Abstract
Geographic range has long been noted to be associated with many organismic and ecological traits such as body size and species richness. However, much less is known about whether and how ecological variation across latitudinal gradients reflects behavioral variation. Ant colonies may also show behavioral variation, and Temnothorax rugatulus show a colony-level behavioral syndrome that seems to reflect risk tolerance across their North American range. While it is presumed that this pattern is the result of adaptation to local environmental conditions, which ecological factors are driving this variation are unknown. Here, we test if colony risk tolerance is affected by competition, predation, resource availability, or environmental stress at each site. Our results show that increased competition, specifically for nest sites, as well as increased spatial clustering of colonies predicts higher risk tolerance. Additionally, the spatial clustering of colonies influences the structure of the risk-taking syndrome, i.e., which colony-level behaviors are correlated and how strongly. This emphasizes the need for understanding large-scale geographic variation in behavior, as it may explain how ecological factors drive the evolution and maintenance of intraspecific behavioral variation across populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large-scale geographic latitudinal gradients are commonly associated with variation in a number of organismic traits. These patterns often reflect adaptive intraspecific variation in phenotype (Conover and Schultz 1995; Angilletta Jr et al. 2003; Ashton 2004). For example, most mammals, birds, and amphibians are larger at higher latitudes while lizards and snakes are larger at lower latitudes (Ashton and Feldman 2003; Ashton 2004). In eusocial insects, species richness decreases and colony size (worker number) increases with increasing latitudes (Kaspari and Vargo 1995; Kaspari et al. 2004). Though morphological variation has been the primary focus, behavioral differences have also been reported across latitudinal clines. For example, the social structure of the spider Anelosimus studiosus changes from solitary female webs to multifemale cooperative webs with increasing latitude (Riechert and Jones 2008). In decorator crabs (Libinia dubia), there is a shift away from chemical defense at higher latitudes, which coincides with decreased predation pressure (Stachowicz and Hay 2000). However, compared to morphological traits, behavioral clines across latitudinal gradients are relatively understudied.
In the rock ant Temnothorax rugatulus, there is a latitudinal gradient in several behavioral traits, which are in turn correlated to one another (Bengston and Dornhaus 2014). Across the western United States, colonies show consistent variation in foraging distance, foraging effort to different resource types, aggression, and activity in response to threat. Colonies at more northern latitudes travel farther, respond more aggressively but invest less (in terms of the number of ants responding) in each given threat incident or resource. Colonies at more southern latitudes deploy more ants to closer resources, are more responsive to threat, avoid traveling far distances, and avoid aggressively engaging conspecific invaders. Despite this clear pattern, the ecological factors underlying it are unknown. Additionally, these behavioral traits are independent of many colony properties that are often important to social insect biology, such as colony size, queen number, and brood to worker ratio (Bengston and Dornhaus 2014).
This correlated suite of behaviors (behavioral syndrome; (Sih et al. 2004) in T. rugatulus may reflect variation in risk tolerance, with risk tolerance positively correlated with increased latitude (Bengston and Dornhaus 2014). Inter-individual, or inter-colony, as in this case, variation in risk tolerance has been widely noted in animals (Blumstein 2006). Clearly, latitude cannot have a direct effect on behavior and is instead a proxy for ecological conditions that may correlate with latitude, but it is not well understood how these ecological variables drive behavior. Four hypothetical driving forces can be drawn from the literature. First and perhaps most intuitively, the intensity of predation may create local adaptations in behavior (Lima 1998; Biro et al. 2004). Indeed, stickleback populations show differential patterns and consistency of risk tolerance between populations with and without predators (Bell and Stamps 2004). Similarly, social parasitism, where one social group benefits at the expense of another, has been shown to affect colony defensive behavior in Temnothorax ants (Pamminger et al. 2012; Jongepier et al. 2014), though such social parasitism has not been investigated in T. rugatulus. A second hypothesis is that competition for resources may drive the level of risk tolerance (Grand and Dill 1999). For example, in two ecologically similar species of sympatric hermit crabs, Clibanarius digueti and Paguristes perrieri, which require similar resources, C. digueti consistently showed more aggressive (and perhaps risky) behaviors while foraging than P. perrieri. C. digueti also consistently won more competitions against P. perrieri in inter-specific interactions (Tran et al. 2014). In Temnothorax longispinosus, nest site limitation has been proposed as a potential driver of increased aggression at higher population densities (Modlmeier and Foitzik 2011). Third, the more ephemeral the resource (either spatially (Grant and Guha 1993), or temporally (Bryant and Grant 1995)), the more aggressive individuals become in attempts to utilize and defend them (Goldberg et al. 2001). Fourth, environmental stressors, such as heat and humidity, may drive behavior. Perhaps best studied in humans, it has been noted that increasing average temperature has been correlated to increased aggressive and violent behaviors since the 1700s (reviewed in Anderson 2001). A similar pattern has been found in domesticated hogs, Sus scrofa domesticus, where increased temperature positively correlated with increased aggressive encounters with littermates (McGlone et al. 1987). These four hypothetical driving forces underlying variation in risk tolerance are not mutually exclusive, and may all contribute to the latitudinal gradient found in Temnothorax (Bengston and Dornhaus 2014).
