Abstract
Conditional mating strategies enable individuals to modulate their mating behaviour depending on ‘individual status’ to maximise fitness. Theory predicts that variation in individual quality can lead to differences in mating preferences. However, empirical evidence is scarce particular in terms of variation in male and female strategies. Here, we experimentally investigated quality-dependent variation in mating preferences concerning reliable quality indicators in Pelvicachromis taeniatus, a colourful cichlid fish with mutual mate choice and ornamentation. Males as well as females were artificially manipulated in phenotypic quality by different feeding regimes. Ornamentation was connected to individual quality in both sexes. Males and females showed conditional mating strategies in different directions. Males showed prudent choice by preferring females of similar quality. In contrast to males, low-quality females preferred highly ornamented males, whereas high-quality females showed neither preferences for high- nor for low-quality males. The results suggest that individuals aim for specific benefits depending on individual quality. Furthermore, the conflicting conditional mating preferences of males and females might lead to sexual conflict, implicating a highly dynamical mating system that evolves even in absence of environmental changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Theory suggests that variation in quality of the choosing individuals can lead to a range of potentially evolutionarily stable mate choice strategies (ESS) (Härdling and Kokko 2005), instead of a general preference for the mate of highest quality that is available on the mating market (Jennions and Petrie 1997; Widemo and Saether 1999). Especially in species in which reproduction is associated with high costs, e.g. due to strong competition over mates (Fawcett and Johnstone 2003), intense brood care (Kokko and Monaghan 2001) or viability costs connected to the development of the sensory apparatus (Cotton et al. 2006a), phenotypic variation may lead to conditional mating strategies that depend on individual quality (Cotton et al. 2006b). The resulting ESS may differ depending on the mating system or the benefits of choice.
Recent studies provided the first empirical evidence that quality-related mating preferences exist in females (Bakker et al. 1999; Holveck and Riebel 2010; Holveck et al. 2011). For instance, preferences for mates of similar quality can evolve, resulting in assortative mating patterns (e.g. Franceschi et al. 2010), i.e. that high-quality individuals prefer high-quality individuals, whereas low-quality individuals show preference for low-quality ones (Härdling and Kokko 2005). These studies largely support the theoretical predictions that females optimally balance their costs and benefits of mate acquisition. High-quality females may indeed prefer high-quality males that offer the highest benefits, whereas low-quality females prefer low-quality males (Holveck and Riebel 2010) or show no mating preferences at all to optimally outweigh the costs connected with choosiness (Cotton et al. 2006b) .
However, sexual selection is a complex co-evolutionary process in which the mating strategies of males and females interact. For example, in species with mutual mate choice conflicting preferences between the sexes might lead to sexual conflict over mating which, however, can be resolved in assortative mating patterns (Parker 2006; Baldauf et al. 2009a), or female preferences for high-quality individuals may conflict with the outcomes of male intrasexual competition. Striking examples are species with antagonistic co-evolution in which highly competitive males impose costs for females (e.g. Friberg and Arnqvist 2003), or species where different male morphs exists due to environmental conditions (Tomkins et al. 2011). However, knowledge about variation of male and female condition-dependent mating preferences still remains poorly explored, particularly in species with mutual mate choice, which we will address in this study. In biparental species with mutual mate choice, both sexes should show co-evolving mating strategies (that might be related to individual quality) (Burley 1986; Kokko and Johnstone 2002), thus making them ideal model systems to investigate the variation between male and female strategies.
