Abstract
Olfactory signals can contain information, such as age and social status, and play a vital role in competitor assessment. In many territorial species, subordinates must leave their natal colony to obtain their own territory and mate. These individuals could be aggressive opponents in agonistic encounters, as they will have little to lose (the desperado effect). In this study, we tested the hypothesis that dominance and age are coded in the anal gland secretion (AGS) of the monogamous and highly territorial Eurasian beaver (Castor fiber), and if this information is used by conspecifics to evaluate the potential threat posed by an intruder. Territorial intrusions were simulated by presenting residents with a two-way choice test of AGS from an unknown male territory owner (i.e., dominant) and his son (i.e., subordinate; either 1 or ≥2 years old). Residents spent more time investigating AGS from subordinates than their fathers and responded more aggressively to subordinates than their fathers when subordinates were ≥2 years old. Chemical analyses gas chromatography and multivariate data analysis supported our behavioral findings and revealed differences between chemical profiles of territory owners and subordinates, as well as between the subordinates in different age classes. This study reveals that information about age and social status is coded in AGS of beavers and that this information is used to determine the level of an eventual future response to the signaler.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owning a territory can ensure exclusive access to food, mates, and nest sites, but defending these resources against other individuals is costly (Maher and Lott 1995; Bradbury and Vehrenkamp 1998). The risk of injury or even death in a physical conflict with an intruder makes it crucial for a territory owner to make the “correct” decision regarding the costs and benefits of a potential fight. It is therefore beneficial to collect information conveyed by different signals to assess the threat posed by an intruder (e.g., Temeles 1994; Müller and Manser 2007), and invest more aggression towards an opponent that poses a higher risk (Parker 1974).
In many mammals, chemical signals play an important role in competitor assessment (Ralls 1971; Gosling and Roberts 2001; Wyatt 2003; Müller-Schwarze 2006), and these signals can convey honest information about, e.g., the age class (e.g., Buesching et al. 2002; MacDonald et al. 2008; Kean et al. 2011; Caspers et al. 2011), body condition (Buesching et al. 2002), and dominance status (e.g., Hayes et al. 2001; Scordato and Drea 2007; Burgener et al. 2009) of the sender. Age, body size, and dominance status are often correlated with fighting ability or resource-holding potential (RHP), which is frequently used to settle contests (Parker 1974; Archer 1987; Kemp and Wiklund 2004). The probability of an escalated contest is generally predicted to increase between individuals with similar RHP (Parker 1974), and it is often observed that when the asymmetry in RHP between two contestants is very large, the individual with the lowest RHP (the probable loser) will retreat without escalating a fight in the first place (e.g., Smith et al. 1994). There are, however, an increasing number of examples where smaller individuals choose a risky tactic and initiate fights they are most likely to lose (e.g., Smith et al. 1994; Moretz 2003; Jenssen et al. 2005; Callander et al. 2012; Svensson et al. 2012), a puzzling behavior referred to as the “Napoleon complex” (Just and Morris 2003). For instance, in the lizard Anolis carolinensis, smaller males did not try to avoid conflict with bigger opponents, but rather invaded their habitats, showed high levels of aggressive signaling and engaged in fights despite losing 90 % of these encounters (Jenssen et al. 2005).
There are several explanations why smaller individuals could behave aggressively, even with a small chance to win. They might be unable to assess their own RHP relative to that of the opponent prior to an interaction (Smith et al. 1994) or could misperceive their own RHP as higher than it is and initiate an escalation by mistake (Bradbury and Vehrenkamp 1998). Being the one who attacks first has also been shown to increase the chance of winning (e.g., Taylor et al. 2001), possibly giving smaller individuals an advantage by initiating the fight. Asymmetries in fighting motivation is another important factor in determining contest behaviors and outcomes (Parker 1984; Bergman et al. 2010), and a high motivation to fight might help in overcoming inferior RHP (e.g., Barnard and Brown 1984; Haley 1994). One scenario where a high level of motivation by an intruder could be expected is when territory ownership is a prerequisite for reproductive success, and vacant territories are rare. If the smaller individual is not able to reproduce without access to a territory, it is expected to be an aggressive opponent, as it will have little to lose (“the desperado effect,” Grafen 1987).
In some territorial mammals, subordinates do not breed while living with their natal family unit but often disperse to find a territory and a mate (e.g., Sun et al. 2000). During dispersal, they are bound to encounter unfamiliar adults with established territories (delBarco-trillo et al. 2011). If established territory owners are able distinguish between other territory owners and subordinates that are bound to acquire a territory, it might be beneficial to invest more aggression towards the latter. However, interactions between young dispersers and established adults have received little theoretical or experimental attention (delBarco-trillo et al. 2011), and to our knowledge, no other studies have investigated the responses of territorial residents towards males of dispersing age versus other established territorial males.
Beavers (Castor spp.) are long-lived, highly territorial, socially monogamous rodents (Schulte 1993; Campbell et al. 2005), and the basic family unit consists of a dominant pair in a long-term relationship and their subordinate offspring living in a stable territory (Wilsson 1971). All family members, except from kits of the year, defend their territory by scent marking (Wilsson 1971; Buech 1995). Offspring will not be sexually active while living with their parents (Wilsson 1971; Svendsen 1980; Campbell et al. 2005) and normally disperse as 2-year-olds (e.g., Hartman 1997; Sun et al. 2000). However, delayed dispersal is common in high-density populations, and in low-density populations, yearlings may also disperse (Hartman 1997). Secondary dispersal by established (dominant) adults is rare in stable populations, but dominant individuals can make exploratory trips outside their own territory (Campbell et al. 2005). The main dispersal period is in spring and early summer (normally April–June) (Svendsen 1980; Rosell et al. 1998; Sun et al. 2000; DeStefano et al. 2006) and coincides with the peak in resident beavers’ territorial behavior (Svendsen 1980; Rosell et al. 1998). Aggressive encounters between conspecifics are common during this period (Piechocki 1977; Svendsen 1989; Nolet and Rosell 1994), and injuries inflicted by other beavers, and resulting infections, can be a major cause of death, especially in dispersing subadults (Piechocki 1977; Nolet et al. 1997).