Ecological factors such as predation, competition, spatial and temporal resource variability, and abiotic factors also may interact with one another to generate broader evolutionary patterns such as different life history strategies. Biro and Stamps (Biro and Stamps 2008) specifically propose that risk tolerance levels may be the result of variation in life history strategy. Interestingly, life history strategy has been shown to vary across a latitudinal gradient in some other cases (Conover 1992; Blanckenhorn and Fairbairn 1995).
Here, we measure which, if any, of these environmental factors may explain variation in risk tolerance measured in colonies of T. rugatulus ants. We primarily focus on predation (measured by large arthropod abundance), competition (measured by proportion of nest sites occupied, spatial clustering of colonies), food resource availability (measured by leaf litter depth and small/juvenile arthropod abundance), and environmental stressors (soil moisture, canopy cover, and nest temperature).
Methods
Model species
T. rugatulus is a Myrmicine ‘rock ant.’ Colonies range in size from usually 50 to 400 ants, with some very large colonies reaching 1300 ants (Bengston and Dornhaus 2013). Generally, this species is found nesting in preformed crevices in rocks (Rüppell et al. 1998; Bengston and Dornhaus 2013). Colonies can overwinter, producing new queens and males for several years. In the field, colonies have been observed foraging for small arthropods such as Collembola and insect larvae, as well as scavenging larger arthropods such as crickets, spiders, and beetles (personal observation, SEB). The small size of the colonies and the locations of their nests (in cracked or flaking rocks) allow for easy collection of the entire colony, including queen and brood. The tolerance of many species in the Temnothorax genus for laboratory conditions allows for empirical studies in controlled conditions, resulting in this genus being used successfully used in behavioral studies in many contexts, meaning well-established methods for empirical studies exist (e.g., emigrations (Cao and Dornhaus 2012), group decision making (Sasaki and Pratt 2013), and intra-colony interactions (Dornhaus et al. 2008, 2009)).
Collection sites
Forty-six colonies were collected and environmental measures were taken during July 2012 from 12 sites across the North American range of the species (Table 1). Each collection site was defined as a 10 m × 10 m plot. There was a minimum of 2 km between any two sites. The total latitudinal range covered from the most northern to the most southern collection site was approximately 2170 km. These sites included the seven reported in Bengston and Dornhaus (2014), as well as five additional collection sites.
Ecological measures
Here, we measure which, if any, of these environmental factors may explain variation in risk tolerance measured in colonies of T. rugatulus ants.
Arthropods (predation, food resource availability)
Two methods were used to search for arthropods that may be a source of food, predators, or competitors for T. rugatulus. First, the Winkler leaf litter sampling method (Fisher 1999) was used to measure surface arthropods. Three 1 m × 1 m plots chosen randomly were raked together and the leaf litter was put into a Winkler trap and left for 12–18 h. Alcohol-filled containers at the bottom of each trap preserved any arthropods from the samples.
The second method searched for arthropods living at or just below the soil level, and thus the most likely to come into contact with T. rugatulus. After the leaf litter had been removed for the Winkler traps, the top centimeter of soil was removed from this 1 m2 plot and bagged. This sample was then sorted by hand under ×10 magnification.
In both methods, collected arthropods were sorted into three categories: larvae/pupae, potential food sources (e.g., Collembola), and potential predators (e.g., Camponotus sp.). Each category was then counted, yielding a count for each site.
Proportion of nest sites occupied (competition)
At each collection site, all potential nest sites in rocks were counted as occupied or not. A potential nest site was considered as any two rocks overlapping one another with at least a 1 mm crevice but smaller than a 1 cm crevice. Rocks directly against the soil were not included, as T. rugatulus typically does not nest in such sites. In one site, a felled log partially decomposed enough to contain appropriately sized cavities within the 10 m × 10 m plot. In this instance, no colonies were found and as such it was not included in the count. Potential nest sites were searched for by turning over rocks and by breaking apart rocks with cracks. As they generally do not excavate their own nest sites, the percentage of unoccupied spaces may reflect the amount of nest space competition.