Here, we investigated quality-dependent mating preferences of both sexes and their interaction in the cichlid fish Pelvicachromis taeniatus, a socially monogamous fish with biparental brood-care and mutual mate choice (Thünken et al. 2007a). Both sexes develop a distinct nuptial belly coloration that underlies sexual selection (Baldauf et al. 2009b; Baldauf et al. 2011). Females prefer males that show yellow belly coloration over dull males (Baldauf et al. 2009b), whereas males prefer females that show a large extent of their belly coloration (Baldauf et al. 2011). Female belly ornamentation predicts female fecundity as well as maternal quality, suggesting that body coloration is a reliable indicator of female quality (Baldauf et al. 2011). Body size and genetic relatedness are further important mate choice criteria in P. taeniatus: the largest potential mating partner is preferred independent from own body size (Baldauf et al. 2009a), and kin is preferred over non-kin (Thünken et al. 2007a, b). Both sexes face high costs of reproduction: after intrasexual competition (Baldauf et al. 2011; Thünken et al. 2011) and mutual mate choice the female cares for the eggs in the breeding cave, while the male attempts long-range defence against intruders. After hatching, the fry are guarded by both parents for a period of several weeks (Thünken et al. 2010). Since both males and females greatly invest in the offspring, both sexes are expected to be choosy according to variation in quality of mating partners (Johnstone et al. 1996). However, mating preferences may further depend on own quality.
We investigated variation in mating preferences of male and female P. taeniatus for high- and low-quality partners with test fish that varied in phenotypic quality. To this end, we manipulated body condition of the focal individuals by keeping fish under different feeding regimes, i.e. high or low energy intake. Individual body condition is an effective proxy to estimate individual quality in fishes (see Bolger and Connolly 1989 for review; Schütz and Taborsky 2005; Taborsky et al. 2007; Lehtonen 2012), especially when changing nutritional balance during food supply. We tested whether ornamentation of both sexes was related to body condition, thus representing reliable indicators of quality in both sexes. Subsequently, we used the variation in condition-dependent colour ornaments identified in the diet manipulation experiment in mate choice experiments under highly controlled conditions. To achieve a high degree of standardisation between stimuli during mate choice experiments, virtual computer animations of fish were developed and were used in dichotomous choice experiments. A set of two virtual stimuli that imitated a high- or low-quality mating partner with respect to the honest visual ornament was presented to each test fish. We would expect that males as well as females show conditional mating preferences depending on their own quality, suggesting quality-assortative mating.
Methods
Experimental animals
Experimental fish were the F1 and F2 generation of fish that were collected in 2007 from the Moliwe river, Cameroon (04°04′N/09°16′E). Fish from 14 different families were housed in family tanks prior to the start of the feeding regimes. Mean offspring sex ratio of P. taeniatus is at 1:1 (SAB, TT, personal observation), and reproduction was prevented due to missing breeding spots. All fish started experimental treatments at the age of 300 ± 10 days as adult virgins and were haphazardly chosen from family tanks. Fish that showed no courtship coloration were replaced with a reproductively active family member. All experiments were conducted between January and June 2010.
Feeding regimes
In order to manipulate phenotypic quality and to identify costly indicator ornaments, fish were randomly assigned into a high (7% of body mass/day) or low (3% of body mass/day) feeding regime over a period of 43 days prior to the mate choice experiments. Whenever possible, four males and four females were taken from a family; however, two families allowed only three reproductive animals per sex. Thus, in each feeding regime 27 males and 27 females were present (n = 108). Two males and two females were introduced in a single aquarium (50 × 30 × 30 cm) that was divided in four parts (25 × 15 cm) by transparent, perforated plastic sheets, thus allowing visual and olfactory communication between the fish. Fish within a single tank originated from the same family and received the same feeding regime. Fish were fed with a standardised mixture of defrosted Chironomus larvae and Artemia.
Fish mass (g) and standard length (mm) of each individual were measured before and after the feeding regime. Subsequently, body condition was calculated as a function of mass and standard length [(100 × mass)/standard length3], following Bolger and Connolly (1989).