Chemical signals play an important role in intraspecific communication and territorial behavior in beavers (Campbell-Palmer and Rosell 2010), and both the North American (C. canadensis) and Eurasian beaver (C. fiber) use anal gland secretion (AGS) for scent marking (Rosell and Bergan 1998; Rosell and Sundsdal 2001). Previous studies have shown that AGS of beavers codes for a variety of information, like species (Rosell and Sun 1999; Rosell 2002), subspecies (Rosell and Steifetten 2004; but see Peterson et al. 2005), gender (Grønneberg and Lie 1984; Sun 1996, Rosell and Sundsdal 2001), individuality (Sun 1996), and kinship (Sun and Müller-Schwarze 1997; Sun and Müller-Schwarze 1998).
The aim of this study was to investigate the hypothesis that information about social status (i.e., territory ownership) and age/body size is coded in the AGS of male Eurasian beavers and that the information will be used by conspecific residents to determine the level of the eventual future aggressive responses towards the signaler. In a free-living population, we simulated simultaneous territorial intrusions by an established dominant territory owner (TO) and his subordinate son (i.e., son that had not yet dispersed and still lived with his father), with the son being either (1) between 2 and 7 years old (old son—OS), or (2) a yearling (young son—YS). We specifically tested two alternative predictions:
-
1.
Residents would show a stronger territorial response towards scent from OS despite their lower RHP based on the fact that they, in contrast to TO, should be highly motivated to acquire a territory. We did not predict a stronger response towards YS, as yearlings are much smaller in body size and still below the common dispersal age of two.
-
2.
Residents would show a stronger territorial response towards scent from TO, as they have a higher RHP than their sons and could constitute a bigger physical threat in the case of a fight.
Chemical analyses of the secretions used in chemical communication can provide useful information about differences in the chemical profiles between groups. To support any findings of behavioral discrimination, we therefore analyzed the AGS samples used in the field experiment with gas chromatography–mass spectrometry (GC-MS) and multivariate data analyses.
Materials and methods
Study area and study animals
The field experiment was conducted in 2008 (10 April–17 July) and 2009 (15 April–20 July) using a population of free-ranging Eurasian beavers in the rivers Straumen (59°29′ N, 09°153′ E), Gvarv (59°386′ N, 09°179′ E), and Saua (59°444′ N, 09°307′ E) in Telemark County, southern Norway. All three rivers form part of the catchment of Lake Nordsjø (Campbell et al. 2005). The study area has been inhabited by beavers since the 1920s (Olstad 1937), and hunting pressure is either low or nonexistent (Parker and Rosell 2003; Campbell et al. 2012). The population is stable and appears to be at carrying capacity (Rosell and Hovde 2001; Campbell et al. 2012). All territories are adjacent to each other with no unoccupied stretches of river in between, and territory overlap is small to nonexistent (Herr and Rosell 2004).
The beavers in the study area have been monitored between March and November every year since 1997 through an extensive live-trapping program, using landing nets from a motor boat (Campbell et al. 2012). The trapping records, radio tracking, and observational data collected include information about territorial borders, family composition, family member replacements, and lengths of pair bonds, as well as breeding and dispersal events. Dominance status is determined by previous trapping and sighting history, body mass, and incidences of lactation in females (see Campbell 2010; Campbell et al. 2012 for details). As beavers live in discrete family groups, individuals that are seen and/or trapped inside the territory of a known dominant pair on more than one occasion are assumed to be their subordinate offspring (Campbell et al. 2005, 2012).
Captured individuals were physically restrained in cloth sacks while measurements and samples were collected. We assigned all captured individuals to an age-class (0 years = kit, 1 years = yearling, 2 years = subadult, and ≥3 years = adult) based on their body weight (Rosell et al. 2010) and trapping record, determined sex based on the color of their AGS (Rosell and Sun 1999), tagged them with a microchip (Avid® or Trovan®), and marked them with unique color combinations of plastic (Dalton) and metal (National Band and Tag Co.) ear tags for individual recognition (Rosell and Hovde 2001; Campbell et al. 2005, 2012). To obtain AGS samples, we lifted the beaver’s tail (at the open end of the cloth sack) and evacuated the rectum. We rinsed the cloaca area with distilled water and then separately pushed out each of the two papillae of the anal gland and squeezed out the AGS (Rosell and Bjørkøyli 2002). All samples were collected in glass vials with Teflon-lined lids and stored at −20 °C. The beavers were released after 20–30 min.
Scent donors
As relatedness is coded in the AGS of North American beavers (Sun and Müller-Schwarze 1997), we used father–son pairs as scent donors to minimize potential effects of genetic factors, as well as habitat and food differences on the chemical composition of AGS within each pair. We used AGS samples obtained during the main dispersal period (April–June) from 1999–2009 in the field experiment and chemical analyses. AGS is very stable over time (Sun 1996), and preliminary multivariate analyses did not reveal any significant differences between samples based on the time they had been stored in the freezer (results not shown). Samples in a father–son pair were taken either within the same year or 1 year apart, and all pairs were living in the same family unit at the time samples were obtained. Each sample was only used once.