Spatial clustering (competition)
When a Temnothorax colony was located, a marker was placed at the location it was found. After all colonies in a site had been located, for every colony the distance to its nearest neighbor was measured. After returning to the lab, colonies were all identified to species and non T. rugatulus colonies were excluded from the behavioral analysis. However, as many Temnothorax species use similar types of nest sites, and thus may compete for such sites, all Temnothorax spp. colonies were included when determining the number of occupied nest spaces and clustering. The amount of clustering was determined by calculating the clustering index by the nearest neighbor distance method, in which 1 signifies random distribution in space, a lower value indicates clustering, and a higher value indicates hyperdispersion (Clark and Evans 1954; Dornhaus and Chittka 2004).
Leaf litter depth (food resource availability)
Three random points were selected in each plot and the depth from the top of the leaf litter to the soil was measured. The average leaf litter depth provides a measure of environmental complexity, and may reflect the amount and type of food resources available (e.g., small arthropods).
Soil carbon content (food resource availability)
The samples dried in the soil moisture measurement were weighed, then left overnight in a 227 °C oven (as per EPA soil carbon content ‘loss-on-ignition’ methods, (Schumacher 2002)) and weighed again. This temperature releases trapped organic carbon but does not release non-organic carbon such as that found in carbonate minerals. The weight difference measures the amount of organic material in soil, and thus may give an indication of food resources available to ground-living arthropods.
Soil moisture (environmental stressor)
Three soil samples (excluding leaf litter) of a standard volume (6 cm × 6 cm × 3 cm) were taken at every plot and held in air-tight containers and frozen until they could be analyzed. Soil moisture is highly dependent upon time since last rainfall, which could not be controlled for. However, it had not rained for a minimum of 5 days before soil samples were collected, minimizing the effect of recent precipitation. Samples were weighed before and after being placed in an oven at 105 °C for 5 h. This process allows for a measure of soil water content (Kelley et al. 1946).
Canopy cover (environmental stressor)
For each 100 m2 plot, we counted the number of 1 m2 sections that were more than half shaded to determine the percentage canopy cover for the plot. Thus, the plot could be between 0 and 100 % shade, and 40 % shade indicated that 40 m2 were mostly shaded. Canopy cover was measured between 1100 and 1300 hours to standardize the effect of sun angle.
Internal and external nest temperature (environmental stressor)
Using a Mastercool infrared laser thermometer, both the external rock temperature and the internal nest temperature were measured for every colony collected. External temperature was measured on the exposed surface of the rock closest to the nest entrance. Internal temperate was taken on the rock forming the wall of the nest immediately upon exposure. If colonies are persistently heat or cold stressed, this may affect the metabolic requirements, as well as changing the colonies’ need or ability to procure resources.
Determining risk tolerance
To see how these ecological traits relate to risk tolerance, the behavioral phenotype of colonies was established. As detailed in Bengston and Dornhaus (2014), each colony collected at each of the sites was tested for activity level, response to threat, aggression level, foraging distance, and response to both novel and familiar resources. (1) Activity level uses an optic flow algorithm developed by Hoan Nguyen and Min Shin (University of North Carolina, Charlotte) to measure the amount of pixel movement per adult ant over a 5-min interval. This was done when colonies where not perturbed or otherwise exposed to a specific stimuli. Colonies were filmed between 1200 and 1600 hours at 24 frames/second with a Nikon D700 and a Nikon AF-S Micro-Nikkor 60 mm f/2.8G lens. (2) Response to threat uses the same optic flow algorithm to measure movement per adult ant in a colony after a conspecific intruder is introduced to the colony. It is reported as the proportional change in movement per ant after an intruder is introduced compared to the activity level assay. (3) Because movement in response to threat may reflect both aggressive and passive behaviors, the aggression assay quantitatively scores the behavior of workers within the colony after the introduction of an intruder. Behaviors range from passive crouching and fleeing the nest to aggressive biting, stinging, and pinning down the intruder. (4) Foraging distance was measured in the field, tracking the farthest point foragers traveled after leaving the nest before returning to the colony. (5) Response to familiar resource was measured as the number of workers exploiting the standard laboratory diet (freeze-killed cockroaches, fruit flies, springtails, and honey solution) 90 min after it was made available to the colony. Response to novel resources was measured in the same way, but with the standard diet replaced with unfamiliar food items (canned tuna and applesauce).
It was found that response to threat, aggression level, foraging distance, and foraging for novel and familiar resources formed a behavioral syndrome, i.e., correlated with each other. The data was then uniformly scaled (so no one variable was overly contributing) by dividing each score for every assay by the overall mean value for that assay. A principal component analysis (PCA) of the scaled data was used to establish a behavioral type score for each colony by calculating its loading in the first principal component (PC1) of the syndrome. A high score indicated that a colony showed an increased foraging effort and response to threat and a decreased level of aggression and shorter foraging distances, a set of traits consistent with low risk tolerance. A low score indicated lower foraging effort, but with longer foraging distances and higher levels of aggression. These colonies contain 18 of the same colonies as Bengston and Dornhaus (2014) and an additional 28 new colonies.