Quantification of male and female ornamentation
Photospectrometrical readings between 300 and 700 nm (Avantes AVS-USB2000 spectrometer, connected to an Avantes DH-2000 light source) of each individual were taken before and after the feeding regime in a dark room. Prior to photospectrometrical measurements, each fish was gently captured and remaining water on lateral side was removed. Subsequently, scans were collected under standardised conditions from the lateral side were the nuptial coloration is located. Spectra were measured after dark calibration relative to a 98 % Spectralon white standard with a bifurcated 200-μm small-tip fibre optic that was held at a 90° angle to the body surface. For each individual, 15 measurements were recorded with Spectrawin 5.1 (Avantes) and imported into Microsoft Excel in which the measurements of each individual were averaged. The procedure was carried out within 1 min in order to minimise stress and changes in colour. Additionally, we took digital photographs (camera—Nikon D70s) of each individual prior to mate choice experiments in order to measure the area of the female belly ornamentation. Photographs were taken under standardised conditions (light source—two Somikon LP001 lights illuminating the lateral side), including a Munsell white standard within the scene. The pictures were saved in RAW format and were white balanced during import into Photoshop CS2. For image processing, they were saved in TIF format without compression to avoid the loss of coloration data due to compression algorithms. Images were analysed using SigmaScan Pro 5.0. We selected the number of pixels of the blue colour component (SigmaScan hue blue, 128–192/hue) as well as the number of pixels forming the body area (excluding the fins) to calculate the relative extent of the nuptially coloured area.
Mate choice experiments
Manipulated males and females were habituated individually in a tank (30 × 20 × 20 cm) for the period of mate choice experiments in which a breeding cave (males) or a plastic plant (females) was installed. Each fish continued to receive an amount of food according to its feeding regime. Males and females performed mating preference experiments in a dichotomous design in which they were given the choice between a computer-animated high- and a low-quality stimulus of the opposite sex. Experimental procedure followed the protocol described in Baldauf et al. (2011). Previous studies showed that P. taeniatus reliably responds to computer stimuli (Baldauf et al. 2009b). We calibrated monitors ensuring that they emitted light that corresponded to spectral data of the nuptial area using a digital colorimeter (Quato Silver Haze pro). To achieve moving animations of the models, we used ‘The GIMP 2.2.17 with animation package’ (http://www.gimp.org/). A neutral grey background (1,024 × 400 px) was shown (RGB = 238, 238 and 238) including a plant as a size reference object in the middle of the image. Each animation consisted of 30 frames per second, which is an established method to present artificial stimuli to fishes (Künzler and Bakker 1998; Baldauf et al. 2009b). Models were constructed from digital photographs of five males or females, respectively. By creating such an artificially mixed phenotype, we aimed to reduce the probability (or theoretical possibility) that the elicited response arises only because the manipulated trait were presented with a single specific natural phenotype (McGregor 2000). Subsequently, we virtually manipulated the quality traits using Adobe Photoshop CS4 in RGB colour mode. Males received a pair of female stimuli that differed only in their extent of the nuptial coloured belly area, whereas virtual male stimuli of different yellow belly brightness were presented to the females. By using computer-animated stimuli, other confounding variables were ruled out, e.g. behavioural interactions with live stimuli or differences in body size that play an important role in mate choice of this species since individuals prefer the largest mating partner independent from own body size (Baldauf et al. 2009a). Both stimuli moved synchronously from one side of the monitor to the other within a period of 15 s, including a 2-s stop in the middle of each screen. After that, stimuli moved their pathway back in the same time frame. Each fish was tested twice with reversed position of the stimuli, thereby controlling for potential side biases. Fish behaviour was recorded with a camera and the mean relative time over both trials spent in the association zone in front of the virtual stimuli was quantified by a naïve observer. Association time with live stimulus fish reliably predicts mating decisions in P. taeniatus (Thünken et al. 2007a).
Although the feeding regime had a significant impact on the body condition of the fish (see results), there was still a large overlap between treatment groups (e.g. because some individuals with an initially high body condition that got the low feeding regime maintained a relative high condition, or individuals with initially low condition in the high feeding regime maintained a relative low condition). For such individuals, food manipulation did not seem to notably affect the phenotypic quality and thus might have an ambiguous impact on their mating preferences. Because our main interest was on the effect of condition-related preferences (and not the effect of food manipulation per se), we only included individuals above the mean body condition in the high-quality feeding regime (‘high-quality individuals’) and vice versa individuals below the mean body condition of the low-quality feeding regime (‘low-quality individuals’), i.e. individuals of definitive high or low phenotypic quality into the statistical analysis. Two females that showed the lowest body condition and thus a body coloration below 5 % of the body area were considered as not reproductively active and were removed from the analysis, thus leading to a total number of n males = 25 from 13 different families (10 per feeding regime) and n females = 23 from 12 different families (10 different families in the high-quality group and eight in the low-quality group). Post hoc selected males were significantly different in body condition prior to food manipulation (LRT, χ 2 = 4.24, p = 0.04), whereas females were not (LRT, χ 2 = 0.624, p = 0.43). However, in each sub-group of both sexes body condition after food manipulation was not significantly explained by initial body condition (males, LRT, χ 2 = 2.436, p = 0.12; females, LRT, χ 2 = 0.65, p = 0.42) but by feeding regime (all p < 0.001). Furthermore, initial as well as final body condition did not differ significantly between families of the post hoc selected subgroups (all p > 0.6), suggesting that the variation in the post hoc selected males is caused by the food manipulation rather than by intrinsic genetic differences.