Design of field experiment
In the field experiment, we used AGS from a TO (age, 6.5 ± 2.5 years; weight, 21.3 ± 2.5 kg; N = 12) and his OS (age, 3.3 ± 1.8 years; weight, 17.6 ± 2.5 kg; N = 12), or from a TO (age, 4.2 ± 1.9 years; weight, 21.5 ± 1.6 kg; N = 10) and his YS (age, 1 year; weight, 10.3 ± 1.7 kg; N = 10). To minimize the probability that responding residents and scent donors had previous contact and/or were genetically related, we used AGS from scent donors caught in a different watershed >15 km away, which is greater than the average dispersal distance for males (e.g., Sun et al. 2000).
We constructed two adjacent experimental scent mounds (ESM) inside each residents territory with AGS from a TO and either OS or YS. This simultaneous presentation controls for temporal variation in motivation of the resident and thus provides a more sensitive test of discriminatory abilities (e.g., Sun and Müller-Schwarze 1997; Rosell and Bjørkøyli 2002; White et al. 2003). A blank control was not included in the experimental design because other studies have shown that beavers do not respond to untreated mounds (Schulte et al. 1995; Rosell et al. 2000).
We placed 0.25 g of AGS into a white plastic bottle cap (2.5 cm top diameter, 1.5 cm high) to hold the sample and control the evaporation surface area (4.9 cm2) (Rosell and Bjørkøyli 2002; Rosell and Steifetten 2004). This presentation has been previously shown to be sufficient to trigger a response (e.g. Rosell and Bjørkøyli 2002; Rosell and Steifetten 2004). Bottle caps were pushed into the center of each ESM, so they were leveled with the surface of the mound.
We constructed ESMs in the center of the territory within 50 m from the active lodge (i.e., the lodge the residents used most frequently during the study period), in a location where the beavers could easily walk onto land. As beavers make distinct tracks where they use to go onto the shore, the ESMs were constructed on each side of a track so that the beaver would come up in the middle of the two AGS samples. If the terrain on either side of the lodge was not suitable for construction of ESMs (e.g., too steep), a location on the opposite river bank was used. We constructed ESMs with a handful of mud and debris from the river bottom. We used clean latex gloves to avoid transmission of human odor when handling the material. Each ESM was approximately 10 cm high and 15 cm wide and made as identical as possible regarding size and the material used. The centers of a pair of ESMs were 40 cm apart and within 50 cm from the edge of the water (Herr et al. 2006). The chosen distance within a pair of ESMs ensured that once a beaver responded to one of them, it could also respond to the other; hence, between-treatment effect could be compared (Sun and Müller-Schwarze 1997).
To control for side preferences, left and right placement of the samples were chosen at random and the position in relation to the lodge was chosen according to the terrain. Scent was placed out ∼60 min before the beavers emerged from the lodge in the evening (1800–2000 hours) (Rosell and Bjørkøyli 2002). Observations ended between 2100 and 2300 hours due to insufficient daylight. After a response was recorded, we removed the ESMs. Each trial lasted until the responding beaver decided to leave. If a beaver was disturbed during a response (e.g., by a conspecific or a boat), the response was not included in the analysis. If no response was recorded during a trial, ESMs were removed, and we returned another evening. Responses were recorded with a tripod-mounted digital camera (Sony DCR-SR35E) set to record continuously. The camera was placed ∼10 m from the ESMs and was always handled with latex gloves to avoid human odor.
Response measures
Video recordings were analyzed with Microsoft Windows Media Player (Microsoft®). The observer was blind to treatment assignments. We recorded the duration of two response patterns to ESMs in seconds: (1) sniffing response, defined as when the beaver’s nose was within 5 cm of the ESM), and (2) the physical response, defined as the beaver standing with its hind feet on the ESM, or pawing and/or over-marking an ESM with castoreum and/or AGS, with longer responses leading to more destruction of the ESM (Rosell and Bjørkøyli 2002). Duration of sniffing was used as a measure of the time a beaver needed to identify the scent, as well as an indication of the level of interest, whereas the duration of the physical response was used to describe how strong an aggressive behavior a scent on the ESM triggered; the longer the response, the more agonistic. Interpreting the duration of the physical behavior and destruction of ESMs as an indication of an agonistic response in beavers can be justified based on previous experimental and observational evidence. Beavers are highly territorial and have been shown to exhibit a longer physical response towards scent from, e.g., strangers than neighbors (Rosell and Bjørkøyli 2002) or non-kin than kin (Sun and Müller-Schwarze 1997). The length of the response is therefore perceived to reflect the aggression level of the responder.
Only the first response by the first resident beaver to investigate ESMs was included into the analysis because physical damage to ESMs might lead to carry-over biases in subsequent responses (Sun and Müller-Schwarze 1997). Only responses from residents >2 years old were included in the analyses (Rosell and Steifetten 2004). We also included subordinate responding residents as long as they were >2 years old, since they are fully grown physically (Rosell et al. 2010). Three residents responded in both a TO–OS and a TO–YS trial. The ID of a responding resident was therefore included as a factor in the statistical analyses. We attempted to identify all responders based on data recorded during captures, such as ear tags, tail scars, and body size.
Chemical sample preparation
Chemical analyses were performed in December 2008. We used toluene–methanol 3:1 as the solvent to extract compounds from AGS (Rosell and Sundsdal 2001). We transferred 0.10 g of AGS into a clean glass vial and added 2.5 ml of solvent. The solutions were left for 2 h at room temperature, then filtrated through a filter paper (Schleicher and Schuell no. 595 ½), and transferred to a gas chromatography (GC) vial. To avoid loss of volatile compounds, samples were covered with aluminum foil during extraction.
