Abstract
Animals gather noisy information about the world and then process it to trigger appropriate behavioural responses. To identify the best action to take, both the costs and benefits associated with each action should be taken into account. Social insects are known to be good at solving such trade-offs, since they rely on the ‘wisdom of the crowd’. In other words, by pooling the decisions of many individuals, they can reach an optimal collective decision. However, this process can lead to the assumption that adaptive flexibility in decision-making resides entirely at the colony level, whereas decision-making by individuals is constrained by their limited cognitive capacities. Here, we show that ant colonies are able to respond flexibly and adaptively to their environment when making decisions and that this feature is accomplished by individuals also showing such flexibility. We presented Temnothorax albipennis colonies with the opportunity to move to a better home and measured how emigration dynamics were affected by varying the value of both current and target nests. Colonies take less time to commit to a new nest when the value difference between current and target nest is bigger, i.e. greater benefit. Furthermore, this is accomplished by individuals manipulating their recruitment speed either by moving faster or recruiting sooner. This observation indicates that, regardless of the degree of difficulty of the choice, an individual that has sufficient time and information can make good decisions that will ultimately confer its group the ability to solve more complicated dilemmas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In their natural environment, animals must decide the best action to take in a variety of circumstances to maximize their fitness (Pelé and Sueur 2013). Whether they are looking for food, a mate or a new home, animals invest both time and energy to assess their environment accurately and to make appropriate commitments (Mori and Nakata 2008). However, behaviour might benefit the animal in one way and be costly in another (McNamara and Houston 1996). In ants, for example, a higher-quality nest site is likely to confer higher fitness, but individuals must decide if this benefit outweighs the costs and risks of abandoning their current home and moving to a new one (Dornhaus et al. 2004). Ants are able to reach a consensus regarding when and where to emigrate by combining the information of many individual ants (Franks et al. 2002). There are many examples of consensus decision-making in animal groups, which compared to lone individuals, reduce errors and increase the chances of finding the best overall solution (Conradt and Roper 2005, 2007; Sumpter and Pratt 2009). However, studies of animal collective behaviour, particularly in social insects, often assume that groups are composed of individuals with only the most basic cognitive capabilities (Deneubourg and Goss 1989; Dornhaus and Franks 2008; Sasaki and Pratt 2011). Here, we challenge this view by showing that when deciding whether to emigrate to a superior nest, ants flexibly and adaptively change their collective behaviour due to adaptive changes occurring at the individual level.
Ant colonies are a powerful model to analyse collective choice mechanisms. They facilitate observation of their component parts as well as the collective outcome. This feature allows analysis not only of the final choice but also of the process by which members of the group contribute to the decision. Furthermore, ants are unrivalled subjects for behavioural experiments in carefully controlled environments (Pratt et al. 2002; Franks et al. 2003a, b). Field observations have shown that in various species of the genus Temnothorax, minor disturbances easily induce a colony to abandon their home and move to a new cavity (Moglich 1978).
Emigrations can be divided into three distinctive phases (Fig. 1): (a) scouts discover and assess potential nest sites; (b) these informed scouts teach the location of the new nest to other ants by a one-to-one recruitment process called tandem running (Franks and Richardson 2006); and (c) once a quorum of nestmates is present in the new nest, individual ants switch to faster recruitment by swiftly carrying their nestmates, including the queen and brood, to the target nest (Pratt et al. 2002; Hölldobler and Wilson 2009). This quorum is reached by individuals deciding independently that a nest is suitable, and this is the moment when the colony has reached a collective decision (Pratt et al. 2002).
Emigration phases. In the searching phase, individuals leave their current nest to search for a better home. Once an ant finds a target nests and decides to recruit another nestmate to assess it, the recruitment phase begins. In this phase, informed ants teach the way to a naive ant to a target nest. The naive ant will assess the target nest and potentially become a recruiter. The population in the target nest starts increasing until it reaches a quorum threshold. At this point, the moving phase begins, and all the remaining individuals (ants, brood or queen) are physically carried to the new nest
Temnothorax albipennis colonies will also ‘move-to-improve’, emigrating to a new nest even when their current nest remains undisturbed (Dornhaus et al. 2004). This behaviour occurs only when the target nest represents a sufficient increase in quality over their current nest. During an emigration in the wild, the risk of predation is increased, and the majority of the colony members, the queen and all the brood items have to be physically carried to the new nest. The colony should therefore only move if the improvement in nest quality compensates for the costs and risks of emigrating (Pratt et al. 2002; Dornhaus et al. 2004). Previous studies have revealed the characteristics T. albipennis seeks in a nest site. Colonies typically prefer narrow entrances, high ceilings and dark nests (Franks et al. 2003b). Moreover, they appear to weight each of these attributes differently and so will, for example, prefer a dark nest to one with a high ceiling and high ceilings to narrow entrances (Franks et al. 2003b). Ultimately, the value of each nest will be the combination of the value of its attributes (Franks et al. 2003b).