Statistical analysis
All analyses were performed using R 2.11.0 for Windows (R Development Core Team 2009). Linear mixed-effect models (LME) with family as random factor were fitted using maximum likelihood (Pinheiro et al. 2009). We assessed whether the removal of a variable or interaction term caused a significant decrease in the model fit (Zuur et al. 2009) using likelihood ratio tests (LRT). Reported p values of models thus refer to the increase in deviance when the respective variable was removed. Thus, degrees of freedom differ by one. QQ plots of model residuals were inspected visually for deviations from normality and were additionally tested with Kolmogorov–Smirnov test with Lilliefors correction.
In order to examine the effect of the feeding regime on body condition, we fitted models with ‘body condition’ as dependent variable, ‘feeding regime’ (high/ low) as explanatory factor and ‘family’ as random factor. Similarly, we analysed the relationship between ornaments and body condition after the feeding regime. In this case, the male or female ornament, respectively, was the dependent variable, body condition the explanatory term and family as random factor. Initially, there were no significant differences between treatment groups concerning body condition or ornamentation of each sex (all p > 0.2).
The main aim of the study was to test potential differences in conditional strategies between the sexes. To achieve this, we first calculated a preference index for each individual as response variable (mean relative time a test fish spent with the high-quality stimulus minus that spent with a low-quality stimulus). We then examined the interactive effect between ‘sex’ (male/female) and ‘feeding regime’ (high/low) on the mating preferences.
Additionally, we investigated the nature of the conditional strategies in detail. First, we tested—for each sex separately—if males and females exhibit conditional preferences when facing mating partners of different quality. Here, the mean relative time each individual spent in front of a stimulus type over both trials was the dependent variable, stimulus quality (high/low quality) and feeding regime (high/low) were explanatory variables, and individual ID nested in family was the random factor. We then tested the interaction between feeding regime and stimulus quality to see whether the preference for a certain stimulus quality depends on own quality. Subsequently, we analysed separately whether high- or low-quality individuals discriminated between both stimuli.
The post hoc selected subsamples were furthermore analysed to disentangle the effects of manipulation and initial quality (see above). Here, the final ‘body condition’ was the dependent variable, ‘feeding regime’ (high/low) and ‘initial body condition’ explanatory factors, and ‘family’ was the random factor.
Results
Condition dependence of individual quality
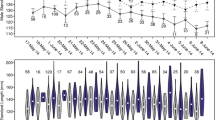
The feeding regime had a significant impact on body condition of males (LRT, n high = 27, n low = 27, χ 2 = 9.418, p = 0.002) as well as females (LRT, n high = 27, n low = 27, χ 2 = 9.598, p = 0.002). Ornamentation of both sexes was significantly related to the manipulated body condition (male brightness, LRT, χ 2 = 4.447, p = 0.03, Fig. 1a; female extent of belly ornament area, LRT, χ 2 = 13.259, p < 0.001, Fig. 1b).
