli 2009; Cronin 2012; Yamamoto and Takimoto 2012). However it is often difficult to determine the actual motivations that bring about food sharing (de Waal and Suchak 2010), or to reliably identify whether the food is transferred voluntarily (e.g., Bullinger et al. 2013). Therefore, we conducted our analysis on the behavioral instead of motivational level to categorize transfers with regard to their tolerance, identified by certain request and resistance behaviors and the possessor’s active contribution to the transfer. This approach allows for ascribing directedness and intentionality to active food transfers at least (Pelé et al. 2009). Moreover, the two neighboring groups in Dortmund engaged repeatedly, mostly actively, in food transfers through the mesh. These instances indicated that sharing occurred voluntarily, because the possessor would have had the opportunity to feed outside the reach of their neighbors, but instead stayed close to the mesh and actively transferred food to particular individuals. To gain deeper insight into proximate mechanisms of food sharing and bonding, future studies on socially housed orangutans should account for the dynamic of social relationships within groups and the relationship between food sharing and relationship quality.
Methodological issues
The test setting did not allow for investigating the value of sharing partners with regard to direct, short-term reciprocity, because food possession was not equally distributed among the individuals. Most individuals who received food were rarely in a position to give food. Nonetheless, we do not consider this as a flaw of the study design. In contrast to previous studies with pre-established dyads (e.g., de Waal 1997b; Burkart et al. 2007; Hare and Kwetuenda 2010), the current study—as in similar studies with chimpanzees and bonobos (e.g., de Waal 1989; Jaeggi et al. 2010c; Crick et al. 2013; Silk et al. 2013)—enabled the individuals to potentially interact with all other individuals of their respective group and to choose with whom to share.
Summary
In this study, we investigated the behavior of captive, group-living orangutans in the context of food sharing. We found that orangutans, when provided with monopolizable food, shared frequently, selectively, and actively with close social partners. A comparison with the results of previous studies demonstrated a significantly higher proportion of active transfers than found for captive chimpanzees. These results support recent findings from long-term studies on wild populations regarding the role and evolution of social bonds in orangutans. Moreover, since permanent group living differs from their social organization in the wild, the observed complex food interactions indicate a remarkable flexibility of orangutans’ social behavior in captive settings.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 40:227–266
Amici F, Aureli F, Call J (2008) Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol 18:1415–1419
Amici F, Call J, Aureli F (2012) Aversion to violation of expectations of food distribution: the role of social tolerance and relative dominance in seven primate species. Behaviour 149:345–368
Arora N, van Noordwijk M, Ackermann C, Willems E, Nater A, Greminger M, Nietlisbach P, Dunkel L, Utami Atmoko S, Pamungkas J (2012) Parentage‐based pedigree reconstruction reveals female matrilineal clusters and male‐biased dispersal in nongregarious Asian great apes, the Bornean orang‐utans (Pongo pygmaeus). Mol Ecol 21:3352–3362
Baayen RH (2008) Analyzing linguistic data. A practical introduction to statistics using R. Cambridge University Press, Cambridge
Bates D, Maechler M, Bolker B, Walker S (2015) lme4: linear mixed-effects models using ‘Eigen’ and S4. R package vers 1.1-10, http://CRAN.R-project.org/package=lme4