Chemical analyses
We used an auto-injection system (Agilent 7683 Series Injector) to inject 1 μl of the AGS solution into a Hewlett-Packard (HP) 6890 Series II gas chromatograph. The gas chromatograph was equipped with a non-polar HP-5 MS 5 % phenyl-methyl-siloxane column (30.0 m long × 0.25 mm ID × 0.25 μm film thickness) and connected to a HP 5973 Series mass spectrometer detector with a split/splitless inlet used in the splitless mode. Helium was used as the carrier gas at a constant flow of 0.7 ml/min. The initial oven temperature was set at 130 °C and then increased 4 °C/min to 310 °C. The temperature was then kept constant at 310 °C for 15 min. A delay of 2 min was set for every run to prevent the solvent from damaging the detector. Samples were analyzed in random order. After every nine samples, a blank sample was run to ensure that the column was clean. AGS samples from three adult male beavers were run repeatedly through the analyses to control for shifts in retention time. Two father–son pairs used in the field experiment were obtained in 2009 after the chemical analyses were conducted; thus, they were not included in the chemical analyses. Therefore, a total of 40 samples were analyzed (N TO = 20, N OS = 10, N YS = 10).
Statistical analysis
We evaluated the reaction time of a sniffing response and a physical response (in seconds) of a resident territorial beaver towards AGS from intruding beavers with generalized linear mixed effects models with a Poisson error structure. The model evaluating the reaction time of a sniffing response of a resident territorial beaver included the variables class of the intruding beaver (as factor, with the levels TO, OS, and YS), sex of the resident territorial beaver (as factor, with the levels male and female), body mass of the intruding beaver (in kilograms), and the interaction between class of the intruding beaver × sex of the resident territorial beaver. We included sex of the responding resident beaver into the analysis because resident males and females may react differently towards intruding males. We included body mass of the intruding beaver into the analysis because some intruding OS were as large as intruding TO, and the response of a resident beaver may also be related to the size and not just the social status of the intruding beaver. We included the interaction between class of the intruding beaver × sex of the resident territorial beaver into the analysis to better understand the intersexual variation in the response of the residents in relation to the social status of the intruding individual.
A physical reaction seemed to be dependent upon the presence of a preceding sniffing reaction, i.e., six trials where a resident beaver showed a 0-s sniffing response resulted also in a 0-s physical response. The model evaluating the reaction time of a physical response of a resident territorial beaver therefore included the variables class of the intruding beaver (as factor, with the levels TO, OS, and YS) and sex of the resident territorial beaver (as factor, with the levels male and female), as well as the duration (in seconds) of a sniffing response preceding a physical response. The variables mass of the intruding beaver and the interaction sex of the resident beaver × age category of the intruding beaver were not included into this analysis to avoid overparameterization of the model due to the reduced sample size and because they were not significant in explaining sniffing response. Due to the pairwise presentation of the AGS samples to residents, and because three residents responded in two trials, we nested the random effects of the resident ID within the random effects of the trial ID in all explanatory models (Bates 2010). We used a backward procedure to select the best models, based on p values with a significance level of α = 0.05, starting with a full model of all covariates and relevant second-order interactions (Crawley 2007). The “lme4” package in R 2.12.0 (www.R-project.org) was used to fit mixed effects models.
In order to investigate the difference between chemical profiles, the total ion current for each time unit on the retention scale (165 time units/min) was used, forming a GC matrix of X-variables (40 samples × 9,561 time measurements). The data were square-root transformed to reduce the influence of the most abundant peaks (Clarke and Warwick 2001). We calculated Euclidean distances between every pair of samples to produce a resemblance matrix that formed the basis of the analyses. We used principal coordinate (PCO) analysis based on the Euclidean resemblance matrix (Gower 1966) to visualize the patterns of differences in the multivariate chemical profiles among samples. The AGS profiles from the three groups were then compared with a single-factor PERMANOVA (Anderson 2001; McArdle and Anderson 2001) using 9,999 permutations. Differences between groups were investigated further using canonical analysis of principal coordinates (CAP; Anderson and Willis 2003). The software PRIMER V6.1.13 (Clarke and Gorley 2006) with the PERMANOVA + V1.0.3 add-on package (Anderson et al. 2008) was used to investigate differences between chemical profiles.
Results
Behavioral bioassays
In total, 22 responses were recorded, but in three cases we were not able to individually identify the responder. Four responses were by subordinate offspring in the territory (N Male = 2, N Female = 2), and the remaining 15 were by dominant residents (N Male = 6, N Female = 6; i.e., 2 males and 1 female were used in 2 trials, respectively). In 92 % of the trials (N = 12) evaluating the difference in sniffing response towards intruding OS and TO, the resident beavers responded first to OS. In 70 % of the trials (N = 10) evaluating the sniffing response of residents towards intruding YS and TO, the residents responded first to YS. Beavers were never observed in proximity (filming range, approximately 10–15 m) to the marks without going onto the shore and responding to at least one ESM. No yearlings were observed to respond, and all the identified subordinate responders were >2 years old.
There was no significant sex difference in body mass or age of resident beavers that responded in the field experiment (body mass: two sample t test, t = −0.062, df = 2.161, P = 0.956; age: Mann–Whitney test, W = 26.5, P = 0.135). TO were significantly heavier than OS (Mann–Whitney test, W = 20.5, P = 0.003).
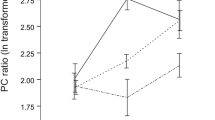
Resident beavers spend significantly more time sniffing AGS from intruding OS and intruding YS than of intruding TO (Fig. 1a, b; Table 1). The variables sex of the resident beaver, body mass of the intruding beaver, and the interaction sex of the resident beaver × age class of the intruding beaver were not significant and removed from the analysis.