Condition-dependent expression of the ornamental traits. P. taeniatus develops a sex-specific belly coloration. The male yellow brightness was related to male body condition (a), and the extent of the area of the female belly coloration was related to female body condition (b), thus rendering the ornaments of both sexes a reliable indicator of individual quality. Grey triangles indicate individuals in the low feeding regime, and black circles show individuals in the high feeding regime
Quality-dependent mating preferences
The strategies of males and females differed significantly (interaction between ‘feeding regime’ and ‘sex’, LRT, n males = 25, n females = 23, χ 2 = 8.182, p = 0.004; Fig. 2). Males preferred females of similar quality (interaction between ‘feeding regime’ and ‘stimulus quality’, LRT, χ 2 = 8.444, p = 0.003; Fig. 2). Low-quality males preferred low-quality females (LRT, χ 2 = 4.277, p = 0.04, Fig. 2) whereas high-quality males preferred high-quality females (LRT, χ 2 = 4.134, p = 0.04, Fig. 2).
Conflicting conditional mating preferences of both sexes. Males as well as females showed conditional strategies depending on own quality but in opposite directions. The Y-axis shows the mean relative amount of time + SE over both trials that individuals spent in front of high-quality (black bars) and low-quality stimuli fish (white bars) of the opposite sex. For levels of significance, see “Results” section
Females also showed conditional mating preferences (interaction between ‘feeding regime’ and ‘stimulus quality’, LRT, χ 2 = 8.711, p = 0.003, Fig. 2). However, the low-quality females preferred high-quality males (LRT, χ 2 = 7.198, p = 0.007, Fig. 2), whereas females of high quality showed no significant preferences (LRT, χ 2 = 0.276, p = 0.6, Fig. 2).
Discussion
Both sexes showed conditional mating preferences. Males adjusted their mating preferences depending on individual quality suggesting positive assortment. The male strategy is in accordance with theory and empirical work that previously dealt with conditional strategies (Härdling and Kokko 2005; Bel-Venner et al. 2008; Venner et al. 2010). Although low-quality males would largely benefit when mating with a high-quality female, e.g. in terms of number of offspring and maternal care, the costs to gain access to such females may be high. The potential costs of developing the sensory apparatus did not seem to be significantly affected by the feeding regime since in both sexes low-quality individuals were able to discriminate between the two stimuli. Instead, in nature searching for a high-quality mate might be time and energy consuming, and intrasexual competition to gain access to high-quality females is expected to be strong in many species (Venner et al. 2010) which is also true for males of P. taeniatus (Thünken et al. 2011). Hence, it could be adaptive for low-quality males to show conditional preference for low-quality females in order to increase their mating chances on the mating market by directing their mating efforts towards a female which is more likely to accept their courtship and to avoid direct competition with males in better condition (see below). In contrast, high-quality males should be expected to prefer high-quality females since such males are most likely successful in both intra- and intersexual competition. The viability costs associated with conspicuous ornamentation (Pomiankowski 1987) should be compensated by the direct and indirect benefit of mating with high-quality females (Andersson 1994) since female ornamentation is a reliable indicator that reflects fecundity and maternal quality in P. taeniatus (Baldauf et al. 2011).
Surprisingly, though, females exhibited a different conditional strategy than males by preferring high-quality males only when their own quality was low. Variation in female choice has recently been found by Griggio and Hoi (2010), who reported that in house sparrows, Passer domesticus, only low-quality females were selective but showed preference for males of average quality, whereas high-quality females were not choosy. Such female strategies contradict the common hypothesis that only high-quality females should be able to show the strongest mate preference, whereas low-quality females should not be able to pay the costs associated with choosiness (Cotton et al. 2006b). The current results might suggest that females seek specific benefits depending on individual quality. Low-quality females might aim at indirect benefits according to good genes or sexy son hypotheses (Weatherhead and Robertson 1979; Hamilton and Zuk 1982), whereas high-quality females might seek for direct benefits that may not be indicated by the investigated ornament. Mate choice in P. taeniatus is based on several criteria [ornamental traits like body coloration, exaggerated body extensions, body size and compatibility, i.e. genetic relatedness (Thünken et al. 2007a; Baldauf et al. 2009a, 2010, 2011)], whose relative importance seems to be sex specific (Thünken et al. 2012). Furthermore, multiple and multimodal cues may play a role in mate choice by interacting one with each other (e.g. visual and olfactory cues as in Thünken et al. 2011). Although it is not known whether the different ornaments signal different benefits, high-quality females may focus in mate choice on other indicator traits or criteria than investigated in the present study. For instance, the genetic relatedness to a male seems to be a more important criteria in female mate choice than male body size (Thünken et al. 2012), suggesting that the ornamental trait might be less important compared to other traits not investigated in our study.