Statistical analysis
We used a Kruskal-Wallis test to confirm that there was between-site variation in behavioral phenotype score (using each colony’s score as a data point).
We calculated the correlation between latitude and the behavioral phenotype of all the colonies to confirm that there was variation across a latitudinal gradient.
To test what ecological factors affect behavioral type, we again used each colony as a data point with its behavioral phenotype score (see above) as a response variable and the site-specific environmental measures as predictor variables. For the ecological traits that have multiple measures, e.g., leaf litter depth, the average value per site was used. We used a linear Gaussian model to assess the effect of the ecological traits (predictor values) on behavioral phenotype (response variable). Because we may have predictor variables that correlated with one another, we performed a stepwise model selection test (both forwards and backwards) to calculate the model with the highest Akaike’s information criterion (AIC). This allowed us to remove predictor variables that were the least informative (Derksen and Keselman 1992). We then performed a Shapiro-Wilk test on the residual values to test for normality. Using the AIC method allows us to reduce the number of predictive variables (by eliminating those that are highly correlated) and build a reliable model without the need for a generalized linear model and associated P values (Anderson and Burnham 2002; Mundry 2010). We then used a Pearson’s correlation to assess if any of these environmental variables correlated with latitude.
Next, we examined how ecological factors influence the syndrome structure, i.e., which behaviors were correlated and how strongly. First, a PCA was performed on the behavioral traits in the risk-taking syndrome separately for each site (yielding 12 separate PCAs). We then calculated the loadings of each behavioral trait on the significant principal component, giving us a measure of how much variation in colony behavior is explained by each. Next, we used a series of separate stepwise GLMs where the loading of each behavioral trait was the response variable and each ecological trait found to be predictive in the stepwise model selection assay was included as a predictive variable. Meaning, for example, one GLM was formed to test if the loadings of aggression at each site were predicted by percentage of nest sites occupied and the clustering index. Another GLM used the loadings of response to threat, etc.
All statistical analysis was done with R version 2.12.1 with the Rstudio interface, using the ‘MASS’ and ‘stats’ packages.
Results
The ecological traits, including mean values and standard deviations, are summarized in Table 1.
Sites varied significantly in colony behavioral type score (Kruskal-Wallis P = 0.0071). Additionally, latitude and risk-taking phenotype score were significantly negatively correlated; meaning colonies at more northern latitudes were more risk-tolerant than colonies at more southern latitudes (P = 0.0002, R 2 = 0.6210, n = 46 colonies), which is consistent with the pattern reported in Bengston and Dornhaus (2014).
Ecological factors affecting risk tolerance
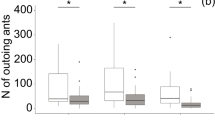
The stepwise model selection test eliminated several variables; leaf litter depth, canopy cover, temperature difference, arthropods—predatory, food, and larvae/pupa—in both the Winkler trap and soil, soil moisture, and soil carbon (supplemental material 1; Burnham et al. 2010). The remaining model, with behavioral phenotype scores predicted by the percentage of nest spaces occupied and the clustering index had an AIC score of −146.9. The residuals of this model were normally distributed (Shapiro-Wilk test P = 0.10). As either competition or clustering increased, colonies became more risk tolerant (Fig. 1).
Competition for nest space and the clustering index score predicts behavioral type. In each row on the Y-axis, the response to a behavioral assay for colonies is summarized, with each box representing a site. The X-axis shows the sites measured, ordered according to the proportion of nest spaces occupied, as well as the clustering index for that site. Colonies with lower behavioral type scores (lower foraging effort, longer foraging distances, and higher levels of aggression) were found in sites that had a higher percentage of occupied nest spaces and increased spatial clustering
We compared both of these predictive variables against latitude to assess if they also varied across latitude. Competition was positively correlated with latitude (P = 0.0006, R 2 = 0.7115, df = 11) while clustering index was not (P = 0.0135, R 2 = 0.2093, df = 11).
Ecological effect on behavioral syndrome structure
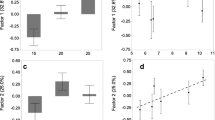
The structure of the syndrome generally stays similar across all sites (Fig. 2). For all of the sites, the first principal component (PC1) was the only significant component (Kaiser-Guttman stopping rule where only components with an eigenvalue greater than the mean are accepted, Guttman 1954; Jackson 1993). Therefore, we used the PC1 loading of each behavioral trait as the response variable in a series of GLMs to test if it is predicted by any of the environmental variables. The only significant predictor of any behavioral trait loading was the effect of clustering on aggression (GLM P = 0.0007, df = 11, all other P > 0.05, Fig. 2), which were negatively related. As a lower colony clustering coefficient indicates a higher level of clustering (Clark and Evans 1954), this means that as clustering increases, aggression explains more of the variation within the syndrome. Again, in these GLM’s, a Gaussian error structure was used and the residuals were checked for normality with a Shapiro-Wilk test, and all were normal (P > 0.05).