Batson CD, Powell AA (2003) Altruism and prosocial behavior. In: Millon T, Lerner MJ (eds) Handbook of psychology, vol 5. Wiley, Hoboken, pp 463–484
Brown GR, Almond REA, van Bergen Y (2004) Begging, stealing and offering: food transfer in non-human primates. Adv Study Behav 34:265–295
Bullinger AF, Burkart JM, Melis AP, Tomasello M (2013) Bonobos, Pan paniscus, chimpanzees, Pan troglodytes, and marmosets, Callithrix jacchus, prefer to feed alone. Anim Behav 85:51–60
Burkart JM, Fehr E, Efferson C, van Schaik CP (2007) Other-regarding preferences in a non-human primate: common marmosets provision food altruistically. Proc Natl Acad Sci U S A 104:19762–19766
Calcutt SE, Lonsdorf EV, Bonnie KE, Milstein MS, Ross SR (2014) Captive chimpanzees share diminishing resources. Behaviour 151:1967–1982
Carter GG (2014) The reciprocity controversy. Anim Behav Cogn 1:368–386
Carter GG, Wilkinson GS (2013) Food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Proc R Soc B 280:20122573
Cheney DL (2011) Extent and limits of cooperation in animals. Proc Natl Acad Sci U S A 108(Suppl 2):10902–10909
Clutton-Brock TH (2009) Cooperation between non-kin in animal societies. Nature 462:51–57
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
R Core Team (2015) R: a language and environment for statistical computing. vers 3.2.2, R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Crick J, Suchak M, Eppley TM, Campbell MW, de Waal FBM (2013) The roles of food quality and sex in chimpanzee sharing behavior (Pan troglodytes). Behaviour 150:1203–1224
Cronin KA (2012) Prosocial behaviour in animals: the influence of social relationships, communication and rewards. Anim Behav 84:1085–1093
de Kort SR, Emery NJ, Clayton NS (2003) Food offering in jackdaws (Corvus monedula). Naturwissenschaften 90:238–240
de Waal FBM (1989) Food sharing and reciprocal obligations among chimpanzees. J Hum Evol 18:433–459
de Waal FBM (1992) Appeasement, celebration, and food sharing in the two Pan species. In: Nishida T, McGrew WC, Marler P, Pickford M, de Waal FBM (eds) Topics in primatology, vol 1, Human origins. University of Tokyo Press, Tokyo, pp 37–50
de Waal FBM (1997a) The chimpanzee’s service economy: food for grooming. Evol Hum Behav 18:375–386
de Waal FBM (1997b) Food transfers through mesh in brown capuchins. J Comp Psychol 111:370–378
de Waal FBM (2008) Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol 59:279–300
de Waal FBM, Suchak M (2010) Prosocial primates: selfish and unselfish motivations. Phil Trans R Soc B 365:2711–2722
Dovidio JF, Piliavin JA, Schroeder DA, Penner LA (2006) The social psychology of prosocial behavior. Lawrence Erlbaum Associates Inc., Mahwah
Edwards SD, Snowdon CT (1980) Social behavior of captive, group-living orangutans. Int J Primatol 1:39–62
Eisenberg N, Fabes RA, Spinrad TL (2007) Prosocial development. In: Eisenberg N (ed) Handbook of child psychology, vol 3. Wiley, Hoboken, pp 646–718
Eppley TM, Suchak M, Crick J, de Waal FBM (2013) Perseverance and food sharing among closely affiliated female chimpanzees. Primates 54:319–324
Feistner ATC, McGrew WC (1989) Food-sharing in primates: a critical review. In: Seth PK, Seth S (eds) Perspectives in primate biology, vol 3. Today’s and Tomorrow’s Printers & Publishers, New Delhi, pp 21–36
Fox EA (2002) Female tactics to reduce sexual harassment in the Sumatran orangutan (Pongo pygmaeus abelii). Behav Ecol Sociobiol 52:93–101
Fox J, Weisberg S (2011) An {R} companion to applied regression. Sage, http://socserv.socsci.mcmaster.ca/jfox/Books/Companion
Freidin E, Carballo F, Bentosela M (2015) Direct reciprocity in animals: the roles of bonding and affective processes. Int J Psychol. doi:10.1002/ijop.12215
Gamer M, Lemon J, Fellows I, Puspendra S (2012) irr: various coefficients to interrater reliability and agreement. R package vers 0.84, http://www.r-project.org
