Abstract
We study the plasticity of collective decision-making in ants by blocking key aspects of pheromone communication across entire colonies. To achieve this, droplets of paint were applied over the gaster tips of entire worker populations within colonies of the rock ant, Temnothorax albipennis. This treatment should prevent pheromone release potentially from each ant’s Dufour’s, poison, and pygidial glands in addition to the hindgut. We then examined the collective decision-making abilities of treatment and control colonies over alternative new nest sites in binary choice experiments. The performance of treatment colonies was compared with that of control colonies that had also been marked with paint but in such a way as not to disrupt their pheromone excretions from the gaster tip. Our results reveal the importance of “gaster-tip” pheromones during colony emigrations. Treatment-colony emigrations were significantly less successful than those of the controls, as the quality of their nest site assessments was reduced. However, treatment ants presented an extraordinary example of behavioral plasticity as they reduced their quorum thresholds in order to maintain normal emigration completion times. Hence, the ants whose communication systems have been compromised can still emigrate swiftly and maintain low levels of colony exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ants are extremely successful ecologically. Much of this success can be attributed to their highly organized social structures, and their very effective division of labor. These traits enable ants to manipulate their surroundings to create local environments that suit them better (Hölldobler and Wilson 1990; Wilson and Hölldobler 2005). The communication systems of ants and other social insects also favor, under certain environmental conditions (Sherman and Visscher 2002), very effective collective decisions. Such decisions can be made without any centralized control, such that ant colonies are able to distribute cognitive tasks across a multitude of workers (Pratt et al. 2002).
An ideal method to demonstrate the importance of communication during collective processes in ant colonies would be to create a class of ant colony in which key communication was largely prevented. The behavior of these “Incommunicado” colonies could then be compared to that of ant colonies with unimpaired communication. Here, we use just such an experimental procedure. We develop and apply an experimental procedure that extirpates “gaster-tip” pheromone excretions in entire colonies of the rock ant, Temnothorax albipennis. We applied droplets of paint directly to the gaster tip where the sting protrudes in an attempt to block “gaster-tip” pheromone excretions. These treatment colonies were then induced to emigrate in a binary choice experiment, where their behavior was directly compared to that of ant colonies that had also been marked with paint, but in a way as not to disrupt “gaster-tip” pheromone excretion.
The approach of testing the importance of communication systems in social insects by disabling such communication is well established. For example, Karl von Frisch sealed the Nasanov glands of certain honeybees with shellac (von Frisch 1967). Indeed, such work might suggest that pheromones are not very important in honeybee foraging—though as both Karl von Frisch (1967) and Gould (1976) pointed out that this has to be treated with caution because the honeybees in these experiments seemed to be more attracted to the smell of shellac than to heavily scented flowers and given that the “shellac odor was present only in the vicinity of the forager station, these experiments do not rule out olfactory recruitment” (Gould 1976). More recent work with honeybees by Sherman and Visscher (2002) disabled their dance communication by re-orientating the combs upon which scouts dance—and this work suggested that dance communication is only valuable to the bees under certain foraging conditions. Later modeling studies by Schürch and Grüter (2014) suggest, however, that the value of waggle dance communication in honey bees can be underestimated if suitable time frames are not taken fully into account. Blocking the production or transfer of pheromones has also been used successfully in studies of ants. For example, Soroker et al. (1998) blocked the mouths of Pachycondyla workers with beeswax and thus showed that the transfer of potential colony odors to the post-pharyngeal gland was based more on allogrooming than trophallaxis. Maschwitz and Schönegge (1977, 1983) also used wax to block the excretion of certain pheromones used by Leptogenys ants during nest relocations. For a recent review of the pros and cons of social information use by social insects, see Grueter and Leadbeater (2014).
Our study system is collective decision-making by ants over new nest sites. Our focal species is T. albipennis. Colonies of this species reside in rock crevices and other natural preformed cavities (Franks et al. 2002). These nest sites are often ephemeral, compelling frequent emigrations. Such nest sites are easily replicated in laboratory conditions and emigrations can be induced experimentally (Franks et al. 2003). T. albipennis colony emigrations encompass a set of behavioral stages whose progression is governed by consistent behavioral stimuli (Pratt et al. 2002). The chronological order of such sequentially progressive emigrations is likely to be organized, in part, by pheromone communication, even though T. albipennis do not use recruitment trails (Pratt et al. 2001; McLeman et al. 2002). Moreover, colonies are typically able to establish appropriate levels of opinion polling that result in an emigration commitment that is often both accurate (i.e., to the best available nest; Franks et al. 2003) and cohesive (i.e., all the ants in a colony end up in the same new nest site even if identical alternatives have been available; Franks et al. 2013). Therefore, T. albipennis and their emigrations form a good model for collective behavioral analyses. The behavioral stages of the emigration include scouting, nest site assessment, tandem run recruitment, quorum achievement, social carrying recruitment, and emigration completion (Fig. 1).