Several studies have shown that T. albipennis ants adjust their search effort in accordance with their current housing conditions, indicating that they decide how much to invest in information gathering depending on how much they can potentially benefit from finding a new home (Stroeymeyt et al. 2011; Doran et al. 2013). In the current paper, by maintaining constant costs whilst varying the benefit, we manipulate the benefit-to-cost ratio and investigate how this affects search effort. Furthermore, we also investigate how the different emigration stages are affected by this benefit increase and how this is underpinned by individual behaviour.
Methods
Experiments were carried out with 12 colonies collected in October 2012 from Dorset, UK. Colonies were cultured and fed according to standard procedures (Sendova-Franks and Franks 1993). Colony size ranged between 67 and 220; nine of the colonies each had one queen, and the remaining three colonies had no queen.
Experiments
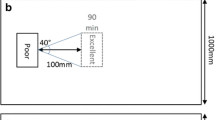
Each experiment began when a new target nest was added to the Petri dish containing the focal colony, so that they could either decide to emigrate to the target nest or remain in the current nest (Fig. 2a). Four different nest types were used: ‘Poor’, ‘Satisfactory’, ‘Medium’ and ‘Good’; however, the current nest was always light, and the target nest always dark (Fig. 2b). Ten different types of ‘move-to-improve’ emigrations were performed (Table 1); in all these cases, the target nest was of higher quality than the current nest, to ensure that there was some benefit to be gained (but also costs incurred) by moving. We used a modified Latin-square design so that each colony faced five of the ten different treatments in a randomized sequence (raw data file). Colonies were allowed to settle into their testing nest for 1 week before experiments. Each experiment ran for 24 h. During the first 6 h, the number of ants in the arena and in the target nest was counted every 15 min, and several components of the dynamics of the emigrations were timed: target nest discovery, first tandem run, first and last transport of a nestmate and carry of last brood item. The number of ants in the arena and target nest was also recorded at the time of these events. The total number of tandem runs was counted for each emigration type. Each colony was tested only once a week to prevent improvement in emigration due to experience (Langridge et al. 2007, 2008). After each experiment, colonies were forced to move into their next testing nest. This was accomplished by removing the top glass slide of their current nest and placing the bottom slide containing the colony on top of the new ‘current nest’ to which colonies quickly emigrate (always within 1 or 2 h). Colonies were fed 1 day before testing, and food and water were always kept in the same location in the arena (Fig. 2a).
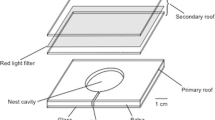
Experimental design. a Diagram of the arena; dotted line represents the position of ‘Poor’ and ‘Poor (dark)’ nests which were different to those of the other nest qualities to account for the different shapes of such nests and to ensure that the distance between the current nest entrance and target nest entrance was the same for all emigration types. b–e Different current nests tested. All the current nests were light. f–i Different target nests tested. All the target nests were kept dark by placing a red filter cover on top. Nest quality characteristics were taken from Doran et al. (2013)
Statistical analysis
We analysed the effect of treatment on the behaviour of colonies with Fisher’s exact test in SPSS (v21) (IBM Corp. 2012).
The effect of current nest value on the number of ants outside was analysed with a generalized linear mixed model by using the function glmer with a Poisson response and log link from the lme4 package and lmerTest for p value estimation of R v3.2.1 (R Development Core Team 2015). The response variable was the number of ants, and the predictors were current nest value (fixed factor) and colony (random factor with variation around the intercept). We compared this model with a simpler model that did not include the fixed factor and ran an analysis of variance using the ANOVA function to check for significant differences between the two models.
We analysed the effect of both current nest and target nest value (treatment) on total emigration time, time of target nest discovery, time of first tandem run, time between first tandem run and first carry, time between the first and last carry, number of tandem runs and quorum (number of ants in the target nest at time of first carry event). In all cases, we ran a linear mixed model using the function lmer from the lme4 package and lmerTest for p value estimation of R v3.2.1 (R Development Core Team 2015). The response variable was the log10 of, either time, number of tandem runs or number of ants. The predictors were treatment (fixed factor) and colony (random factor with variation around the intercept). Again, for all cases, we compared these models with a simpler model that did not include the fixed factor and ran an ANOVA to check for significant differences between the two models.