Responses of beavers in seconds to experimental scent mounds with anal gland secretion from a a territory owner (TO) and his old son (OS) and b a TO and his young son (YS). The line in the boxplot indicates the median; the lower and upper ends indicate the 25 and 75 % values, respectively; and the two whiskers indicate the distance from the end of the boxplot to the largest and smallest observed values that are less than 1.5 box lengths from either end of the box, diamond outliers (1.5–3 box lengths from the end of the box); circle extreme values (>3 box lengths from the end of the box); N = number of responses, *P < 0.05
The physical response time of a resident beaver towards AGS increased significantly with the duration of the preceding sniffing response and was significantly higher for AGS of intruding OS than of intruding TO (Fig. 1a; Table 1). The variable sex of the resident beaver was not significant and therefore excluded from the analysis. There was no significant difference in the physical response time towards AGS of intruding YS and of intruding TO (Fig. 1b; Table 1).
GC comparisons
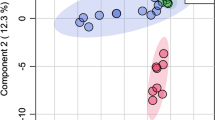
An unconstrained 2D PCO explained 60.5 % of the variation in the data (Fig. 2a), and the third axis explained further 13.3 %, but there was no clear separation between groups. However, the PERMANOVA comparing the three groups was statistically highly significant (pseudo F = 3.47, P ≤ 0.001). The CAP analysis classified 92.5 % of the chemical profiles into the correct group using leave one out cross-validation and m = 22 axes (δ 1 2 = 0.94, P = 0.0001, Fig. 2b). The number of axes is chosen to minimize the probability of misclassifying a new point to the wrong group, and a high number of axes needed to separate the classes suggest that the difference between groups is complex.
Bidimensional PCO ordination (a) and CAP analyses showing 92.5 % correct discrimination (b) of anal gland secretion profiles from Eurasian beavers (Castor fiber) used in behavioral bioassay and chemical analyses. Note that b displays only 2 CAP axes of the 22 axes produced by the model. Each point corresponds to one chemical profile, being either a territory owner (triangle TO, N = 20), an old son (inverted triangle OS, N = 10), or a young son (square YS, N = 10)
Discussion
The results from the behavioral bioassay combined with the chemical analyses supported our hypotheses of a chemical difference between AGS from dominant Eurasian beaver territory owners and their subordinate sons, as well as between sons in different age classes. The results of the field experiment showed that conspecifics can use this information for behavioral discrimination.
Sniffing response from residents
Resident beavers spent significantly more time investigating (sniffing) AGS from yearling sons and older sons than their fathers. This suggests that residents were able to discriminate between dominant and subordinate individuals based on the difference in chemical cues alone, consistent with results in several other species, such as Iberian rock lizards (Lacerta monticola; Martín et al. 2007), house mice (Mus domesticus; Drickamer 1992), and bank voles (Clethrionomys glareolus; Rozenfeld and Rasmont 1991; Kruczek 1997). The fact that residents spent more time sniffing AGS from YS and OS than from TO also suggests that scent from subordinates might be considered more interesting, or takes more time to identify, but not necessarily more threatening than scent from territory owners, as sniffing alone normally is not considered an agonistic response (e.g., Raynaud and Dobson 2011).
Physical response from residents
There was no significant difference in the time residents spent responding physically to AGS from yearling sons and their fathers. We interpret physical responses to scent on ESMs as agonistic because beavers are highly territorial and scent mounds containing odor from a stranger are typically destroyed by residents (e.g., Sun and Müller-Schwarze 1997; Rosell and Bjørkøyli 2002). This suggests that yearlings are not considered to pose a greater territorial threat than their fathers. The lack of observable discrimination most likely has a true biological basis. First, the body size of yearlings is very small compared to older individuals (Rosell et al. 2010), and there is little chance that yearlings will be able to compete physically with older conspecifics. In territorial or dominance-based social systems, scent marks should convey honest information about the signaler’s competitive ability, whether superior or inferior, and honest signals can minimize the potential costs of agonistic encounters (Gosling and Roberts 2001).
Yearling beavers will probably also differ in dispersal behavior and motivation compared to older individuals because of a less pronounced dispersal instinct (Hartman 1997). Wilsson (1971) observed that beaver yearlings in captivity only showed some uneasiness in the dispersal season, while 2-year-olds became very restless and tried to leave their enclosures, indicating that 2-year-olds might be more “desperate” to leave their natal site than yearlings. The phenomenon of yearling dispersal in beavers is indeed considered to be a result of the availability of vacant habitat and rather the exception than the rule (Hartman 1997). This supports that yearlings are not able to compete for resources and therefore do not constitute a serious threat to residents. Male beavers do not reach sexual maturity until the age of about 21 months (Wilsson 1971); thus, the yearlings in this study were not yet sexually mature. The old son scent donors, on the other hand, could be sexually mature but sexually suppressed by their father; this will probably be reflected in the AGS profile. For instance, Caspers et al. (2011) found in male greater sac-winged bats (Saccopteryx bilineata) that the wing sac odorant of males encoded information about age and/or sexual maturity. In male Syrian hamsters (Mesocricetus auratus), established adult territorial males only showed aggression towards unfamiliar young males above an age threshold (50 days or older), coinciding with their sexual maturity. Young males below this age threshold did not elicit aggression from residents (delBarco-Trillo et al. 2011). The authors suggested that one reason might be that young males display more aggressive and less submissive behaviors as their age increases and also that the odors influencing aggression changes at this time.