In P. taeniatus as well as other fish species, female belly coloration has been shown to signal both readiness to spawn and relative fecundity (Massironi et al. 2005; Baldauf et al. 2011). Thus, a possible explanation for the lack of preference in high-quality females may be due to their urgency to mate. In contrast, low-quality females may be less close to spawn and thus could increase their choosiness by conducting a finer mate assessment. Female choosiness is expected to increase with higher levels of benefits and decrease with costs of choice (Jennions and Petrie 1997). Consequently, females from the high feeding regime may have higher costs of choice when choosiness would delay their reproduction, whereas low-quality females might not pay this cost because they have more time before reproduction. Alternatively, high-quality females might not need to show any preference since they will be preferred by high-quality males anyway. However, the lack of preference of high-quality females is puzzling but might reflect results from the dynamics and complexity of systems with mutual mate choice.
Here, we only tested for conditional mating preferences for a relatively short experimental period. Hence, not all potential costs such as intrasexual competition were present during the trials, which have been shown to affect or even reverse initial mating preferences in other species (e.g. Plath et al. 2008; Jeswiet et al. 2012). Possibly, low-quality females of P. taeniatus might reverse their preference towards less preferred males in presence of high-quality competitors (Mautz and Jennions 2011; Wada et al. 2011). Although this possibility cannot be ruled out, prior to the start of the experiments fish were raised in mixed-sex family tanks in which intrasexual competition was allowed, thus rendering fish not naïve to potential competitive costs. Intrasexual competition is expected to be strong within family tanks since P. taeniatus prefers to mate with related individuals (Thünken et al. 2007a).
Our main focus was on condition-dependent mating preferences which we were able to demonstrate. However, it remains unsolved whether the variation in mating preference between high- and low-quality males is a consequence of the feeding regime since males of the analysed sub-group were different in body condition before entering the experimental manipulation. Nevertheless, the feeding regime had a stronger impact on final male body condition than initial body condition, suggesting that the diet manipulation had a stronger impact than other factors, such as genetic effects.
Several consequences of the conflicting preferences of males and females could be outlined. High-quality individuals are expected to easily find a mating partner, whereas low-quality individuals may be rejected, and thus remain unmated at first. However, as males are expected to have higher potential reproductive rates than females, high-quality males could abandon the current brood, which is often observed in biparental cichlids (Keenleyside 1983; Jennions and Polakow 2001) and try to attract additional females including low-quality females who are still available on the mating market. Under this scenario, the low-quality females’ preference for high-quality males makes sense because competition with high-quality females is reduced if the latter have already mated. Low-quality females may also receive a relatively high fitness gain from an attractive father that would provide indirect benefits to the offspring. Hence, the strongest selective pressure lies upon low-quality males, which show indeed ‘prudent’ mating preferences, but might receive no or low fitness benefits.
The conflicting preferences of males and females may lead to a sexual conflict resulting from conflicting reproductive interests, which is especially expected to emerge in biparental species (Chapman et al. 2003). In the case of interlocus sexual conflict, it might indeed be in the interest of an allele in a low-quality female that the female mates with a high-quality male to increase the female’s fitness. However, such a female strategy is initially not in the male’s interest since it would reduce fitness of high-quality males. Thus, the expected rejection of low-quality females should generate direct negative selection on female preference for high-quality males.
Still, it remains unknown whether the observed mating strategies represent an evolutionary equilibrium, implicating that high-quality males may aim to mate with multiple females or whether we observe a certain phase of an co-evolutionary arms race in the evolutionary cycle. Nevertheless, the current results suggest that in a species with conventional sex roles the conditional mating strategies implicate highly evolutionary dynamics that might involve antagonistic co-evolution (Chapman et al. 2003; Chapman 2006) or Red-Queen processes in the evolution of the mating system (Parker 2006). Such highly dynamic mating systems are expected to evolve even in absence of environmental changes (Chapman et al. 2003), suggesting that the observed results might contribute to the understanding of the role of sexual selection and rapid speciation (Salzburger 2009).