We ran all the models with polynomial contrast in order to assess the significance of a linear, quadratic and cubic trend. This was appropriate because the categorical factor, nest value, is ordinal (i.e. the nest values have an inherent order). Furthermore, we also ran the models with a treatment contrast to assess differences between the treatments so that we could have pairwise comparisons, always correcting for the number of comparisons with Bonferroni corrections.
Results
Over the course of 5 weeks, a total of 60 emigrations were conducted. Of these, 41 occurred within the observation period (first 6 h), 6 occurred overnight, 4 colonies split between the current and target nests and 9 did not emigrate at all. These results were not distributed equally, Fisher’s exact test showed a significant association between treatment and whether emigrations were successful or not (F = 35.008 and p = 0.001). The majority of non-emigrations occurred when the current and the target nest were of the same structural type, differing only in that the target nest was darker (fig S1). These emigrations are the ones with the biggest difference between expected and observed counts in the different behaviours and hence the ones that most contribute to this result (supplementary material section a).
The number of ants in the arena decreased significantly with increasing quality of the current nest, in the absence of a target nest (p < 0.001, = 0.527, = 0.403 for a linear, quadratic and cubic trend, respectively, with the inclusion of treatment contributing significantly to the model, p < 0.001; supplementary material section b and fig S2). All 60 trials were counted for this section. Thus, in accordance with previous findings, a smaller effort is put into searching for a new nest when the colony’s current nest is of high quality (Stroeymeyt et al. 2011; Doran et al. 2013).
Colonies emigrate faster when the benefit of moving is greater
We analysed the effect of varying the value of current nests or target nests on total emigration time for 33 cases where emigrations were successfully complete (raw data). Emigrations were considered complete at the moment the last brood item was placed inside the target nest.
The influence of the current nest value was assessed by comparing the total emigration time to a Good (dark) target nest from the four different types of current nests (Fig. 3a). Low-quality nests are expected to be valued less. Decreasing the current nest quality significantly decreased total emigration time linearly (p < 0.001, quadratic and cubic trends were not significant p = 0.558 and 0.441, respectively), with the inclusion of treatment in the model having a highly significant effect (p < 0.001, Fig. 3a, supplementary material section c); furthermore, pairwise comparisons showed that emigration times from current nests that were Poor were significantly different in comparison to emigrations from Good and Medium current nests. Total emigration times between Satisfactory, Medium and Good current nests and the Good target nests were not significantly different from each other (Fig. 3a (capital letters) and supplementary material section c).
Current nest devaluation and target nest attraction. a Effect of decreasing current nest value on total emigration time; all emigrations were to ‘Good (dark)’ target nests. b Effect of attraction to the target nest on total emigration time; all emigrations were from ‘Poor (light)’ current nests. Capital letters represent significant differences between the different treatments after a Bonferroni correction
The influence of target nest value was assessed by comparing total emigration time from a Poor (light) current nest to the four different types of target nests. High-quality nests should be more attractive to the ants, and indeed, there was a significant linear decrease in total emigration time with target nest value increase (p = 0.0121, quadratic and cubic trends were not significant p = 0.6153 and 0.6966, respectively) with the inclusion of treatment in the model having a significant effect (p = 0.01139, Fig. 3b, supplementary information section d). Here, there were no significant differences between the emigration times except between those associated with Good (dark) target nests and Satisfactory target nests (Fig. 3b, supplementary material section d). Overall, emigration times were influenced much more by varying current nest values (Fig. 3b) than varying target nest values (Fig. 3a), and in both cases, the easier the decision, i.e. the bigger the value difference between current and target nests, the faster were colonies in reaching a consensus and moving.