Gintis H, Bowles S, Boyd R, Fehr E (2003) Explaining altruistic behavior in humans. Evol Hum Behav 24:153–172
Goetz JL, Keltner D, Simon-Thomas E (2010) Compassion: an evolutionary analysis and empirical review. Psychol Bull 136:351–374
Gomes CM, Boesch C (2009) Wild chimpanzees exchange meat for sex on a long-term basis. PLoS One 4:e5116
Gurven M, Jaeggi AV (2015) Food sharing. In: Scott R, Kosslyn S (eds) Emerging trends in the social and behavioral sciences. Wiley, Hoboken, pp 1–12
Hamilton WD (1964) The genetical evolution of social behaviour. I & II. J Theor Biol 7:1–52
Hardus ME, Lameira AR, Zulfa A, Atmoko SSU, de Vries H, Wich SA (2012) Behavioral, ecological, and evolutionary aspects of meat-eating by Sumatran orangutans (Pongo abelii). Int J Primatol 33:287–304
Hare B, Kwetuenda S (2010) Bonobos voluntarily share their own food with others. Curr Biol 20:R230–R231
Hockings KJ, Humle T, Anderson JR, Biro D, Sousa C, Ohashi G, Matsuzawa T (2007) Chimpanzees share forbidden fruit. PLoS One 2:e886
Hohmann G, Fruth B (2008) New records on prey capture and meat eating by bonobos at Lui Kotale, Salonga National Park, Democratic Republic of Congo. Folia Primatol 79:103–110
Jaeggi AV, Gurven M (2013a) Natural cooperators: food sharing in humans and other primates. Evol Anthropol 22:186–195
Jaeggi AV, Gurven M (2013b) Reciprocity explains food sharing in humans and other primates independent of kin selection and tolerated scrounging: a phylogenetic meta-analysis. Proc R Soc B 280:20131615
Jaeggi AV, van Schaik CP (2011) The evolution of food sharing in primates. Behav Ecol Sociobiol 65:2125–2140
Jaeggi AV, van Noordwijk MA, van Schaik CP (2008) Begging for information: mother-offspring food sharing among wild Bornean orangutans. Am J Primatol 70:533–541
Jaeggi AV, Burkart JM, van Schaik CP (2010a) On the psychology of cooperation in humans and other primates: combining the natural history and experimental evidence of prosociality. Phil Trans R Soc B 365:2723–2735
Jaeggi AV, Dunkel LP, van Noordwijk MA, Wich SA, Sura AAL, van Schaik CP (2010b) Social learning of diet and foraging skills by wild immature Bornean orangutans: implications for culture. Am J Primatol 72:62–71
Jaeggi AV, Stevens JMG, van Schaik CP (2010c) Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. Am J Phys Anthropol 143:41–51
Jaeggi AV, de Groot E, Stevens JMG, van Schaik CP (2013) Mechanisms of reciprocity in primates: testing for short-term contingency of grooming and food sharing in bonobos and chimpanzees. Evol Hum Behav 34:69–77
Jantschke F (1972) Orang-Utans in Zoologischen Gärten. R. Piper & Co. Verlag, München
Levine RV, Norenzayan A, Philbrick K (2001) Cross-cultural differences in helping strangers. J Cross-Cult Psychol 32:543–560
Liebal K, Pika S, Tomasello M (2006) Gestural communication of orangutans (Pongo pygmaeus). Gesture 6:1–38
Maple TL (1980) Orangutan behavior. Van Nostrand-Reinhold, New York
Martin P, Bateson P (2007) Measuring behaviour. An introductory guide. Cambridge University Press, Cambridge
Maynard Smith J (1964) Group selection and kin selection. Nature 201:1145–1147
Mitani JC (2006) Reciprocal exchange in chimpanzees and other primates. In: Kappeler PM, van Schaik CP (eds) Cooperation in primates and humans: mechanisms and evolution. Springer, New York, pp 107–119
Mitra Setia T, Delgado RA, Utami Atmoko SS, Singleton I, van Schaik CP (2009) Social organization and male–female relationships. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variations in behavioral ecology and conservation. Oxford University Press, New York, pp 245–253
Nieuwenhuis R, te Grotenhuis M, Pelzer B (2012) Influence.ME: tools for detecting influential data in mixed effects models. R J 4:38–47