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Bakker TCM, Künzler R, Mazzi D (1999) Condition-related mate choice in sticklebacks. Nature 401:234
Baldauf SA, Bakker TCM, Herder F, Kullmann H, Thünken T (2010) Male mate choice scales female ornament allometry in a cichlid fish. BMC Evol Biol 10:301
Baldauf SA, Bakker TCM, Kullmann H, Thünken T (2011) Female nuptial coloration and its adaptive significance in a mutual mate choice system. Behav Ecol 22:478–485
Baldauf SA, Kullmann H, Schroth SH, Thünken T, Bakker TCM (2009a) You can’t always get what you want: size assortative mating by mutual mate choice as a resolution of sexual conflict. BMC Evol Biol 9:129
Baldauf SA, Kullmann H, Winter S, Thünken T, Bakker TCM (2009b) Computer animation as a tool to study preferences in the cichlid Pelvicachromis taeniatus. J Fish Biol 75:738–746
Bel-Venner MC, Dray S, Allaine D, Menu F, Venner S (2008) Unexpected male choosiness for mates in a spider. Proc R Soc Lond B 275:77–82
Bolger T, Connolly PL (1989) The selection of suitable indices for the measurements and analysis of fish condition. J Fish Biol 34:171–182
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445
Chapman T (2006) Evolutionary conflicts of interest between males and females. Curr Biol 16:744–754
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Cotton S, Rogers DW, Small J, Pomiankowski A, Fowler K (2006a) Variation in preference for a male ornament is positively associated with female eyespan in the stalk-eyed fly Diasemopsis meigenii. Proc R Soc Lond B 273:1287–1292
Cotton S, Small J, Pomiankowski A (2006b) Sexual selection and condition-dependent mate preferences. Curr Biol 16:755–765
Fawcett TW, Johnstone RA (2003) Mate choice in the face of costly competition. Behav Ecol 14:771–779
Franceschi N, Lemaitre JF, Cezilly F, Bollache L (2010) Size-assortative pairing in Gammarus pulex (Crustacea: Amphipoda): a test of the prudent choice hypothesis. Anim Behav 79:911–916
Friberg U, Arnqvist G (2003) Fitness effects of female mate choice: preferred males are detrimental for Drosophila melanogaster females. J Evol Biol 16:797–811
Griggio M, Hoi H (2010) Only females in poor condition display a clear preference and prefer males with an average badge. BMC Evol Biol 10:261
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Härdling R, Kokko H (2005) The evolution of prudent choice. Evol Ecol Res 7:697–715
Holveck MJ, Geberzahn N, Riebel K (2011) An experimental test of condition-dependent male and female mate choice in zebra finches. PLoS One 6:e23974
Holveck MJ, Riebel K (2010) Low-quality females prefer low-quality males when choosing a mate. Proc R Soc Lond B 277:153–160
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev Camb Philos Soc 72:283–327
Jennions MD, Polakow DA (2001) The effect of partial brood loss on male desertion in a cichlid fish: an experimental test. Behav Ecol 12:84–92
Jeswiet SB, Lee-Jenkins SSY, Godin JGJ (2012) Concurrent effects of sperm competition and female quality on male mate choice in the Trinidadian guppy (Poecilia reticulata). Behav Ecol 23:195–200
Johnstone RA, Reynolds JD, Deutsch JC (1996) Mutual mate choice and sex differences in choosiness. Evolution 50:1382–1391
Keenleyside MHA (1983) Mate desertion in relation to adult sex-ratio in the biparental cichlid fish Herotilapia multispinosa. Anim Behav 31:683–688
Kokko H, Johnstone RA (2002) Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Phil Trans R Soc Lond B 357:319–330
Kokko H, Monaghan P (2001) Predicting the direction of sexual selection. Ecol Lett 4:159–165
Künzler R, Bakker TCM (1998) Computer animations as a tool in the study of mating preferences. Behaviour 135:1137–1159
Lehtonen TK (2012) Signal value of male courtship effort in a fish with paternal care. Anim Behav 83:1153–1161