Before the ants can initiate any emigration behavior, a new nest site has to be found. This is achieved through scouting. Workers are always in search of new potential nests of higher quality than the one they currently occupy (Dornhaus et al. 2004). Here, it is likely that a worker improves its own navigation between the original and new potential nest sites by laying orientation pheromone trails. Once a new nest site has been found, nest site assessment can take place. It has been established that T. albipennis prefer a narrow entrance leading to a dark nest cavity of appropriate size. Indeed, such preferences remain consistent regardless of colony size (Franks et al. 2006). For full details of nest site preferences, see Franks et al. (2003). Ants have an ability to estimate area by utilizing the Buffon’s needle algorithm (Mallon and Franks 2000). If a scout recognizes potential in a new nest site, it will leave and re-enter the nest several times. During its first visit, an ant explores a potential nest cavity laying her own specific trail both across the center of the nest cavity and around its periphery. On a subsequent visit without further trail laying, she estimates the frequency that she crosses her previous path. This Buffon’s needle algorithm enables an ant to estimate the area of a cavity: small spaces would have crossing frequencies that are too high; excessively large spaces would have crossing frequencies that are too low. Using this as a proxy, the algorithm permits the ant to make an estimation of nest cavity area and thus contributes significantly to their nest site assessment (Mallon and Franks 2000).
If the scout recognizes in a new nest site attributes superior to those of their current nest, that scout may go on to initiate recruitment by tandem-running. Recruitment is defined as communication that brings nest mates to some point in space where work needs to be done (Wilson 1971). Tandem-run recruitment occurs when a knowledgeable scout individually leads a worker to the new resource (Franks and Richardson 2005). Tandem running is a slow and careful process (Franklin 2014). Intermittent antennal contact between the recruit and recruiter is required to maintain a tandem run; however, it is likely that the required recruitment signal to entice a follower is pheromonal (Möglich 1979; Basari et al. 2014). Tandem run recruitment teaches the recruit the location of the new nest site. This means the recruit can in turn become a recruiter (Franks and Richardson 2005). The better the new nest site, the sooner a scout will begin tandem-run recruitment (Mallon et al. 2001). Therefore, high quality nest sites will receive more assessors sooner than nest sites of low quality. This creates a positive feedback loop that facilitates well-informed discrimination among a multitude of potential sites (Franks et al. 2002). Eventually, ants will begin to accumulate in potentially suitable new nest sites. This can lead to quorum achievement.
Ants entering a new nest site are able to estimate the number of individuals within the nest through encounter rates (Pratt 2005). If they find a sufficient abundance of nest mates, a quorum threshold is met and the ants commit to emigrating to that nest site. Such quorum sensing triggers a switch from slow tandem run recruitment to fast social carrying recruitment. Social carrying recruitment is when fellow workers, brood, and the queen are carried directly to the new nest site (Möglich and Hölldobler 1974). This is a fast form of recruitment where carriers take a direct route between original and new nest sites. This switch from slow to fast recruitment indicates full commitment to an emigration as carried ants do not learn the route between nest sites, such that they are unlikely to return to the original nest site (Möglich and Hölldobler 1974). This is the final step culminating in emigration completion, when the majority of workers, the colony queen, and all brood items have been safely established within the new nest site. Pheromone communication is likely to be an important factor in the navigation and communication used throughout these emigrations. However, little research has focused on the specific importance of pheromones at each behavioral stage of an emigration and how plastic this behavior can be.
Our goal here is to not only to gain further knowledge about the processes that help build a consensus during T. albipennis emigrations, but also to determine some of the behavioral mechanisms that underpin such processes. In addition to this, we aim to assess the ability of the ants to adapt to constrained communication, furthering our understanding of the ants’ capacity for plastic behavior. We hypothesize first colonies that are not able to use pheromones will show behavioral plasticity in the process of emigrating to achieve a successful collective decision; second, the ants will be able to compensate for the loss of communication by adjusting their behavior in the emigration stages were pheromone use is less important.
Methods
Collecting and culturing of experimental colonies
Thirty-two queen right T. albipennis colonies were collected from a site in Dorset, UK, in September 2012. All colonies had brood present at varying developmental stages. Colony sizes ranged from 36 to 86 workers in both the control and the treatment. Colonies were housed in small petri dishes (10 × 10 × 1.9 cm) with Fluon®-coated walls to prevent escape. Within the petri dishes, colonies resided in artificial nests (Sendova-Franks and Franks 1995) consisting of a cardboard perimeter (38 × 59 × 1 mm) sandwiched by two microscope slides. A cardboard cover was used to darken the nest cavity. Colonies were resourced with an ad libitum supply of honey solution, three Drosphila flies, and they were given water once a week (Sendova-Franks and Franks 1995).