Behavioral syndrome structure at each site. The relationships between foraging distance (FD), response to threat (RT), response to a familiar resource (RF), response to a novel resource (RN), and aggression (Ag) were similar across all 12 sites. A lower clustering index (CI) indicates colonies are more closely clustered within the site (see text)
Discussion
In this study, we tested four hypotheses about which types of ecological factors may drive the evolution of risk tolerance in ant colonies: predation, competition, food resource abundance, and environmental stressors. We found that competition, as reflected both through the percentage of nest sites occupied and the spatial clustering of colonies, predicts risk tolerance. As competition increased, so did the risk tolerance of colonies. As competition for nest sites increases with latitude, this most likely explains the latitudinal gradient seen here and in Bengston and Dornhaus (2014). We also found that spatial clustering additionally affects phenotype, though it did not vary in a consistent way across latitudes. Predation, food resource availability, and environmental stress did not predict colony risk tolerance. Additionally, we found that densely clustered colonies correlate with the amount of variation within the syndrome explained by aggression. In other words, in highly clustered sites, aggression explains more of the syndrome variation compared to sites with more evenly spaced colonies.
Competition can come in many forms. We differentiate competition (the amount of either food or nest spaces available per ant colony) from resource abundance. This is a necessary distinction. Even in the absence of competition, starvation due to ephemeral or patchy resources may alter behavior (Croy and Hughes 1991; Inger et al. 2006); on the other hand, even with abundant resources, competition may be intense in dense populations. Here, we found nest site competition to be an important factor in shaping colony behavior; colonies in high levels of competition for nest sites show more risk tolerance. This suggests ecological conditions affect the maintenance of behavioral variation between colonies. A similar pattern was shown by Modlmeier and Foitzik (2011), who found that average colony aggressiveness in T. longispinosus increased with higher population density (more neighbors, which may be similar to our clustering index measure).
Given this, it is likely that nest site competition is a strong selective factor for many Temnothorax spp. behavior as a result of their specific ecology. Due to the specific ecology of Temnothorax ants (many species do not excavate their own nests, and are instead completely dependent upon preformed nest sites), it is likely that nest site competition is a strong selective factor driving behavior in many species of this genus (Partridge et al. 1997; Foitzik and Heinze 1998; Bengston and Dornhaus 2014). If a colony is evicted or cannot find an open nest site, desiccation or predation (which pose significant challenges for many ant species (Hood and Tschinkel 1990; Holway 1998; Pinter-Wollman et al. 2012; Gordon 2013)) may result in colony death. Additionally, T. rugatulus is exceptionally starvation resistant (Rueppell and Kirkman 2005). This may provide a buffer for colonies both when food is scarce as well as when it is too hot or dry to forage (as seen in harvester ants; (Pinter-Wollman et al. 2012; Gordon 2013)). As a result, this could decrease the selective pressures of both resource availability and environmental stress on behavior.
Additionally, we have found a significant effect of colony clustering on the risk-taking syndrome, with colonies increasing risk tolerance as they become more spatially clustered. This may reflect a colony’s need to increase their foraging range to decrease the competition associated with high clustering, or reflect an increased importance of aggressive responses to conspecific invasion. This is particularly interesting, given the potential association between spatial clustering and aggression, further discussed blow. Little is known about the dispersal patterns or relatedness of Temnothorax ants in nature (but see (Rüppell et al. 2003)). However, if daughter colonies are not dispersing long distances and are instead founding close to their parent colony, we would expect decreased aggression between these closely related colonies (Chapuisat et al. 1997). Even if colonies are not closely related, if they are not directly competing with one another, then colonies should additionally decrease antagonistic interactions with familiar neighbors (‘dear enemy’ hypothesis; Fisher 1954). Instead, colonies at higher spatial clustering are more risk-tolerant. This may suggest that colonies are not closely related to their neighbors or are directly competing with them for resources (perhaps for nest site quality).
Here, the risk tolerance of a colony is a measure reflecting a suite of correlated traits, also known as a behavioral syndrome (Sih et al. 2004). Specifically, this syndrome shows a correlation among foraging distance, foraging effort to both novel and familiar resources, aggression and the response to threat (Bengston and Dornhaus 2014). Syndromes essentially describe behavioral variation using a single behavioral axis (Sih et al. 2004; Jandt et al. 2014; Bengston and Dornhaus 2014). This single measure of a linked set of behaviors may be more driven by one behavioral trait within the syndrome than others. Here, we have demonstrated that in areas of higher spatial clustering, aggression is a stronger driver of colony phenotype. While it is not clear what this pattern means, it does suggest that more complicated processes underlie behavioral type. This also provides evidence that behavioral syndromes can change in their structure between populations, evidence perhaps that the link between these behaviors is not fixed as a result of constraints (Bengston and Jandt 2014). A similar phenomenon has been seen in both stickleback fish (Gasterosteus aculeatus, (Bell and Stamps 2004)) and red-kneed tarantulas (Brachypelma smithi, Bengston et al. 2014), where the presence of specific environmental cues can trigger the formation of correlations between specific, consistent behavioral traits. Together, these results suggest further emphasis should be placed on understanding both the development of behavioral syndromes and the role of local environment.