Individuals recruit faster when the decision is easier
Although both the current nest and target nest value affected the overall emigration time, the different phases of the emigration were not affected equally. The time it took colonies to find the target nest and start recruiting was not affected significantly neither by varying current nest value (p = 0.6302, Fig. 4a) nor varying target nest value (p = 0.0719, Fig. 4b). In contrast, the recruitment phase duration significantly decreased both when the current nest value decreased (p < 0.001, = 0.767, = 0.178 for a linear, quadratic and cubic trend, respectively, with the addiction of treatment to the model having a significant effect, p < 0.001) and when the target nest value increased (p = 0.0189, 0.405, 0.9717 for a linear, quadratic and cubic trend, respectively, with the addiction of treatment in the model also having a significant effect, p < 0.001). Colonies committed more quickly to the target nest when the quality difference was large and so easier to distinguish between the two nests. This is the phase of the emigration in which individual ants are being recruited to the target nest, assessing it and deciding whether it is a suitable nest or not. Furthermore, the time between the first and the last transport event showed a significant linear decrease with current nest value decrease (p = 0.0107, 0.2523, 0.7127 for a linear, quadratic and cubic trend); however, the effect of adding treatment to the model was marginally non-significant (p = 0.05191, Fig. 4e). This phase of the emigration was significantly shorter when the quality of the target nest increased (adding treatment to the model had a significant effect, p = 0.01225, with a significant linear trend, p < 0.001, = 0.864, = 0.590 for linear, quadratic and cubic trend, respectively, Fig. 4f).
Emigration dynamics for current next value decrease and target nest value increase (Fig. 3). a, b Time taken for colonies to find and assess the target nest. c, d Time between the first recruitment by tandem run until the first carrying behaviour. e, f Duration of recruitment by carrying. Capital letters represent significant differences between the different treatments after a Bonferroni correction
Although the speed of recruitment was influenced by the value of both current and target nests, other aspects of the emigration were not. The total number of tandem runs did not change significantly with a change in the quality of the current nest, or the target nest as in both cases adding treatment to the model showed no significant differences (p = 0.3098 and 0.7955, respectively, fig S3a and S4a) and neither did the quorum threshold (p = 0.3235 and 0.6016, respectively, fig S3b and S4b). All these analyses were made for colonies that showed the behaviour being analysed; namely, 37 cases for time of first tandem run and 33 for time between first tandem run and first carry, time between first and last carry, number of tandem runs and quorum threshold. Detailed results can be found in supplementary material (section c and d).
Because both the quorum size and the number of tandem runs remained constant, the acceleration in recruitment cannot be attributed to a larger number of recruits. Instead, the decrease in emigration times can be explained by two different scenarios: (1) both tandem running and carrying were performed faster, due to individuals being more motivated to emigrate; and/or (2) individuals decided to start recruiting sooner, so that the same number of tandem runs and carrying events occurred in a shorter time period. Both of these scenarios involve individual ants flexibly adjusting their behaviour to their current situation by recruiting faster and/or sooner.
Discussion
The current study shows that colonies adjust their recruitment times, i.e. the period between the first tandem run and the achievement of a quorum, and the period between the first and last carry event (Fig. 1), predominantly to the value of the final payoff (value difference between current and target nest). Furthermore, we also show that this collective behaviour is accomplished by individuals flexibly adjusting their behaviour.
Behaviour should be favoured by natural selection whenever the benefits of performing the action outweigh the costs (Davies et al. 2012). Several studies have shown that colonies of T. albipennis make nest-site choices in a way that is consistent with the axioms stipulated by economical rationality (Franks et al. 2003b; Edwards and Pratt 2009). However, it has also been reported that although colonies appear to make rational decisions, this can emerge from the behaviour of many irrational individuals. Sasaki and Pratt (2011) showed that isolated individual ants behave irrationally, in the sense that they change their preference between two nest sites in the presence of a third option that is asymmetrically dominated, i.e. inferior to one of the two nest sites in all attributes, and inferior to the other in some attributes but superior in others (Mark and Marley 2006; Sasaki and Pratt 2011). However, to quote Sasaki and Pratt (2011), ‘Collective choice can only limit these errors if it allows individuals to show qualitatively different behaviors in the social context than they do when alone’. In sum, this means that individual ants separated from their colonies are bound to behave very differently than in a group. Social insect colonies function as one adaptive unit, and arguably, a lone individual may not behave rationally because it is in a stressful state (Seeley 1997; Schuck-Paim et al. 2004; Gardner and Grafen 2009). Although selection in such systems acts primarily at the colony level (Gardner and Grafen 2009), the current study provides evidence that, under relaxed time constraints, individuals can also make decisions adaptively and thus significantly improve the power of the collective.
Previous work shows evidence of individual ants adjusting their behaviour depending on the value of their current nest by increasing their acceptance rate in response to a greater need of finding a new home, i.e. a greater benefit to be gained from finding a suitable target nest (Sumpter and Pratt 2009); here, we show that individuals also decrease their recruiting speed when the benefit from moving is greater.