“Gaster-tip” pheromone extirpation and control painting
All workers in 16 of the 32 colonies had their “gaster-tip” pheromones extirpated. This was achieved by applying a small droplet of paint (PACTRA R/C polycarbonate, ketone-soluble, model paint) directly over the gaster tip where an individual’s sting protrudes. These formed the treatment colonies (Fig. 2a). This “blocking” method should prevent the release of pheromones from the hindgut as well as the Dufour’s, poison, and pygidial glands (Fig. 3). All workers were individually anesthetized using low levels of CO2 released into a crystallization dish, and then held unharmed within a slit cut in the surface of a sponge, positioned on the stage of a dissection microscope (Sendova-Franks and Franks 1993). This allowed only the gaster to protrude from the sponge, thus exposing only the part of the ant where paint needed to be applied. Once painted, each ant was kept in isolation in an Eppendorf tube for a minimum of 2 h while the paint dried. This limited paint removal was done via social grooming. A similar procedure was applied to the remaining 16 colonies. However, paint droplets were only applied to the top of the abdomen (Fig. 2b). This resulted in a set of control colonies that had undergone the same “stress” of the painting process, but their “gaster-tip” pheromone excretions had not been manipulated.
Panel a shows a T. albipennis worker with a droplet of paint applied to the tip of its gaster to prevent it from excreting pheromones. This represents the treatment. Panel b shows a T. albipennis worker with a droplet of paint applied to a different region of its gaster as a control. The entire length of the gaster is less than 1 mm
Schematic diagram of an ant’s abdomen, showing relevant gaster tip organs and gland morphology. The red crosses show the areas where excretion pheromones from the hindgut and the Dufour’s, poison, and pygidial glands are prevented from excretion (both Dufour’s and poison gland are excreted through the sting). The top yellow shape represents the paint droplet applied in the control (away from the gaster tip) and the other shows the position of the paint droplet applied in the treatment (covering the gaster tip). Redrawn from Mitra (2013)
Inducing emigrations
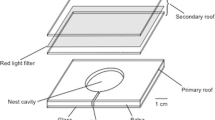
To encourage colonies to initiate emigrations within a relatively short time period, without causing any emergency behavior by destroying the original nest, both treatment (n = 16) and control colonies (n = 16) began each experiment in a nest with poor attributes. This was achieved by building a nest with a removable entrance wall that provided a nest entrance of preferable width when inserted, but an undesirably large nest entrance when it was removed (Fig. 4). Such an increase in entrance size does not induce the emergency behavior caused by the commonly used procedure of roof removal (Franks et al. 2006). Moreover, the procedure used here guaranteed colonization of the nest directly after painting. This method also ensured that ants would search their surroundings, as it has been shown that the lower the quality their original nest site is, the more scouts are committed to searching (Doran et al. 2013).
All emigrations were induced in relatively small arenas (23 × 23 cm; Fig. 5). Emigrating colonies emigrated in a “move to improve” scenario (Dornhaus et al. 2004). The ants were given a binary choice between a “deluxe” and a “good” quality nest. “Deluxe” nests offered a smaller nest entrance and a darker nest (using a dark red semi-transparent cover) cavity than those of the “good” quality nests (has no darkening cover) and the original nest offered the largest entrance and no darkening cover. The “deluxe” and “good” quality nests had entrance tunnels that were 1 and 4 mm wide, respectively: all of the other measurements of all of the nests were the same; cavity depths were 1.6 mm, cavity areas were 33 × 25 mm, and entrance tunnels were 4 mm deep. For further information regarding these nest qualities, see Dornhaus et al. (2004). The relative positions in which these new nest sites were placed in the arena were switched after each experiment. The distance between all three nests (original, good, and deluxe) was kept constant throughout all experiments: 7.7 cm between the original and two new nest sites, and 3.5 cm between the two new nest sites (Fig. 5).
Each experimental replicate began when the colony’s current nest, which contained most if not all the ants, was placed in the experimental arena (Fig. 5). Ants that were not in the nest at that time were picked up using softly sprung forceps (these forceps do not injure the ants) and delicately placed on top of their nest within the experimental arena. As soon as all ants were in the experimental arena, the entrance wall was removed and a stopwatch started. This marked the beginning of the experiment. If the ants had not begun to emigrate after 6 h, the experiments were terminated under the assumption that the colony had failed to make a collective decision. For all emigrations, the arena was illuminated with one LED lamp. The arenas were cleaned thoroughly with alcohol after each emigration to remove any existing pheromone excretions.
Data collection and analyses
All emigrations were directly observed from start to finish. Data was collected in order to analyze the characteristics of each stage in the emigrations and to obtain dynamical data relevant to the ants’ progression throughout the emigration. Emigration dynamics and their characteristics recorded in chronological order included:
Nest site assessment
The number of visits within each of the two new nest sites was recorded from the time the entrance wall was removed (Fig. 4), marking the beginning of the experiment, until the first occurrence of social carrying. The latter indicates that a quorum threshold had been reached. Multiple visits performed by the same individuals were all recorded as separate entry events.
Tandem run recruitment
To determine whether tandem runs could be performed by the treatment and control ants, all initiated tandem runs were recorded. Forward and reverse tandem run initiations were pooled for data analysis. This is because both forms of recruitment contribute to training ants to have an active role in the emigration by carrying nest mates from the old to the new nest site (see: Franks et al. 2009). Initiations of tandem runs are when ants are recruited from the original to the new nest site. Initiation of reverse tandem runs occurs when ants are recruited from the new to the original nest site.