Pelé M, Dufour V, Thierry B, Call J (2009) Token transfers among great apes (Gorilla gorilla, Pongo pygmaeus, Pan paniscus, and Pan troglodytes): species differences, gestural requests, and reciprocal exchange. J Comp Psychol 123:375–384
Penner LA, Dovidio JF, Piliavin JA, Schroeder DA (2005) Prosocial behavior: multilevel perspectives. Annu Rev Psychol 56:365–392
Pika S, Liebal K, Call J, Tomasello M (2005) The gestural communication of apes. Gesture 5:41–56
Poole TB (1987) Social behavior of a group of orangutans (Pongo pygmaeus) on an artificial island in Singapore Zoological Gardens. Zoo Biol 6:315–330
Schino G, Aureli F (2009) Reciprocal altruism in primates: partner choice, cognition, and emotions. Adv Study Behav 39:45–69
Shumaker RW, Palkovich AM, Beck BB, Guagnano GA, Morowitz H (2001) Spontaneous use of magnitude discrimination and ordination by the orangutan (Pongo pygmaeus). J Comp Psychol 115:385–391
Silk JB (2007) The adaptive value of sociality in mammalian groups. Phil Trans R Soc B 362:539–559
Silk JB, House BR (2011) Evolutionary foundations of human prosocial sentiments. Proc Natl Acad Sci U S A 108(Suppl 2):10910–10917
Silk JB, Altmann J, Alberts SC (2006) Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav Ecol Sociobiol 61:183–195
Silk JB, Brosnan SF, Henrich J, Lambeth SP, Shapiro S (2013) Chimpanzees share food for many reasons: the role of kinship, reciprocity, social bonds and harassment on food transfers. Anim Behav 85:941–947
Singleton I, van Schaik CP (2002) The social organisation of a population of Sumatran orang-utans. Folia Primatol 73:1–20
Singleton I, Knott CD, Morrogh-Bernard HC, Wich SA, van Schaik CP (2009) Ranging behavior of orangutan females and social organization. In: Wich SA, Utami-Atmoko SS, Setia TM, van Schaik CP (eds) Orangutans: geographic variations in behavioral ecology and conservation. Oxford University Press, New York, pp 205–213
Stevens JR, Gilby IC (2004) A conceptual framework for nonkin food sharing: timing and currency of benefits. Anim Behav 67:603–614
Stevens JR, Stephens DW (2002) Food sharing: a model of manipulation by harassment. Behav Ecol 13:393–400
Sugardjito J, Nurhuda N (1981) Meat-eating behaviour in wild orang utans, Pongo pygmaeus. Primates 22:414–416
Tajima T, Kurotori H (2010) Nonaggressive interventions by third parties in conflicts among captive Bornean orangutans (Pongo pygmaeus). Primates 51:179–182
Tomasello M, Vaish A (2013) Origins of human cooperation and morality. Annu Rev Psychol 64:231–255
Trivers RL (1971) The evolution of reciprocal altruism. Q Rev Biol 46:35–57
Ueno A, Matsuzawa T (2004) Food transfer between chimpanzee mothers and their infants. Primates 45:231–239
Utami Atmoko SS, Mitra Setia T, Goossens B, James SS, Knott CD, Morrogh-Bernard HC, van Schaik CP, van Noordwijk MA (2009a) Orangutan mating behavior and strategies. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variations in behavioral ecology and conservation. Oxford University Press, New York, pp 235–244
Utami Atmoko SS, Singleton I, van Noordwijk MA, van Schaik CP, Mitra Setia T (2009b) Male-male relationships in orangutans. In: Wich SA, Utami-Atmoko SS, Setia TM, van Schaik CP (eds) Orangutans: geographic variations in behavioral ecology and conservation. Oxford University Press, New York, pp 225–234
Utami SS, van Hooff JARAM (1997) Meat-eating by adult female Sumatran orangutans (Pongo pygmaeus abelii). Am J Primatol 43:159–165
van Noordwijk MA, van Schaik CP (2005) Development of ecological competence in Sumatran orangutans. Am J Phys Anthropol 127:79–94
van Noordwijk MA, van Schaik CP (2009) Intersexual food transfer among orangutans: do females test males for coercive tendency? Behav Ecol Sociobiol 63:883–890
