Abstract
Environmental and social factors are critical to determine the timing and duration of lekking behavior since they provide species with signs to maximize benefits over costs in sexual displays. However, these factors have rarely been studied under different environmental conditions, and thus, it remains unclear whether exogenous factors affecting group displays show a general species-specific pattern or whether they are population-specific. Using audio-trapping techniques, we compared factors influencing the daily occurrence and duration of lekking behavior in two populations of Hyla molleri and two populations of Hyla meridionalis located at the thermal extremes (coldest vs. hottest) of their Iberian distribution range. From 12,240 hourly recordings over one season, multimodel inference revealed that the major determinants of chorus occurrence were similar between populations and species (i.e., chorus size the previous day, daytime air temperature, relative humidity, and barometric pressure), and accounted for 51–79 % of its deviance. In contrast, the major determinants of chorus duration differed between populations and species (i.e., chorus size, number of day, and air temperature and relative humidity at the onset of the chorus), and accounted for 38–69 % of its variance. Our findings suggest that the decision making related to lek attendance is environment-dependent, takes place at time between lekking events, and is associated with exogenous factors that may be both stable across species ranges and population-specific when populations are under different climatic conditions. This intraspecific variation might be underlain by plasticity mechanisms providing tree frogs with means to cope with changing environments. Moreover, social facilitation related to male-male acoustic competition seems to play a relevant role on the daily time invested by males in lek attendance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lek mating systems have been described in a wide range of animals, such as mammals, birds, amphibians, fish, and insects (e.g., Lloyd 1867; Downes 1969; Wells 1977; Clutton-Brock et al. 1993; Höglund and Alatalo 1995). Males breeding in leks or choruses congregate synchronously on reproductive sites to defend non-resource-based territories from competitors and to attract mating partners by advertisement signals (Bradbury 1981; Höglund and Alatalo 1995). Attendance at group displays is crucial for individual fitness in lekking species, although there is often variation in mating success of males within a lek (Widemo and Owens 1995; Mackenzie et al. 1995; Kokko et al. 1998). However, lekking behavior is also highly costly in terms of energy (Taigen and Wells 1985; Prestwich 1994; Schwartz et al. 1995; Grafe and Thein 2001) and predation risk (Ryan et al. 1981; Tuttle and Ryan 1981), and thus, it is expected that selective pressures favor mechanisms in lekking species to determine the optimal time for lek formation (Murphy 1999, 2003; Brooke et al. 2000).
The timing and duration of group displays are influenced by environmental or social factors, or both, that have been found to act as determinants of the temporal patterns of lekking and chorusing behavior in multiple organisms (e.g., Schneider 1971; Pemberton and Balmford 1987; Han and Gatehouse 1991; Baines 1996; Brooke et al. 2000; Berg et al. 2006). Temperature, light intensity, and relative humidity (or rainfall) are the most common environmental factors associated with the onset and maintenance of anuran chorusing (Blair 1961; Heinzmann 1970; Schneider 1971; Obert 1975; Fukuyama and Kusano 1992; Henzi et al. 1995; Navas 1996; Oseen and Wassersug 2002; Steelman and Dorcas 2010), although other weather variables may play a role in chorus formation, including barometric pressure, wind speed, or lunar cycle (Blankenhorn 1972; Henzi et al. 1995; Cree 1989; Oseen and Wassersug 2002; Murphy 2003; Grant et al. 2009; Steelman and Dorcas 2010). Moreover, calling activity from conspecifics can elicit acoustic displays at different scales and levels (e.g., Capranica 1966; Ryan and Rand 1998; Brooke et al. 2000), and thus, social facilitation also affects strongly on the timing and duration of group acoustic displays (Wells and Taigen 1986).
Interspecific comparisons, both in sympatry and allopatry, have suggested that the set of exogenous determinants of lek or chorus formation tend to be species-specific (Blankenhorn 1972; Obert 1975; Salvador and Carrascal 1990; Oseen and Wassersug 2002; Berg et al. 2006; Steelman and Dorcas 2010). Additionally, prolonged breeders seem to respond to more exogenous factors than explosive breeders and be affected by different factors throughout the breeding season (Wells 1977; Oseen and Wassersug 2002), but some exceptions have also been found (Blankenhorn 1972; Salvador and Carrascal 1990).