Quorum achievement
The number of ants within a new nest site was recorded at the time when the first social carrying to that site was observed. This population of ants constitutes the quorum threshold as indicated by the first switch in the behavior of some of the ants from assessment and tandem running to social carrying recruitment (either of adults or brood).
Transport recruitment
The number of adult and brood transports performed was recorded. Adult and brood transports were pooled for data analysis.
Emigration completion
The time between the beginning of the experiment and the portage of the final brood item into the new nest site was recorded. This represented the emigration completion time.
Emigration success
An emigration was deemed successful if a colony collectively emigrated to the “deluxe” new nest site. An emigration was deemed unsuccessful either if a colony remained within the original nest or it emigrated to the “good” new nest site.
Data was analyzed using the Minitab 15 statistical package. Box plots and Mann–Whitney U tests were used to test for statistical significance for all emigration dynamics in addition to colony size comparisons. In certain cases, a data set was used for two comparisons, and in such cases, we applied a Bonferroni correction. Emigration success was tested for significance using a Fisher’s exact test. Non-parametric methods were used because the distribution of data sets was significantly different from normal.
Results
Colony size
The sizes of colonies used in the controls and the treatments were not significantly different (colony sizes ranged from 36 to 86, Mann–Whitney: p = 0.9269, U = 128; control: n = 16; treatment: n = 16). For this reason, we have not normalized the data by expressing the results as percentages or proportions because this is unnecessary and potentially more difficult to interpret.
Nest site assessment
Control-colony scouts made significantly more visits to the “deluxe”quality nest site than did treatment-colony scouts (Mann–Whitney: p = 0.0023, U = 345.5; control: n = 16; treatment: n = 16; Fig. 6). This is probably because scouts visit high quality sites multiple times (Robinson et al. 2009) using their pheromone trails for orientation.
The number of nest site visits to the “good” and “deluxe”new nest sites. The box encompasses the interquartile range, the line across the box is the median, and the whiskers are drawn to the nearest value within 1.5 times the interquartile range. All remaining outlying points are marked with a square. Significance is marked with broken horizontal line
There was, however, no significant difference in the number of visits to the “good” quality nest site between treatment and control colonies (Mann–Whitney: p = 0.1790, U = 300; control: n = 16; treatment: n = 16; Fig. 6). Nevertheless, pooling across both types of new nest site (good and deluxe) revealed that control colony scouts performed significantly more nest site visits than the treatment colony scouts (Mann–Whitney: p = 0.0059, U = 337.5; control: n = 16; treatment: n = 16; Fig. 6). This result remains significant after a Bonferroni correction has been applied, such that the critical p value is 0.025 (0.05/2).
Tandem run recruitment
Control colonies performed significantly more tandem runs than treatment colonies (Mann–Whitney: p < 0.00001, U = 392; control: n = 16; treatment: n = 16; Fig. 7). Control colony tandem run initiation frequency ranged from 3 to 36, whereas treatment colony tandem run initiation frequency ranged from 0 to 2. Treatment colony tandem run leaders (i.e., only 3 ants in total from 16 emigrations) were removed from their experimental replicates and their gasters were analyzed under a dissection microscope. In each case, these leaders no longer had paint on their gaster tip. Hence, these unusual cases also seem to confirm the general efficacy of the paint treatment—i.e., they seem to be the exceptions that prove the rule.
The number of tandem runs performed during each attempted emigration by both control and treatment colonies. Conventions as in Fig. 6
Quorum achievement
Among those colonies that emigrated successfully (i.e., to the deluxe new nest site), control colonies used significantly higher quorum thresholds than those of treatment colonies (Mann–Whitney: p = 0.0004, U = 309.5; control: n = 16; treatment: n = 11; Fig. 8). Control colony quorum thresholds ranged from 4 to 21, whereas treatment colony quorum thresholds ranged from 3 to 7. There was no significant difference between the quorum threshold achievement times of control and treatment colonies (Mann–Whitney: p = 0.4033, U = 250.5; control: n = 16; treatment: n = 11).
The number of ants within the chosen new nest site at the time of the first transport behavior (quorum threshold) during the successful emigrations performed by the control and treatment colonies. Conventions as in Fig. 6
Transport recruitment
There was no significant difference in the number of transports performed by emigrating control and treatment colonies (Mann–Whitney: p = 0.4897, U = 238.5; control: n = 16; treatment: n = 11).
Emigration completion
There was no significant difference in the emigration completion times of the control and treatment colonies (Mann–Whitney: p = 0.9803, U = 223; control: n = 16; treatment: n = 11).