Residents did however show a significantly stronger physical response towards scent from old sons than from their fathers, supporting our first prediction that these subordinates are perceived to constitute a greater threat than established territory owners despite their lower age and body weight. This result indicates that sexually mature male beavers without a territory could be more likely to involve in a physical confrontation with established residents than other territory owners. Aggression from lower RHP individuals is expected particularly when resources are scarce and valuable and the difference in RHP is not very large (Eshel and Sansone 2001; Morrell et al. 2005). As subordinate beavers do not reproduce while living with their parents (e.g., Campbell et al. 2005), and all territories in the study area are adjacent to each other with no unoccupied stretches of river in between (Herr and Rosell 2004), a scenario where dispersers take the risk of a physical conflict might be expected. Unfortunately, no observational data exist on direct interactions between dispersers and residents in the study population, but dispersers are often trapped in spring and summer with severe biting injuries, showing that they have been involved in physical conflicts with conspecifics. Few studies have investigated the behavior of young dispersing males and their interactions with territorial residents, but in banded mongooses (Mungo mungo), dispersing groups (i.e., groups of subordinates that were either evicted or left voluntarily) were in more than four times as many fights with rival packs compared to established groups (Cant et al. 2001). In a study of dispersal in young tigers (Panthera tigris), Smith (1993) observed several aggressive interactions between dispersing males and resident males. In both examples, the risk of injury or death was high for the dispersers, who still took the risk of fighting residents.
The effect of motivation on willingness to fight has been demonstrated in, e.g., hermit crabs (Pagurus longicarpus), where individuals in lower quality shells were more willing to initiate and escalate fights than those with higher quality shells, as an attempt to overtake and occupy a higher quality shell (Gherardi 2006). We argue that old sons should be more motivated to engage in a physical conflict than their fathers and that this difference was perceived by residents.
Chemical analyses
Scent can communicate information through a mixed composition of chemicals, and the high number of compounds in beaver AGS (Sun 1996; Rosell and Sundsdal 2001) allows the potential coding of a wealth of information. The composition of glandular secretions are partly controlled by hormones (Ebling 1977), and there are often hormonal correlates with age (Bales et al. 2006), dominance (Creel 2001; Bales et al. 2006), and dispersal status (Holekamp and Smale 1998; Young and Montfort 2009). It was not the goal of this study to identify the compounds involved in information coding, but merely whether the chemical profiles differed between groups. The results from our chemical analyses support our hypothesis that GC profiles differ significantly between dominant and subordinate beavers as well as between old and young subordinates.
In conclusion, resident territorial beavers showed the strongest territorial response towards older subordinate sons, suggesting that they are considered a bigger territorial threat. These results indicate that territory owners can be identified by scent. Further chemical studies should be conducted to provide better insight about the exact information coded in the AGS and identify the chemical differences between the different age classes.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Archer J (1987) The behavioural biology of aggression. Cambridge University Press, Cambridge
Bales KL, French JA, McWilliams J, Lake RA, Dietz JM (2006) Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia). Horm Behav 49:88–95
Barnard CJ, Brown CAJ (1984) A payoff asymmetry in resident–resident disputes between shrews. Anim Behav 32:302–304
Bates DM (2010) lme4: mixed-effects modeling with R. Springer, Berlin
Bergmann M, Olofsson M, Wiklund C (2010) Contest outcome in a territorial butterfly: the role of motivation. Proc R Soc Lond B 277:3027–3033
Bradbury JW, Vehrencamp SJ (1998) The principles of animal communication. Sinauer Associates, Inc., Sunderland
Buech RR (1995) Sex difference in behaviour of beavers living in near-boreal lake habitat. Can J Zool 73:2133–2143
Buesching CD, Waterhouse JS, Macdonald DW (2002) Gas-chromatographic analyses of the subcaudal gland secretion of the European badger (Meles meles). Part I: chemical differences related to individual parameters. J Chem Ecol 28:41–56
Burgener N, Dehnhard M, Hofer H, East ML (2009) Does anal gland scent signal identity in the spotted hyaena? Anim Behav 77:707–715
Callander S, Bolton J, Jennions MD, Backwell PRY (2012) A farewell to arms: males with regenerated claws fight harder over resources. Anim Behav 84:619–622
Campbell RD (2010) Demography and life history of the Eurasian beaver Castor fiber. Ph.D. thesis, Merton College, Oxford
Campbell RD, Rosell F, Nolet BA, Dijkstra VAA (2005) Territory and group size in Eurasian beavers (Castor fiber): echoes of settlement and reproduction? Behav Ecol Sociobiol 58:597–607
Campbell RD, Nouvellet P, Newman C, Macdonald DW, Rosell F (2012) The influence of mean climate trends and climate variance on beaver survival and recruitment dynamics. Glob Chang Biol 18:2730–2742
Campbell-Palmer R, Rosell F (2010) Conservation of the Eurasian beaver Castor fiber: an olfactory perspective. Mamm Rev 4:293–312