Massironi M, Rasotto M, Mazzoldi C (2005) A reliable indicator of female fecundity: the case of the yellow belly in Knipowitschia panizzae (Teleostei: Gobiidae). Mar Biol 147:71–76
Mautz BS, Jennions MD (2011) The effect of competitor presence and relative competitive ability on male mate choice. Behav Ecol 22:769–775
McGregor PK (2000) Playback experiments: design and analysis. Acta Ethol 3:3–8
Parker GA (2006) Sexual conflict over mating and fertilization: an overview. Phil Trans R Soc Lond B 361:235–259
Pinheiro J, Bates D, DebRoy S, Sarkar D, R-Core-team (2009) nlme: Linear and nonlinear mixed effects models. R package version 3.1-92
Plath M, Blum D, Schlupp I, Tiedemann R (2008) Audience effect alters mating preferences in a livebearing fish, the Atlantic molly, Poecilia mexicana. Anim Behav 75:21–29
Pomiankowski A (1987) The costs of choice in sexual selection. J Theor Biol 128:195–218
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Salzburger W (2009) The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Mol Ecol 18:169–185
Schütz D, Taborsky M (2005) The influence of sexual selection and ecological constraints on an extreme sexual size dimorphism in a cichlid. Anim Behav 70:539–549
Taborsky B, Skubic E, Bruintjes R (2007) Mothers adjust egg size to helper number in a cooperatively breeding cichlid. Behav Ecol 18:652–657
Thünken T, Bakker TCM, Baldauf SA, Kullmann H (2007a) Active inbreeding in a cichlid fish and its adaptive significance. Curr Biol 17:225–229
Thünken T, Bakker TCM, Baldauf SA, Kullmann H (2007b) Direct familiarity does not alter mating preferences for sisters in male Pelvicachromis taeniatus (Cichlidae). Ethology 113:1107–1112
Thünken T, Baldauf SA, Kullmann H, Schuld J, Hesse S, Bakker TCM (2011) Size-related inbreeding preference and competitiveness in male Pelvicachromis taeniatus (Cichlidae). Behav Ecol 22:358–362
Thünken T, Meuthen D, Bakker TCM, Baldauf SA (2012) A sex-specific trade-off between mating preferences for genetic compatibility and body size in a cichlid fish with mutual mate choice. Proc R Soc Lond B 279:2959–2964
Thünken T, Meuthen D, Bakker TCM, Kullmann H (2010) Parental investment in relation to offspring quality in the biparental cichlid fish Pelvicachromis taeniatus. Anim Behav 80:69–74
Tomkins JL, Hazel WN, Penrose MA, Radwan JW, LeBas NR (2011) Habitat complexity drives experimental evolution of a conditionally expressed secondary sexual trait. Curr Biol 21:569–573
Venner SI, Bernstein C, Dray S, Bel-Venner MC (2010) Make love not war: when should less competitive males choose low quality but defendable females? Am Nat 175:650–661
Wada S, Arashiro Y, Takeshita F, Shibata Y (2011) Male mate choice in hermit crabs: prudence by inferior males and simple preference by superior males. Behav Ecol 22:114–119
Weatherhead PJ, Robertson RJ (1979) Offspring quality and the polygyny threshold: "the sexy son hypothesis". Am Nat 113:201–208
Widemo F, Saether SA (1999) Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol Evol 14:26–31
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, Berlin
Acknowledgements
We thank I.P. Rick for discussion and his help in the analysis of the photospectrometrical data. This research was funded by the Deutsche Forschungsgemeinschaft (DFG) (BA 2885/2-3).
We thank A. Pilastro and two anonymous reviewers for helpful comments.
Ethical standards
All experiments comply with the current laws of Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Pilastro
Rights and permissions
About this article
Cite this article
Baldauf, S.A., Engqvist, L., Ottenheym, T. et al. Sex-specific conditional mating preferences in a cichlid fish: implications for sexual conflict. Behav Ecol Sociobiol 67, 1179–1186 (2013). https://doi.org/10.1007/s00265-013-1543-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1543-4