Emigration success
The control colonies were more successful in their emigrations than treatment colonies (two-tailed Fisher’s exact test: p = 0.0434, control: n = 16; treatment: n = 16). All control colonies solved the binary choice problem successfully, emigrating to the “deluxe” quality nest site. Treatment colonies did not display the same levels of success. Eleven out of the 16 treatment colonies emigrated to the “deluxe” quality nest site, one colony emigrated to the “good” nest site and four colonies failed to emigrate at all.
Discussion
The results of this study demonstrate that “gaster-tip” pheromone communication provides a key mechanism contributing to the organization of T. albipennis colony emigrations and that such colonies have a remarkable capacity for behavioral plasticity. Emigrations performed by ants with restricted “gaster-tip” pheromone communication had significantly reduced nest site assessment and tandem run recruitment. This consequently hindered emigrations as collective decisions became significantly less successful, i.e. more colonies failed to emigrate to the better of the two new nest sites. Nevertheless, the majority of the treatment colonies did successfully emigrate and in response to a reduction in their pheromone communication treatment ants changed their opinion-polling dynamics. The treatment colonies seemed to trade-off a reduction in the quality of their collective decision-making, i.e., lowered their quorum threshold, to favor greater speed and hence lower levels of exposure to a potentially hostile environment and their natural enemies.
As might be predicted, the first two behavioral stages of emigrations, i.e., scouting and tandem running, were reduced in the treatment colonies. Commitment to scouting, evaluated by differences in the number of visits ants made to the new nest sites (Fig. 6), was performed significantly less by the treatment colonies. There can be three non-mutually exclusive explanations: first, treatment ants may not have been able to assess the state of the original nest site. As the original nest site quality has been shown to be a governing factor effecting commitment to scouting (Doran et al. 2013), this could contribute significantly to the scouting differences between the control and the treatment. Second, the ants in treatment colonies may be unable to give (and hence, also gain) certain information from their nest mates about the condition of the original nest. Third, it is possible that applying a small droplet of paint over the gaster tip might actually harm the ants. However, this seems unlikely because the workers in the treatment and control colonies appear to be equally adept at carrying their nest mates (personal observations) and this strenuous task is likely to have been compromised if the treatment ants had been harmed. Nevertheless, we cannot fully discount the possibility of gaster-tip painting causing some harm but what is intriguing is that the treatment colonies can manage to overcome this potential disability and with some behavioral adjustment successfully emigrate.
There was a significant difference between treatment and control colonies in the number of visits to the “good” quality nest, control colonies visited the “deluxe” quality nest significantly more than the treatment colonies (Fig. 6). This is probably due to the lack of orientation pheromones laid by ants in treatment colonies during scouting. In this case, it is possible that the larger nest entrance (4 mm) provided by the “good” quality nest increased the potential for finding that entrance and this compensated for the lack of orientation cues. Conversely, the smaller sized nest entrance (1 mm) of the “deluxe” quality nest may have been significantly more difficult to find without orientation cues. This is a simple example of how pheromone communication can affect a colony’s success in finding and exploiting the best available resource. Of additional importance during nest site assessment, treatment colonies were unlikely to be able to utilize the Buffon’s needle algorithm, as their extirpated “gaster-tip” pheromones may have eliminated their ability to lay area assessment marking trails. This would result in an inability to make an estimation of nest cavity size and may explain the lower success rate of treatment colony emigrations. However, 11 out of the 16 treatment colonies still emigrated to the optimum nest site, potentially without a Buffon’s needle assessment. Neither gaster-tip pheromones nor the associated Buffon needle algorithm are likely to be used to assess the width of nest entrances. The Buffon’s needle algorithm is used to measure nest cavity areas (Mallon and Franks 2000), and it is not suitable for measuring linear properties such as widths. Moreover, it is likely that nest entrances, being so narrow, can easily be assessed by individual ants using attributes of their own bodies as a yard stick. That is, an ant could easily touch both sides of a 1-mm wide nest entrance simultaneously with its antennae and discriminate against a 4-mm wide entrance because such contact cannot be made. Nest choice preferences in terms of entrance widths and numbers have been extensively studied in T. albipennis by Franks et al. (2006). Thus, the clear preference of treatment colonies for the better new nest site is almost certainly due to the different light levels present within the “good” and “deluxe”quality nest sites. It is already known that T. albipennis colonies prioritize darkness as a desirable nest site trait (Franks et al. 2003). Also, studies of ants even with small eyes such as those of Temnothorax ants have shown sophisticated color discrimination abilities and that workers can clearly assess varying light intensities (Cammaerts and Cammaerts 2009). Thus, discriminating between the light levels of the new potential nest sites was likely to have been sufficient, in the absence of cavity size measurement, for a choice in favor of the “deluxe” quality nest over the “good” quality nest. This ability to assess a nest site with regards to light quality may also be utilized when judging nest entrance size. This could be possible as larger nest entrances will allow more light to enter the nest. Thus, it is likely that an effective assessment of both light levels and nest entrance width can be based on light levels alone within the nest cavity and neither of these assessments would be influenced by the presence or absence of pheromones.