In contrast with the abundant literature about between-species variation, little attention has been addressed to investigate the within-species variation in the responses to exogenous factors mediating lek attendance. Studies measuring the effects of environmental variables on chorusing activity have mostly been conducted at single locations, where one or more species respond to the same climatic conditions (Blair 1961; Heinzmann 1970; Schneider 1971; Blankenhorn 1972; Obert 1975; Salvador and Carrascal 1990; Fukuyama and Kusano 1992; Henzi et al. 1995; Moreira and Barreto 1997; Hatano et al. 2002; Oseen and Wassersug 2002; Steelman and Dorcas 2010). Therefore, it remains unclear whether such variables and their effects may be extrapolated onto populations across the geographic distribution of the species and hence under different environmental or social conditions. The few intraspecific studies available were not specially focused on studying the effect of clearly divergent climate regimes, since they compared populations at small spatial scales (Ritke et al. 1992; Navas 1996; Brooke et al. 2000).
The comprehension of factors affecting the timing and duration of these sexual displays under different environments provides insight into a remaining open question: whether the exogenous factors determining lek formation show a general species-specific pattern or whether they are population-specific. Since exogenous factors eliciting lekking behavior must reflect optimal local conditions for lek formation (e.g., Höglund and Alatalo 1995; Brooke et al. 2000), it may be expected that species respond to different exogenous factors when they inhabit in different environmental or social contexts. Such response would imply the occurrence of mechanisms of local adaptation or phenotypic plasticity providing lekking species with means to confront changing environments. Thus, the study of intraspecific variation of exogenous determinants of group displays may contribute to reveal the occurrence of such mechanisms and helps in understanding of how species cope with environmental heterogeneity, which is a grand and urgent task for current biology (Schwenk et al. 2009). To our knowledge, this is the first study that examines the intraspecific variation of environmental and social determinants of anuran lekking behavior (chorus occurrence and duration).
The technical difficulty to monitor simultaneously distant populations has been so far one of the reasons of the lack of intraspecific long-term acoustic surveys. As used in this study, the recently developed automated recording systems (ARS; Peterson and Dorcas 1994; Frommolt et al. 2008; Obrist et al. 2010) now facilitate the simultaneous monitoring of calling activity over complete seasons and in multiple populations, even if the populations are located at the latitudinal margins of the species' distribution range. Moreover, this sampling method prevents changes to the behavior of the study species owing to observer presence as well as enables subsequent thorough analyses of the recordings with enhanced detection and reduced observer bias (Bridges and Dorcas 2000; Oseen and Wassersug 2002; Acevedo and Villanueva-Rivera 2006; Steelman and Dorcas 2010; Llusia et al. 2011).
Here, we examine the environmental and social factors that determine lek formation in two species of Palearctic tree frogs, Hyla molleri and Hyla meridionalis. With audio-trapping techniques based on ARS, chorusing activity and weather conditions were monitored in four populations (two per species) located at the thermal extremes (coldest vs. hottest) of their Iberian distribution range and exposed to different climatic conditions. Thereby, we attempt to test whether exogenous determinants of lekking behavior show a general species-specific pattern or whether they are population-specific. Specifically, we address the following two questions: (1) what environmental and social factors are associated with the daily occurrence and duration of tree frog chorus? (2) Do these factors differ across populations (hot vs. cold), species (H. molleri vs. H. meridionalis), and indexes of chorus formation (occurrence vs. duration)? Furthermore, we discuss the implications of our findings for predictions on the effects of current climate change on anuran populations.
Material and methods
Species and study sites
Iberian tree frog (H. molleri Bedriaga, 1890) and Mediterranean tree frog (H. meridionalis Boettger, 1874) share numerous morphological, behavioral, and ecological traits. They are both prolonged breeders and aquatic egg-layers that aggregate in choruses around ponds, streams, or other water bodies from early spring to summer during their breeding season (García-París et al. 2004). Within this period, males are typically hidden in bushes or underground hideouts at daytime, and before sunset they migrate to breeding points, where they vocalize while floating on water or perched on emergent vegetation (Paillette 1967; Schneider 1974; Márquez and Tejedo 1990; Márquez et al. 2005). Lek-like mating system has been described in these species (Friedl and Klump 2005; Jaquiéry et al. 2009); therefore, they are suitable models to study the mechanisms underlying lekking behavior, as recent studies have showed with some closely related hylids (e.g., Hyla arborea) and other lek-breeding anurans (Grafe 1997; Friedl and Klump 2005; Broquet et al. 2009; Knopp et al. 2008; Castellano et al. 2009; Jaquiéry et al. 2009). Sympatric populations of the study species occur in central and western Iberia, where hybridization takes place occasionally (Oliveira et al. 1991; Barbadillo and Lapena 2003).