Cant MA, Otali E, Mwanguhya F (2001) Eviction and dispersal in co-operatively breeding banded mongooses (Mungo mungo). J Zool 254:155–162
Caspers BA, Schroeder FC, Franke S, Voigt CC (2011) Scents of adolescence: the maturation of the olfactory phenotype in a free-ranging mammal. PLoS One 6:e21162
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd, Plymouth
Clarke KR, Warvick RM (2001) Change in marine communities, 2nd edn. PRIMER-E Ltd, Plymouth
Crawley MJ (2007) The R book. John Wiley, Chichester
Creel S (2001) Social dominance and stress hormones. Trends Ecol Evol 16:491–497
delBarco-Trillo J, McPhee ME, Johnston RE (2011) Syrian hamster males below an age threshold do not elicit aggression from unfamiliar adult males. Aggress Behav 37:91–97
DeStefano S, Koenen KKG, Henner CM, Strules J (2006) Transition to independence by subadult beavers (Castor fiber) in an unexploited, exponentially growing population. J Zool 269:434–441
Drickamer LC (1992) Estrous female house mice discriminate dominant from subordinate males and sons of dominant from sons of subordinate males by odor cues. Anim Behav 43:868–870
Ebling FJ (1977) Hormonal control of mammalian skin glands. In: Mozell MM (ed) Chemical signals in vertebrates. Plenum Press, New York, pp 17–39
Eshel I, Sansone E (2001) Multiple asymmetry and concord resolutions of a conflict. J Theor Biol 213:209–222
Gannon WL, Sikes RS (2007) Guidelines of the American Society of Mammalogists for the use of wild animals in research. J Mammal 88:809–823
Gherardi F (2006) Fighting behavior in hermit crabs: the combined effect of resource-holding potential and resource value in Pagurus longicarpus. Behav Ecol Sociobiol 59:500–510
Gosling LM, Roberts SC (2001) Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv Stud Behav 30:169–217
Gower JC (1966) Some distance properties of latent root and vector methods used in multivariate analyses. Biometrika 53:325–338
Grafen A (1987) The logic of divisively asymmetric contests: respect for ownership and the desperado effect. Anim Behav 35:462–467
Grønneberg TØ, Lie T (1984) Lipids of the anal gland secretion of beaver, Castor fiber. Chem Scr 24:100–103
Haley MP (1994) Resource-holding power asymmetries, the prior residence effect, and reproductive payoffs in male Northern elephant seal fights. Behav Ecol Sociobiol 34:427–434
Hartman G (1997) Notes on age at dispersal of beaver (Castor fiber) in an expanding population. Can J Zool 75:959–962
Hayes RA, Richardson BJ, Wyllie SG (2001) Increased social dominance in rabbits, Oryctalus cuniculus, is associated with increased secretion of 2-phenoxyethanol from the chin gland. In: Marchlewska-Koi A, Lepri JJ, Müller-Schwarze D (eds) Chemical signals in vertebrates 9. Plenum Press, New York, pp 335–341
Herr J, Rosell F (2004) Use of space and movement patterns in monogamous adult Eurasian beavers (Castor fiber). J Zool 262:257–264
Herr J, Müller-Schwarze D, Rosell F (2006) Resident beavers (Castor canadensis) do not discriminate between castoreum scent marks from simulated adult and subadult intruders. Can J Zool 84:1–8
Holekamp KE, Smale L (1998) Dispersal status influences hormones and behaviour in the male spotted hyena. Horm Behav 33:205–216
Jenssen TA, Decourcy KR, Congdon JD (2005) Assessment in contests of male lizards (Anolis carolinensis): how should smaller males respond when size matters? Anim Behav 69:1325–1336
Just W, Morris MR (2003) The Napoleon Complex: why smaller males pick fights. Evol Ecol 17:509–522
Kean EF, Müller CT, Chadwick EA (2011) Otter scent signals age, sex, and reproductive status. Chem Sens 36:555–564
Kemp DJ, Wiklund C (2004) Fighting without weaponry: a review of male-male contest competition in butterflies. Behav Ecol Sociobiol 49:429–442
Kruczek M (1997) Male rank and female choice in the bank vole, Clethrionomys glareolus. Behav Process 40:171–176
MacDonald EA, Fernandez-Duque E, Evans S, Hagey LR (2008) Sex, age and family differences in the chemical composition of owl monkey (Aotus nancymaae) subcaudal scent secretions. Am J Primatol 70:12–18
Maher CR, Lott DF (1995) Definitions of territoriality used in the study of variation in vertebrate spacing systems. Anim Behav 49:1581–1597
Martín J, Moreira PL, López P (2007) Status-signalling chemical badges in male Iberian rock lizards. Funct Ecol 21:568–576
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Moretz JA (2003) Aggression and RHP in the northern swordtail fish, Xiphophorus cortezi: the relationship between size and contest dynamics in male–male competition. Ethology 109:995–1008
Morrell LJ, Lindström J, Ruxton GD (2005) Why are small males aggressive? Proc R Soc Lond B 272:1235–1241
Müller CA, Manser MB (2007) 'Nasty neighbours' rather than 'dear enemies' in a social carnivore. Proc R Soc Lond B 274:959–965
Müller-Schwarze D (2006) Chemical ecology of vertebrates. Cambridge University Press, Cambridge
Nolet BA, Rosell F (1994) Territoriality and time budgets in beavers during sequential settlement. Can J Zool 72:1227–1237
Nolet BA, Broekhuizen S, Dorrestein GM, Rienks KM (1997) Infectious diseases as main causes of mortality to beavers Castor fiber after translocation to the Netherlands. J Zool 241:35–42
Olstad O (1937) Beverens (Castor fiber) utbredelse i Norge. Statens viltundersøkelser. Nytt Magasin for Naturvidenskapene 77:217–273
Parker GA (1974) Assessment strategy and evolution of fighting behaviour. J Theor Biol 47:223–243
Parker GA (1984) Evolutionary stable strategies. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell Scientific, Oxford, pp 30–61
Parker H, Rosell F (2003) Beaver management in Norway: a model for continental Europe? Lutra 46:223–234