Tandem run recruitment was heavily restricted by the extirpation of “gaster-tip” pheromones. So much so that the results confirm that “gaster-tip” pheromones have a large role in the facilitation of the initiation of tandem running. Hence, it can be concluded that the tandem initiating pheromone used by T. albipennis originates at the gaster tip (as is the case for other members of this genus: Möglich 1979). Further supporting this point are our personal observations regarding in-nest behavior performed by emigrating colonies. Treatment and control ants were observed to perform gaster “raising” within their original nest. Gaster “raising” is a potential sign of an attempt to entice a tandem follower with “gaster-tip” pheromones. This behavior proved successful for control colonies when enticing a follower. By contrast, in the treatment, gaster “raising” failed to evoke a behavioral response from their conspecifics. This gives an example of how “gaster-tip” pheromones play a role in organizing emigration dynamics. To our knowledge, this is the first study to demonstrate this by direct manipulation. However, to specifically identify the exact exocrine gland responsible for the tandem calling pheromone would require further experimental work.
It might be argued that potential tandem leaders in the treatment colonies may have been disabled because they could not feel antenatal tapping from their potential followers because of the paint on their gaster tips. However, such a scenario seems highly unlikely for the following reasons. First, ants in treatment colonies seemed not to be able to initiate any tandem following at all. This points to a lack of pheromone production rather than a secondary insensitivity to the presence of a follower. Second, tandem leaders from control colonies also had paint on their gasters that might equally have glued up the hairs on their gaster by which they might register antennal touches, yet they could successfully lead tandem runs. Third, tandem followers not only touch their leader’s gaster but they also touch the back legs of leaders (Möglich 1979; Basari et al. 2014) and these were unaffected by our paint treatment.
In the absence of tandem run recruitment, treatment colony emigrations are likely to have been less (energy) efficient. Groups of autonomous “robot” ants that forage for energy packets have been shown to benefit from a tandem run recruitment strategy (Krieger et al. 2000). Robot foragers were programmed to lead fellow workers to previously discovered energy resources in a process designed to mimic tandem running behavior in ants. The success of such a foraging strategy was compared with that of robots foraging without any recruitment communication. Despite the initial energy costs of a seemingly slow and tortuous tandem process, a mean net energy gain of 9.4 % was achieved (Krieger et al. 2000). This suggests that treatment ant colony emigrations may have required more energy than those of the control colonies. Intriguingly, our study suggests communication in ants during emigrations may be steered towards reducing costs, rather than a strategy that increases gains as shown in studies of honeybees (Seeley 1983). Further analyses would be required to confirm the potential varying strategies amongst ants and honeybees, as context dependency is likely to play a significant role in governing behavioral strategy.
Although the treatment colonies were limited in the use of pheromones, they responded behaviorly to the new constraint and show remarkable behavioral plasticity in the quorum threshold they used and they were able to maintain their efficiency social carrying and overall were able to achieve emigration completion times similar to the control colonies. Most probably the lack of tandem run recruitment by the treatment colonies led to differences in quorum thresholds between the treatment and control colonies. Here, time taken to achieve a quorum threshold was not significantly different between treatment and control colonies. However, treatment colonies used a significantly smaller quorum size. Lowering of the quorum threshold demonstrates beautifully that these ants were able to adjust their behavior in response to a constraint. In the absence of tandem-running recruitment, scouts are likely to be exposed for significantly more time while attempting to build a typical quorum threshold (10–15 workers; Pratt et al. 2002) than scouts from colonies with sufficient tandem recruitment. This would increase exposure to predators. Treatment colonies typically used a quorum threshold of four individuals, meaning their commitment to an emigration was reliant on four ants rather than the usual 10–15. This is an example of the ants adjusting their behavior such that the quality of opinion polling is sacrificed to reduce exposure.
As mentioned above, quorum achievement, the initiating stimulus for social carrying recruitment, was accomplished in a similar time by the treatment and control colonies. It is well known that animals are able to monitor the progression of time (Roberts 2002). There have been numerous experiments showing that with the appropriate stimuli, animals are able consistently to return to specific locations at the correct time on separate days (Daan and Koene 1981; Biebach et al. 1989; Wilkie et al. 1996). In this case, a time threshold is defined as the particular amount of time that acts as a stimulus which initiates a change in behavior. There are two clear examples where T. albipennis workers change their behavior in response to the passing of a certain amount of time. Richardson et al. (2007) show that tandem-leading ants will wait for a lost tandem run for consistent amounts of time depending on the progress of the tandem run. Here, the further a tandem run has progressed, the longer a leader will wait for a lost follower to find her. Therefore, the leader ant is in some sense responding to progression of time, as she knows how long to wait with regards to the tandem’s current progress. Moreover, as already described, emigrating ants satisfied with a new nest site will remain still and “wait” within the nest site in order to build a quorum (Pratt et al. 2002). If too much time passes without reaching a quorum threshold, the ants may leave the new nest site as part of the decision-making strategy based on not enough individuals having rated the new resource in a sufficiently short period of time. These examples demonstrate a change in behavior in response to the passing of time. Therefore, individuals committing to a nest lacking a full quorum threshold may be responding to the passing time. In other words, ants that deem the new nest site to be of a higher quality than their current nest site may begin social carrying recruitment after a certain length of time, regardless of current scout presence within the new nest site. However, further experimentation would be needed to understand better what mechanisms facilitate the reduction in quorum sizes employed by the treatment ants.