The interplay between behavior and the environment is critical to behavioral ecologists. Both behavioral plasticity in response to environmental change and long-term selection resulting in local adaptation have significant evolutionary consequences (West-Eberhard 1989; Scheiner 1993; Relyea 2001; Cousyn et al. 2001; Dingemanse et al. 2010). While this has been shown consistently both in the lab, as well as in the field, our understanding of how behavior varies across large geographic ranges is limited. As such, an increased sampling effort of behavior across larger ranges may reveal similar patterns as are seen in morphology. For example, Drosophila melanogaster show a latitudinal cline in the gene period, which is associated with both circadian and ultradian rhythms (Costa et al. 1992). This is suspected to be a result of thermal selection, though the mechanism by which this occurs is not clear (Sawyer et al. 2006). Understanding large-scale behavioral variation is a promising avenue of future research as, in turn, it can better inform hypotheses about the driving factors in natural behavioral variation.
References
Anderson CA (2001) Heat and violence. Curr Dir Psychol Sci 10:33–38. doi:10.1111/1467-8721.00109
Anderson DR, Burnham KP (2002) Avoiding pitfalls when using information-theoretic methods. J Wildl Manag 66:912–918. doi:10.2307/3803155
Angilletta MJ Jr, Wilson RS, Navas CA, James RS (2003) Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol 18:234–240. doi:10.1016/S0169-5347(03)00087-9
Ashton KG (2004) Sensitivity of intraspecific latitudinal clines of body size for tetrapods to sampling, latitude and body size. Integr Comp Biol 44:403–412. doi:10.1093/icb/44.6.403
Ashton KG, Feldman CR (2003) Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57:1151–1163
Bell AM, Stamps JA (2004) Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus. Anim Behav 68:1339–1348. doi:10.1016/j.anbehav.2004.05.007
Bengston SE, Dornhaus A (2013) Colony size does not predict foraging distance in the ant Temnothorax rugatulus: a puzzle for standard scaling models. Insect Soc 60:93–96. doi:10.1007/s00040-012-0272-4
Bengston SE, Dornhaus A (2014) Be meek or be bold? A colony-level behavioural syndrome in ants. Proc R Soc B Biol Sci 281:20140518. doi:10.1098/rspb.2014.0518
Bengston SE, Jandt JM (2014) The development of collective personality: the ontogenetic drivers of behavioral variation across groups. Behav Evol Ecol 2:81. doi:10.3389/fevo.2014.00081
Bengston SE, Pruitt JN, Riechert SE (2014) Differences in environmental enrichment generate contrasting behavioural syndromes in a basal spider lineage. Anim Behav 93:105–110. doi:10.1016/j.anbehav.2014.04.022
Biro PA, Stamps JA (2008) Are animal personality traits linked to life-history productivity? Trends Ecol Evol 23:361–368. doi:10.1016/j.tree.2008.04.003
Biro PA, Abrahams MV, Post JR, Parkinson EA (2004) Predators select against high growth rates and risk–taking behaviour in domestic trout populations. Proc R Soc Lond B Biol Sci 271:2233–2237. doi:10.1098/rspb.2004.2861
Blanckenhorn WU, Fairbairn DJ (1995) Life history adaptation along a latitudinal cline in the water strider Aquarius remigis (Heteroptera: Gerridae). J Evol Biol 8:21–41. doi:10.1046/j.1420-9101.1995.8010021.x
Blumstein DT (2006) Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim Behav 71:389–399. doi:10.1016/j.anbehav.2005.05.010
Bryant MJ, Grant JWA (1995) Resource defence, monopolization and variation of fitness in groups of female Japanese medaka depend on the synchrony of food arrival. Anim Behav 49:1469–1479. doi:10.1016/0003-3472(95)90068-3
Burnham KP, Anderson DR, Huyvaert KP (2010) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. doi:10.1007/s00265-010-1029-6
Cao TT, Dornhaus A (2012) Ants use pheromone markings in emigrations to move closer to food-rich areas. Insect Soc 59:87–92. doi:10.1007/s00040-011-0192-8
Chapuisat M, Goudet J, Keller L (1997) Microsatellites reveal high population viscosity and limited dispersal in the ant formica paralugubris. Evolution 51:475–482. doi:10.2307/2411120
Clark PJ, Evans FC (1954) Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology 35:445–453. doi:10.2307/1931034
Conover DO (1992) Seasonality and the scheduling of life history at different latitudes. J Fish Biol 41:161–178. doi:10.1111/j.1095-8649.1992.tb03876.x