This is revealed by three observations: (a) reduced emigration times are not caused solely by an increase in the number of scouts, since there were differences in total emigration time even when comparing emigration times from the same current nest value, which is the cause of the variation observed in the number of ants outside; (b) the time it takes a colony to make a decision and emigrate is affected by the benefit-to-cost ratio; and (c) both the number of tandem runs and the magnitude of quorum thresholds remain constant across all types of emigrations. The quorum signifies that many ants have concluded independently that the target nest is a suitable one (Pratt et al. 2002), whilst the number of tandem runs influences the number of individuals with an active role in the emigration (Planqué et al. 2007). This means that the change in emigration time reflects changes in the time it takes individuals to make their own assessments and decisions. Even though we did not record the behaviour of individual ants marked or isolated from their colonies, we were able to show that the same number of informed individuals performed the same job at different speeds.
The study of collective cognition in animals is a growing field, embracing ever more species and several levels of complexity (Couzin 2007). Many studies have shown that individuals, by reacting to one another, may confer on the group an enhanced ability to extract and act upon information in a noisy environment (Sumpter and Pratt 2009; Couzin 2009; Marshall et al. 2009). Arguably, flexibility in individual decision-making does not necessarily mean an increase in cognitive capacities. Sumpter and Pratt (2009) showed how individuals are able to adjust their responses depending on their speed vs accuracy needs, using the same underlying behavioural algorithm. Simple rules can often explain the behaviour of individuals but might not represent their full capabilities, and we might be constrained by what we are able to observe experimentally and explore through the use of mathematical modelling. For these reasons, we caution against underestimating the sophistication of the individual.
In a ‘move-to-improve’ scenario, the ants have no need to rush into a decision, and in this case, the colony’s integrity is kept, since we do not physically separate individuals, allowing for rationality to emerge without the need of the power of the collective. The present findings, together with the study by Sasaki and Pratt (2011), highlight the remarkable ability of social insects to respond to challenges in their natural environment. Unquestionably, collectives can accomplish more than their individual components (Sasaki and Pratt 2012). However, there is a pressing need to recognize the cognitive abilities of the individuals that contribute to the collective. We hope this study will ignite further research into collective animal behaviour with an appreciation of sophistication and complexity not only at the group level but also at the individual level.
Data accessibility
All data are available in the supplementary information (‘rawdata.xlsx’).
References
Conradt L, Roper TJ (2005) Consensus decision making in animals. Trends Ecol Evol 20:449–456. doi:10.1016/j.tree.2005.05.008
Conradt L, Roper TJ (2007) Democracy in animals: the evolution of shared group decisions. Proc Biol Sci 274:2317–2326. doi:10.1098/rspb.2007.0186
Couzin I (2007) Collective minds. Nature 445:715. doi:10.1038/445715a
Couzin ID (2009) Collective cognition in animal groups. Trends Cogn Sci 13:36–43. doi:10.1016/j.tics.2008.10.002
Davies NB, Krebs JR, West SA (2012) An introduction to behavioural ecology, 4th edn. Wiley-Blackwell, Chichester, p 520
Deneubourg J, Goss S (1989) Collective patterns and decision-making. Ethol Ecol Evol 1:295–311. doi:10.1080/08927014.1989.9525500
Doran C, Pearce T, Connor A et al (2013) Economic investment by ant colonies in searches for better homes. Biol Lett 9:20130685. doi:10.1098/rsbl.2013.0685
Dornhaus A, Franks NR (2008) Individual and collective cognition in ants and other insects (Hymenoptera: Formicidae). Myrmecological News 11:215–226
Dornhaus A, Franks NR, Hawkins RM, Shere HNS (2004) Ants move to improve: colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Anim Behav 67:959–963. doi:10.1016/j.anbehav.2003.09.004
Edwards SC, Pratt SC (2009) Rationality in collective decision-making by ant colonies. Proc Biol Sci 276:3655–3661. doi:10.1098/rspb.2009.0981