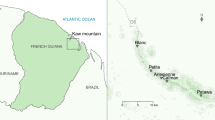
The study was conducted on populations located at the thermal extremes of the Iberian distribution range of tree frogs: two localities of H. molleri (cold and hot, hereafter CHmo and HHmo) and two localities of H. meridionalis (cold and hot, hereafter CHme and HHme; Fig. 1). CHmo occurs above the timberline of the mountain area of the Somiedo Natural Park in northern Iberia (1,490 m.a.s.l., Asturias, Spain), in a small temporary pond (25 × 18 m) surrounded by bushes (Genista florida), alpine prairies, and grasslands. The area is under an oceanic climate, with cool and wet summers (Cfb, Köppen-Geiger climate classification; Peel et al. 2007). HHmo and CHme populations are sympatric in west-central Iberia and breed in a medium-sized permanent pond (62 × 53 m) located at 408 m.a.s.l. within an agricultural land of the São Mamede Natural Park (Portalegre, Portugal). In this site, climate shows transitional features between oceanic and Mediterranean climates, with warm and dry summers (Csb; Peel et al. 2007). HHme is located in the coastal marshes of the Doñana Biological Station in southern Iberia (3 m.a.s.l., Huelva, Spain), in a large-sized temporary pond (160 × 101 m) with sedges (Scirpus spp.) and halophytic vegetation, where it undergoes a typical Mediterranean climate, with hot and dry summers (Csa; Peel et al. 2007). Geographic coordinates, meteorological conditions, and other features of the study sites are listed in Table 1.
Location of study sites and tree frogs ranges in the Iberian Peninsula (Pleguezuelos et al. 2002; Loureiro et al. 2008). Species ranges are plotted through 10 × 10 km UTM squares (Universal Transverse Mercator coordinate system) with occurrence of Hyla molleri (grey open squares) and Hyla meridionalis (black closed squares). CHmo and HHmo: cold and hot populations of H. molleri, respectively. CHme and HHme: cold and hot populations of H. meridionalis, respectively. HHmo and CHme are sympatric populations
To quantify how thermally extreme were the climate regime of each study site, we compared the annual mean temperature of the 10 × 10 km UTM square including the study site (Universal Transverse Mercator coordinate system; Hijmans et al. 2005) with that of the remaining 10 × 10 km UTM squares of the Iberian distribution range of the study species (1,257 squares for H. molleri and 1,074 squares for H. meridionalis; Pleguezuelos et al. 2002; Loureiro et al. 2008). In the Iberian Peninsula, between 73 % and 99 % of the UTM squares of the species ranges had a higher annual mean temperature than that of the UTM square in the cold study sites (7.6 °C in CHmo; 15.4 °C in CHme; Table 1). Conversely, between 78 % and 97 % of the UTM squares had a lower annual mean temperature than that of the UTM square in the hot study sites (15.4 °C in HHmo; 17.9 °C in HHme; Table 1). The hot-cold pairs of sites showed a similar thermal difference, approximately 5 °C, measured as mean temperature of the wettest quarter (4.3 °C vs. 9.2 °C, for H. molleri pair; 9.2 °C vs. 14.3 °C, for H. meridionalis pair; Table 1).