Conover DO, Schultz ET (1995) Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol Evol 10:248–252. doi:10.1016/S0169-5347(00)89081-3
Costa R, Peixoto AA, Barbujani G, Kyriacou CP (1992) A latitudinal cline in a drosophila clock gene. Proc R Soc Lond B Biol Sci 250:43–49. doi:10.1098/rspb.1992.0128
Cousyn C, Meester LD, Colbourne JK et al (2001) Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc Natl Acad Sci 98:6256–6260. doi:10.1073/pnas.111606798
Croy MI, Hughes RN (1991) Effects of food supply, hunger, danger and competition on choice of foraging location by the fifteen-spined stickleback, Spinachia spinachia L. Anim Behav 42:131–139. doi:10.1016/S0003-3472(05)80613-X
Derksen S, Keselman HJ (1992) Backward, forward and stepwise automated subset selection algorithms: Frequency of obtaining authentic and noise variables. Br J Math Stat Psychol 45:265–282. doi:10.1111/j.2044-8317.1992.tb00992.x
Dingemanse NJ, Kazem AJN, Réale D, Wright J (2010) Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol 25:81–89. doi:10.1016/j.tree.2009.07.013
Dornhaus A, Chittka L (2004) Why do honey bees dance? Behav Ecol Sociobiol 55:395–401. doi:10.1007/s00265-003-0726-9
Dornhaus A, Holley J-A, Pook VG et al (2008) Why do not all workers work? Colony size and workload during emigrations in the ant Temnothorax albipennis. Behav Ecol Sociobiol 63:43–51. doi:10.1007/s00265-008-0634-0
Dornhaus A, Holley J-A, Franks NR (2009) Larger colonies do not have more specialized workers in the ant Temnothorax albipennis. Behav Ecol. doi:10.1093/beheco/arp070
Fisher J (1954) Evolution and bird sociality. Evol Process 71–83
Fisher BL (1999) Improving inventory efficiency: a case study of leaf-litter ant diversity of Madagascar. Ecol Appl 9:714–731. doi:10.1890/1051-0761(1999)009[0714:IIEACS]2.0.CO;2
Foitzik S, Heinze J (1998) Nest site limitation and colony takeover in the ant Leptothorax nylanderi. Behav Ecol 9:367–375. doi:10.1093/beheco/9.4.367
Goldberg JL, Grant JWA, Lefebvre L (2001) Effects of the temporal predictability and spatial clumping of food on the intensity of competitive aggression in the Zenaida dove. Behav Ecol 12:490–495. doi:10.1093/beheco/12.4.490
Gordon DM (2013) The rewards of restraint in the collective regulation of foraging by harvester ant colonies. Nature 498:91–93. doi:10.1038/nature12137
Grand TC, Dill LM (1999) The effect of group size on the foraging behaviour of juvenile coho salmon: reduction of predation risk or increased competition? Anim Behav 58:443–451. doi:10.1006/anbe.1999.1174
Grant JWA, Guha RT (1993) Spatial clumping of food increases its monopolization and defense by convict cichlids, Cichlasoma nigrofasciatum. Behav Ecol 4:293–296. doi:10.1093/beheco/4.4.293
Guttman L (1954) Some necessary conditions for common-factor analysis. Psychometrika 19:149–161. doi:10.1007/BF02289162
Holway DA (1998) Factors governing rate of invasion: a natural experiment using Argentine ants. Oecologia 115:206–212. doi:10.1007/s004420050509
Hood WG, Tschinkel WR (1990) Desiccation resistance in arboreal and terrestrial ants. Physiol Entomol 15:23–35. doi:10.1111/j.1365-3032.1990.tb00489.x
Inger R, Bearhop S, Robinson JA, Ruxton G (2006) Prey choice affects the trade-off balance between predation and starvation in an avian herbivore. Anim Behav 71:1335–1341. doi:10.1016/j.anbehav.2005.08.015
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214. doi:10.2307/1939574
Jandt JM, Bengston S, Pinter-Wollman N et al (2014) Behavioural syndromes and social insects: personality at multiple levels. Biol Rev 89:48–67. doi:10.1111/brv.12042
Jongepier E, Kleeberg I, Job S, Foitzik S (2014) Collective defence portfolios of ant hosts shift with social parasite pressure. Proc R Soc B Biol Sci 281:20140225. doi:10.1098/rspb.2014.0225
Kaspari M, Vargo EL (1995) Colony size as a buffer against seasonality: Bergmann’s rule in social insects. Am Nat 145:610–632
Kaspari M, Ward PS, Yuan M (2004) Energy gradients and the geographic distribution of local ant diversity. Oecologia 140:407–413. doi:10.1007/s00442-004-1607-2
Kelley OJ, Hunter AS, Haise HR, Hobbs CH (1946) Comparison of methods of measuring soil moisture under field conditions