Peterson AM, Sun L, Rosell F (2005) Species and sub-species recognition in the North American beaver. In: Mason RT, McMaster L, Müller-Schwarze D (eds) Chemical signals in vertebrates 10. Plenum Press, New York, pp 56–63
Piechocki R (1977) Ökologische todesursachenforschung am Elbebiber (Castor fiber albicus). Beitr Jagd Wildforsch 10:332–341
Ralls K (1971) Mammalian scent marking. Science 171:443–449
Raynaud J, Dobson SF (2011) Scent communication by female Columbian ground squirrels, Urocitellus columbianus. Behav Ecol Sociobiol 65:351–358
Rosell F (2002) The function of scent marking in beaver (Castor fiber) territorial defence. Ph.D. thesis, Norwegian University of Science and Technology, Trondheim
Rosell F, Bergan F (1998) Free-ranging Eurasian beavers (Castor fiber) deposit anal gland secretion when scent marking. Can Field Nat 112:532–535
Rosell F, Bjørkøyli T (2002) A test of the dear enemy phenomenon in the Eurasian beaver. Anim Behav 63:1073–1078
Rosell F, Hovde B (2001) Methods of aquatic and terrestrial netting to capture Eurasian beavers. Wildl Soc Bull 29:269–274
Rosell F, Steifetten Ø (2004) Subspecies discrimination in the Scandinavian beaver (Castor fiber): combining behavioural and chemical evidence. Can J Zool 82:902–909
Rosell F, Sun L (1999) Use of anal gland secretion to distinguish the two beaver species Castor canadensis and C. fiber. Wildl Biol 5:119–123
Rosell F, Sundsdal LJ (2001) Odorant source used in Eurasian beaver territory marking. J Chem Ecol 27:2471–2491
Rosell F, Bergan F, Parker H (1998) Scent-marking in the Eurasian beaver (Castor fiber) as a means of territory defense. J Chem Ecol 24:207–219
Rosell F, Johansen G, Parker H (2000) Eurasian beavers (Castor fiber) behavioral response to simulated territorial intruders. Can J Zool 78:931–935
Rosell F, Zedrosser A, Parker H (2010) Correlates of body measurements and age in Eurasian beaver from Norway. Eur J Wildl Res 56:43–48
Rozenfeld FM, Rasmont R (1991) Odour cue recognition by dominant male bank voles, Clethrionomys glareolus. Anim Behav 41:839–850
Schulte BA (1993) Chemical composition and ecology of the North American beaver (Castor canadensis). Ph.D. thesis. State University of New York, Syracuse
Schulte BA, Müller-Schwarze D, Tang R, Webster FX (1995) Bioactivity of beaver castoreum constituents using principle component analysis. J Chem Ecol 21:941–957
Scordato ES, Drea CM (2007) Scents and sensibility: information content of olfactory signals in the ringtailed lemur, Lemur catta. Anim Behav 73:301–314
Smith JLD (1993) The role of dispersal in the Chitwan tiger population. Behaviour 124:3–4
Smith IP, Huntingford FA, Atkinson RJA, Taylor AC (1994) Strategic decisions during agnostic behaviour in the velvet swimming crab, Necora puber (L.). Anim Behav 47:885–894
Sun L (1996) Chemical kin recognition in the beaver (Castor Canadensis): behavior, relatedness and information coding. Ph.D. thesis. State University of New York, College of Environmental Science and Forestry, Syracuse, New York
Sun L, Müller-Schwarze D (1997) Sibling recognition in the beaver: a field test for phenotype matching. Anim Behav 54:493–502
Sun L, Müller-Schwarze D (1998) Anal gland secretion codes for relatedness in the beaver, Castor canadensis. Ethology 104:917–927
Sun L, Müller-Schwarze D, Schulte BA (2000) Dispersal pattern and effective population size of the beaver. Can J Zool 78:393–398
Svendsen GE (1980) Patterns of scent-mounding in a population of beaver (Castor canadensis). J Chem Ecol 6:133–147
Svendsen GE (1989) Pair formation, duration of pair-bonds, and mate replacements in a population of beavers (Castor canadensis). Can J Zool 67:336–340
Svensson PA, Lehtonen TK, Wong BBM (2012) A high aggression strategy for smaller males. PLoS One 7:e43121
Taylor PW, Hasson O, Clark DL (2001) Initiation and resolution of jumping spider contests: roles for size, proximity, and early detection of rivals. Behav Ecol Sociobiol 50:403–413
Temeles EJ (1994) The role of neighbors in territorial systems—when are they dear enemies? Anim Behav 47:339–350
White MM, Swaisgood RR, Zhang H (2003) Chemical communication in the giant panda (Ailuropoda melanoleuca): the role of age in the signaller and assessor. J Zool 259:171–178
Wilsson L (1971) Observations and experiments on the ethology of the European beaver (Castor fiber). Viltrevy 8:115–266
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press, Cambridge
Young AJ, Monfort SL (2009) Stress and the cause of extra-territorial movement in a social carnivore. Biol Lett 5:439–441
Acknowledgments
We would like to thank Howard Parker, Jon Swenson, and Dan Blumstein for useful comments on a previous version of the manuscript; Bjorn Steen for help with the GC-MS analysis; Frode Bergan for technical assistance; and Moritz Klein, Christian Robstad, and Patricia Graf for beaver trapping. Finally, we are sincerely grateful to Dr. Kathreen Ruckstuhl and two anonymous referees who provided critical comments on the drafts of our paper and improved its quality. This work was funded by Telemark University College.
Ethical standards
All trapping and handling procedures were approved by the Norwegian Experimental Animal Board and the Norwegian Directorate for Nature Management and met the guidelines approved by the American Society of Mammalogists (Gannon and Sikes 2007).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. E. Ruckstuhl
Rights and permissions
About this article
Cite this article
Tinnesand, H.V., Jojola, S., Zedrosser, A. et al. The smell of desperadoes? Beavers distinguish between dominant and subordinate intruders. Behav Ecol Sociobiol 67, 895–904 (2013). https://doi.org/10.1007/s00265-013-1512-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1512-y