Lower quorum threshold may be also a result of treatment scouting ants perceiving their colony to be smaller than its actual size due to the lower levels of communication. This could be significant, as it has been shown that when T. albipennis colonies are split, the two new smaller colonies utilize a lower quorum threshold to that of their original combined larger colony (Dornhaus and Franks 2006). In fact, the smaller colonies adopted quorum thresholds proportionate to the quorum size of their original full sized colony. Therefore, it is possible that population within the new nest site is relative to the population in their current nest site (Dornhaus and Franks 2006). However, further experimentation would be required to understand further how the treatment ants interpret their environment with restricted communication. Here it is important to realize that quorum thresholds are set by individual scouts (Pratt 2005) namely what abundance of nest mates in a new nest site that an individual will accept is a quorum threshold that will switch her own behavior to social carrying. Thus, when attempting to dissect the potential mechanisms that underpin the lower quorum thresholds used by the treatment colonies, it is essential to analyze behavior at an individual level.
Our results show that social carrying recruitment and as mentioned earlier emigration completion times are conserved. This may be indicative of their crucial role in the process of emigration even at the cost of less successful collective decision-making. Furthermore, the observation that there were no significant differences in social carrying between the treatment colonies and the control colonies strongly suggests that “gaster-tip” pheromones are not required to initiate and maintain social carrying. It is likely that when a worker ant picks up a sister worker or the colony queen, the communication involved is mostly, if not entirely tactile (Möglich and Hölldobler 1974). Moreover, it is unlikely that much if any communication is required when carrying brood items.
Conclusions
We have shown that “gaster-tip” pheromones play a significant role in T. albipennis emigrations. Emigrations were hindered significantly by restricted “gaster-tip” pheromone excretion. Orientation and recruitment seem to be the most affected behavioral processes, as “gaster-tip” pheromones appear to facilitate such behavioral mechanisms. This resulted in limited resource assessment. However, treatment ants displayed impressive behavioral plasticity. The ants were able to adjust their behavior to limit the effects of nest site assessment. Here, the ants in the treatment colonies seem to accept an unusually low quorum threshold in order to maintain a “standard” emigration completion time, thus avoiding more exposure than during normal emigrations. This demonstrates plasticity of individual behaviors and reveals yet another example of the behavioral robustness crucial for the ecological success of ants. Moreover, extirpating “gaster-tip” pheromones using paint droplets has revealed new plasticity in behavior as exhibited by the manipulated ants. For these reasons, we believe further work using this manipulation could help elucidate further fundamental mechanisms underlying collective behavior in ants.
References
Basari N, Laird-Hopkins BC, Sendova-Franks AB, Franks NR (2014) Trail laying during tandem-running recruitment in the ant Temnothorax albipennis. Naturwissenschaften 101:549–556
Biebach H, Gordijn M, Krebs JR (1989) Time-and-place learning by garden warblers, Sylvia borin. Anim Behav 37:353–360
Cammaerts MC, Cammaerts D (2009) Light thresholds for colour vision in workers of the ant Myrmica sabuleti. Belg J Zool 139:40–49
Daan S, Koene P (1981) On the timing of foraging flights by oystercatchers, Haematopus ostralegus, on tidal mudflats. Neth J Sea Res 15:1–22
Doran C, Pearce T, Connor A, Schlegal T, Franklin E, Sendova Franks AB, Franks NR (2013) Economic investment by ant colonies in searches for better homes. Biol Lett 9:5
Dornhaus A, Franks NR (2006) Colony size affects collective decision-making in the ant Temnothorax albipennis. Insect Soc 53:420–427
Dornhaus A, Franks NR, Hawkins RM, Shere HNS (2004) Ants move to improve: colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Anim Behav 67:959–963
Franklin EL (2014) The journey of tandem running: the twists, turns and what we have learned. Insect Soc 61:1–8
Franks NR, Richardson T (2005) Teaching in tandem-running ants. Nature 439:153
Franks NR, Pratt SC, Mallon EB, Britton NF, Sumpter DJT (2002) Information flow, opinion polling, and collective intelligence in house hunting social insects. Philos Trans R Soc Lond 357:1567–1583
Franks NR, Mallon EB, Bray HE, Hamilton MJ, Mischler TC (2003) Strategies for choosing between alternatives with different attributes: exemplified by house hunting ants. Anim Behav 65:215–223