Lima SL (1998) Stress and decision-making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Advances in the study of behavior: stress and behavior. Academic Press
McGlone J, Stansbury W, Tribble L (1987) Effects of heat and social stressors and within-pen weight variation on young pig performance and agonistic behavior. J Anim Sci 65:456–462
Modlmeier AP, Foitzik S (2011) Productivity increases with variation in aggression among group members in Temnothorax ants. Behav Ecol 22:1026–1032. doi:10.1093/beheco/arr086
Mundry R (2010) Issues in information theory-based statistical inference—a commentary from a frequentist’s perspective. Behav Ecol Sociobiol 65:57–68. doi:10.1007/s00265-010-1040-y
Pamminger T, Modlmeier AP, Suette S et al (2012) Raiders from the sky: slavemaker founding queens select for aggressive host colonies. Biol Lett. doi:10.1098/rsbl.2012.0499
Partridge LW, Partridge KA, Franks NR (1997) Field survey of a monogynous leptothoracine ant (Hymenoptera, Formicidae) evidence of seasonal polydomy ? Insect Soc 44:75–83. doi:10.1007/s000400050031
Pinter-Wollman N, Gordon DM, Holmes S (2012) Nest site and weather affect the personality of harvester ant colonies. Behav Ecol. doi:10.1093/beheco/ars066
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540. doi:10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2
Riechert SE, Jones TC (2008) Phenotypic variation in the social behaviour of the spider Anelosimus studiosus along a latitudinal gradient. Anim Behav 75:1893–1902. doi:10.1016/j.anbehav.2007.10.033
Rueppell O, Kirkman RW (2005) Extraordinary starvation resistance in Temnothorax rugatulus (Hymenoptera, Formicidae) colonies: demography and adaptive behavior. Insect Soc 52:282–290. doi:10.1007/s00040-005-0804-2
Rüppell O, Heinze J, Hölldobler B (1998) Size-dimorphism in the queens of the North American ant Leptothorax rugatulus (Emery). Insect Soc 45:67–77. doi:10.1007/s000400050069
Rüppell O, Strätz M, Baier B, Heinze J (2003) Mitochondrial markers in the ant Leptothorax rugatulus reveal the population genetic consequences of female philopatry at different hierarchical levels. Mol Ecol 12:795–801. doi:10.1046/j.1365-294X.2003.01769.x
Sasaki T, Pratt SC (2013) Ants learn to rely on more informative attributes during decision-making. Biol Lett 9:20130667. doi:10.1098/rsbl.2013.0667
Sawyer LA, Sandrelli F, Pasetto C et al (2006) The period gene thr-Gly polymorphism in Australian and African drosophila melanogaster populations: implications for selection. Genetics 174:465–480. doi:10.1534/genetics.106.058792
Scheiner SM (1993) Genetics and evolution of phenotypic plasticity. Annu Rev Ecol Syst 24:35–68
Schumacher B (2002) Methods for the determination of total organic carbon (TOC) in soils and sediments
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. doi:10.1016/j.tree.2004.04.009
Stachowicz JJ, Hay ME (2000) Geographic variation in camouflage specialization by a decorator crab. Am Nat 156:59–71. doi:10.1086/an.2000.156.issue-1
Tran MV, O’Grady M, Colborn J et al (2014) Aggression and food resource competition between sympatric hermit crab species. PLoS ONE 9:e91823. doi:10.1371/journal.pone.0091823
West-Eberhard MJ (1989) Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Syst 20:249–278
Acknowledgments
We would like to thank the Dornhaus lab, Stephen Pratt and two anonymous reviewers for helpful feedback on the manuscript, Daniel Charbonneau for advice and feedback on the data analysis, Min Shin and Hoan Nguyen for their development of the optic flow algorithm, as well as NSF (grants no. IOS-1045239 and DBI-1262292 to AD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. O. H. Hughes
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
All of the models tested in the stepwise model selection process and the associated AIC value of each model. The final model selected resulted in the percentage of nest sites occupied and the clustering index as the predictive variables with an AIC score of -146.9. (JPEG 60 kb)
Rights and permissions
About this article
Cite this article
Bengston, S.E., Dornhaus, A. Latitudinal variation in behaviors linked to risk tolerance is driven by nest-site competition and spatial distribution in the ant Temnothorax rugatulus . Behav Ecol Sociobiol 69, 1265–1274 (2015). https://doi.org/10.1007/s00265-015-1939-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1939-4