van Noordwijk MA, Sauren SEB, Abulani Ahbam N, Morrogh-Bernard HC, Utami Atmoko SS, van Schaik CP (2009) Development of independence: Sumatran and Bornean orangutans compared. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variations in behavioral ecology and conservation. Oxford University Press, New York, pp 189–203
van Noordwijk MA, Arora N, Willems EP, Dunkel LP, Amda RN, Mardianah N, Ackermann C, Krützen M, van Schaik CP (2012) Female philopatry and its social benefits among Bornean orangutans. Behav Ecol Sociobiol 66:823–834
van Schaik CP (1999) The socioecology of fission-fusion sociality in orangutans. Primates 40:69–86
van Schaik CP, Burkart JM (2011) Social learning and evolution: the cultural intelligence hypothesis. Phil Trans R Soc B 366:1008–1016
Vlamings PHJM, Uher J, Call J (2006) How the great apes (Pan troglodytes, Pongo pygmaeus, Pan paniscus and Gorilla gorilla) perform on the reversed contingency task: the effects of food quantity and food visibility. J Exp Psychol Anim Behav Process 32:60–70
Weingrill T, Willems EP, Zimmermann N, Steinmetz H, Heistermann M (2011) Species-specific patterns in fecal glucocorticoid and androgen levels in zoo-living orangutans (Pongo spp.). Gen Comp Endocrinol 172:446–457
Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbühler K (2014) Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc R Soc B 281:20133096
Yamamoto S (2015) Non-reciprocal but peaceful fruit sharing in wild bonobos in Wamba. Behaviour 152:335–357
Yamamoto S, Takimoto A (2012) Empathy and fairness: psychological mechanisms for eliciting and maintaining prosociality and cooperation in primates. Soc Justice Res 25:233–255
Zahavi A (1995) Altruism as a handicap: the limitations of kin selection and reciprocity. J Avian Biol 26:1–3
Zahn-Waxler C, Radke-Yarrow M, Wagner E, Chapman M (1992) Development of concern for others. Dev Psychol 28:126–136
Zucker EL, Mitchell G, Maple T (1978) Adult male-offspring play interactions within a captive group of orang-utans (Pongo pygmaeus). Primates 19:379–384
Zucker EL, Dennon MB, Puleo SG, Maple TL (1986) Play profiles of captive adult orangutans: a developmental perspective. Dev Psychobiol 19:315–326
Acknowledgments
We cordially thank the directors, curators, and keepers at Zoo Berlin and Zoo Dortmund for their extraordinary support, especially André Schüle, Christian Aust, and Ruben Gralki from Berlin and Ilona Schappert, Eddy Laudert, Natascha Kurt, Sonja Borchers, and Jörg Woitzik from Dortmund. We are particularly grateful to Martin Schultze for his statistical support. Special thanks go to Adrian Jaeggi for the kind and helpful advice. Many thanks also to Roger Mundry for providing a diagnostic function for R. We are very thankful to Anja Kopper and Laura Thomson for conducting the reliability coding, to Suska Nolte for help with the proximity scan, and to Bruno Suski for his assistance in the coding of food quality. Finally, we thank Maria van Noordwijk and two anonymous reviewers for their comments, which were very helpful to improve earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal welfare
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Both zoos, where this study has been conducted, are members of the European Association of Zoos and Aquaria (EAZA) and of the World Association of Zoos and Aquariums (WAZA). Animal husbandry and research comply with the EAZA Minimum Standards for the Accommodation and Care of Animals in Zoos and Aquaria and the WAZA Ethical Guidelines for the Conduct of Research on Animals by Zoos and Aquariums.
Additional information
Communicated by M. A. van Noordwijk
Rights and permissions
About this article
Cite this article
Kopp, K.S., Liebal, K. Here you are!—Selective and active food sharing within and between groups in captive Sumatran orangutans (Pongo abelii). Behav Ecol Sociobiol 70, 1219–1233 (2016). https://doi.org/10.1007/s00265-016-2130-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2130-2