Franks NR, Dornhaus A, Best CS, Jones EL (2006) Decision-making by small and large house-hunting ant colonies: one size fits all. Anim Behav 72:611–616
Franks NR, Dechaume-Moncharmont F-X, Hanmore E, Reynolds JK (2009) Speed versus accuracy in decision-making ants: expediting politics and policy implementation. Philos Trans R Soc B 364:845–852
Franks NR, Richardson TO, Stroeymeyt N, Kirby RW, Amos WMD, Hogan PM, Marshall JAR, Schlegal T (2013) Speed-cohesion trade-offs in collective decision making in ants and the concept of precision in animal behaviour. Anim Behav 85:1233–1244
Gould JL (1976) The dance-language controversy. Q Rev Biol 51:211–244
Grueter C, Leadbeater E (2014) Insights from insects about adaptive social information use. Trends Ecol Evol 29:177–184
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Harvard
Krieger M, Billeter JB, Keller L (2000) Ant-like task allocation and recruitment in cooperative robots. Nature 406:992–995
Mallon EB, Franks NR (2000) Ants estimate area using Buffon’s needle. Proc R Soc Lond B 267:165–770
Mallon EB, Pratt SC, Franks NR (2001) Individual and collective decision-making during nest site selection by the ant Leptothorax albipennis. Behav Ecol Sociobiol 50:352–359
Maschwitz U, Schönegge P (1977) Recruitment gland of Leptogenys chinensis. Naturwissenschaften 64:589–590
Maschwitz U, Schönegge P (1983) Forage communication, nest moving recruitment, and prey specialization in the oriental ponerine Leptogenys chinensis. Oecologia 57:175–182
McLeman MA, Pratt SC, Franks NR (2002) Navigation using visual landmarks by the ant Leptothorax albipennis. Insect Soc 49:203–208
Mitra A (2013) Function of the Dufour’s gland in solitary and social hymenoptera. J Hymenopt Res 35:33–58
Möglich M (1979) Tandem calling pheromone in the genus Leptothorax (Hymenoptera: Formicidae): behavioural analysis of specificity. J Chem Ecol 5:35–52
Möglich M, Hölldobler B (1974) Social carry behaviour and the division of labour during nest moving of ants. Psyche 81:219–236
Pratt SC (2005) Quorum sensing by encounter rates in the ant Temnothorax albipennis. Behav Ecol 16:488–496
Pratt SC, Brooks SE, Franks NR (2001) The use of edges in visual navigation by the ant Leptothorax albipennis. Ethology 107:1125–1136
Pratt SC, Mallon EB, Sumpter DJT, Franks NR (2002) Quorum sensing, recruitment, and collective decision-making during colony emigration in the ant Leptothorax albipennis. Behav Ecol Sociobiol 52:117–127
Richardson TO, Sleeman PA, McNamara JM, Houston AI, Franks NR (2007) Teaching with evaluation in ants. Curr Biol 17:1520–1526
Roberts WA (2002) Are animals stuck in time? Psychol Bull 128:479–489
Robinson EJ, Smith FD, Sullivan KM, Franks NR (2009) Do ants make direct comparisons? Proc R Soc B 276:2635–2641
Schürch R, Grüter C (2014) Dancing bees improve colony foraging success as long-term benefits outweigh short-term costs. PLoS ONE 9(8):e104660. doi:10.1371/journal.pone.0104660
Seeley TD (1983) Division of labour between scouts and recruits in honeybee foraging. Behav Ecol Sociobiol 12:253–259
Sendova-Franks AB, Franks NR (1993) Task allocation in ant colonies within variable environments (a study of temporal polyethism: experimental). Bull Math Biol 55:75–96
Sendova-Franks AB, Franks NR (1995) Division of labour in a crisis: task allocation during colony emigration in the ant Leptothorax unifiascus. Behav Ecol Soiobiol 36:269–282
Sherman G, Visscher K (2002) Honeybee colonies achieve fitness through dancing. Nature 419:920–922
Soroker V, Fresneau D, Hefetz A (1998) Formation of colony odor in ponerine ant Pachycondyla apicalis. J Chem Ecol 24:1077–1090
Von Frisch K (1967) The dance language and orientation of bees. Harvard University Press, Harvard
Wilkie DM, Carr JAR, Siegenthaler A, Lenger B, Liu M, Kwok M (1996) Field observations of time-place behaviour in scavenging birds. Behav Process 38:77–88
Wilson EO (1971) The insect societies. Cambridge, MA: Harvard University Press
Wilson EO, Hölldobler B (2005) The rise of the ants: a phylogenetic and ecological explanation. PNAS 102:7411–7414
Acknowledgments
We thank Ana Sendova-Franks, Carolina Doran, and Liz Langridge for comments on the manuscript. We thank all the members of the lab for assistance with experiments. Daphna Gottlieb was funded by a Marie Curie research grant 274342. We also wish to thank the anonymous reviewers of this paper for their very helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. O. H. Hughes
Jonathan P. Stuttard and Daphna Gottlieb contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stuttard, J.P., Gottlieb, D. & Franks, N.R. Ants incommunicado: collective decision-making over new nest sites by ants with reduced communication. Behav Ecol Sociobiol 70, 145–155 (2016). https://doi.org/10.1007/s00265-015-2033-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-2033-7