Franks NR, Richardson T (2006) Teaching in tandem-running ants. Nature 439:153. doi:10.1038/439153a
Franks NR, Pratt SC, Mallon EB et al (2002) Information flow, opinion polling and collective intelligence in house-hunting social insects. Philos Trans R Soc Lond B Biol Sci 357:1567–1583. doi:10.1098/rstb.2002.1066
Franks NR, Dornhaus A, Fitzsimmons JP, Stevens M (2003a) Speed versus accuracy in collective decision making. Proc R Soc B Biol Sci 270:2457–2463. doi:10.1098/rspb.2003.2527
Franks NR, Mallon EB, Bray HE et al (2003b) Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Anim Behav 65:215–223. doi:10.1006/anbe.2002.2032
Gardner A, Grafen A (2009) Capturing the superorganism: a formal theory of group adaptation. J Evol Biol 22:659–671. doi:10.1111/j.1420-9101.2008.01681.x
Hölldobler B, Wilson EO (2009) The superorganism: the beauty, elegance, and strangeness of insect societies. W.W. Norton, London p 522
IBM Corp. (2012) IBM SPSS statistics for windows, version 21.0. Armonk, New York
Langridge EA, Sendova-Franks AB, Franks NR (2007) How experienced individuals contribute to an improvement in collective performance in ants. Behav Ecol Sociobiol 62:447–456. doi:10.1007/s00265-007-0472-5
Langridge EA, Sendova-Franks AB, Franks NR (2008) The behaviour of ant transporters at the old and new nests during successive colony emigrations. Behav Ecol Sociobiol 62:1851–1861. doi:10.1007/s00265-008-0614-4
Mark J, Marley AA (2006) Extending the bounds of rationality: evidence and theories of preferential choice. J Econ Lit XLIV:631–661
Marshall JA, Bogacz R, Dornhaus A et al (2009) On optimal decision-making in brains and social insect colonies. J R Soc Interface 6:1065–1074. doi:10.1098/rsif.2008.0511
McNamara JM, Houston AI (1996) State-dependent life histories. Nature 380(6571):215–221
Moglich M (1978) Social organization of nest emigration in Leptothorax (Hym., Form.). Insect Soc 25:205–225
Mori Y, Nakata K (2008) Optimal foraging and information gathering: how should animals invest in repeated foraging bouts within the same patch? Evol Ecol Res 10:823–834
Pelé M, Sueur C (2013) Decision-making theories: linking the disparate research areas of individual and collective cognition. Anim Cogn 16:543–556. doi:10.1007/s10071-013-0631-1
Planqué R, Dechaume-Moncharmont F-X, Franks NR et al (2007) Why do house-hunting ants recruit in both directions? Naturwissenschaften 94:911–918. doi:10.1007/s00114-007-0273-8
Pratt S, Mallon E, Sumpter D, Franks N (2002) Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav Ecol Sociobiol 52:117–127. doi:10.1007/s00265-002-0487-x
R Development Core Team (2015) R: A language and environment for statistical computing. RFoundation for Statistical Computing, Vienna, Austria
Sasaki T, Pratt SC (2011) Emergence of group rationality from irrational individuals. Behav Ecol 22:276–281. doi:10.1093/beheco/arq198
Sasaki T, Pratt SC (2012) Groups have a larger cognitive capacity than individuals. Curr Biol 22:R827–R829. doi:10.1016/j.cub.2012.07.058
Schuck-Paim C, Pompilio L, Kacelnik A (2004) State-dependent decisions cause apparent violations of rationality in animal choice. PLoS Biol 2:e402. doi:10.1371/journal.pbio.0020402
Seeley TD (1997) Honey bee colonies are group-level adaptive units. Am Nat 150:S22–S41. doi:10.1086/286048
Sendova-Franks A, Franks NR (1993) Task allocation in ant colonies within variable environments (a study of temporal polyethism: experimental). Bull Math Biol 55:75–96
Stroeymeyt N, Robinson EJH, Hogan PM et al (2011) Experience-dependent flexibility in collective decision making by house-hunting ants. Behav Ecol 22:535–542. doi:10.1093/beheco/arr007
Sumpter DJT, Pratt SC (2009) Quorum responses and consensus decision making. Philos Trans R Soc Lond B Biol Sci 364:743–753. doi:10.1098/rstb.2008.0204
Acknowledgments
We thank Ana Sendova-Franks, Jamie Freeman and Tim Fawcett for their comments on the manuscript and help with statistical analysis. We thank all the members of the lab for their assistance with experiments. Carolina Doran thanks the Fundação para a Ciência e Tecnologia, Portugal (grant SFRH/BI/51712/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. O. H. Hughes
Rights and permissions
About this article
Cite this article
Doran, C., Newham, Z.F., Phillips, B.B. et al. Commitment time depends on both current and target nest value in Temnothorax albipennis ant colonies. Behav Ecol Sociobiol 69, 1183–1190 (2015). https://doi.org/10.1007/s00265-015-1932-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1932-y