Data collection
Anuran calling activity and weather conditions were monitored during an entire breeding season (from December 2006 to July 2007; Table 2) with audio-trapping techniques (i.e., acoustic ARS) and data loggers. Sound monitoring was extended 10–12 weeks before and after the expected period of calling activity for each population ensuring its complete recording. Weather monitoring was also extended to the previous and the subsequent year to record the meteorological conditions outside the period of reproductive activity. The ARS consisted of an omnidirectional condenser microphone (Fonestar FCM-626) and a solid-state digital recorder (Marantz PMD660, D&M Professional), powered by a 12-V battery and controlled by a custom-built programmable timer (Western Kentucky University; Cambron and Bowker 2006). The equipment was housed in watertight boxes, with an external microphone placed 5–10 m from the shore of the ponds and 1–2 m above ground to maximize the probability of detection of anuran vocalizations. Microphones were protected from rainfall and humidity by a plastic shield. During the whole study period, sound recordings (44.1 kHz, 16-bit, MP3 format) were obtained with a schedule of 3 min per hour, 24 h per day, which it has shown to be an adequate sampling regime for the most anuran calling surveys (Shirose et al. 1997; Dorcas et al. 2009). To standardize the ARS-based sound monitoring (recording levels, detection spaces, etc.), we followed the method described in Llusia et al. (2011). Data download and battery replacement were carried out every 3 or 4 weeks. During the study period, 12,240 3-min-long audio files were collected from 86 monitoring days in CHmo, 175 in HHmo and CHme, and 252 in HHme. In total, the automated recording equipment was inactive in the field 18 days in CHmo, 62 in HHmo and CHme, and 15 in CHme due to different technical problems, thus preventing the inclusion of data from those days. To detect calling activity from either of the two species, we subsequently listened to the recordings in the laboratory, and visually inspected oscillograms and audio-spectrograms (Peak 4.0, BIAS, Inc.; XBAT, Cornell University). Sound recordings were assigned to a calling index value between 0 and 5, following a modified version of the NAAMP protocol (i.e., 0 = absence of calling activity; 1 = single vocalizations lacking continuity from one or two isolated individuals; 2 = individuals can be counted, space between calls; 3 = calls of individuals can be distinguished, lack of overlapping; 4 = intermittent chorus, calls are discontinuous, some overlapping; 5 = full chorus, calls are constant, continuous, and overlapping; Weir and Mossman 2005).
Air and water temperatures (degrees Celsius) and relative humidity (percent) were measured in situ every 5 min with data loggers (Pendant 64 Kb and HOBO Pro V2, Onset Computer). The air data loggers were placed 5–10 m from the shore, in the vegetation that surrounds the ponds, 15–35 cm above ground, and with probes protected from rainfall and direct sunlight. The water data loggers were in the water surface at 1–2 m from the shore of breeding ponds. Accuracy in the measurements was 0.3 °C for temperatures and 2.8 % for humidity. In addition to the data loggers, hourly measurements of barometric pressure (hectopascal) and cloud cover (okta) were obtained from three automatic weather stations of the Spanish and Portuguese National Meteorological Services, Agencia Estatal de Meteorología (AEMET, Spain) and Instituto de Meteorologia (IP, Portugal). The weather stations are located in Oviedo (335 m.a.s.l., 39 km from CHmo), Portalegre (597 m.a.s.l., 11 km from HHmo and CHme), and Mazagón (41 m.a.s.l.; 28 km from HHme). Time and date of the moon phases during 2006 and 2007 were also recorded (Planetary System Laboratory, NASA) because reproductive phenology has been associated with lunar cycle in some anuran species (FitzGerald and Bider 1974; Grant et al. 2009). For each hour of the study period, we assigned an equidistant percentage value that ranged from 0 % (at time of new moon) to 100 % (at time of full moon) as a measure of the moon phases.
Response and predictor variables
From the initial hourly time series of data, two daily response variables were calculated for each study site: the daily occurrence of tree frog chorus (hereafter, chorus occurrence) and the nightly chorus duration (hereafter, chorus duration). European tree frog males attend choruses mostly between sunset and sunrise (Schneider 1971) and tend to move in and out of the breeding points between chorusing events (Grafe and Meuche 2005). Thus, the temporal patterns of chorusing activity within the breeding season are expected to be associated with a daily decision making that may be based at least on twofold decision: (1) whether to migrate to the breeding ponds to attend a chorus, which determines chorus occurrence (presence/absence), and (2) when cease to call and leave the chorus, which determines chorus duration (number of hours). Moreover, the daily time series were selected instead of the hourly information because hourly time series may suffer from autocorrelation in errors of the explanatory variables, so that they fail the assumption of independent values required for regression methods (Sokal and Rohlf 1995).
The response variable chorus occurrence was assigned a value of 1 (presence) when any recording within the day (i.e., from sunrise to sunrise) achieved a value of the calling index between 2 and 5, whereas it was assigned a value of 0 (absence) when all recordings within the day had a value of the calling index between 0 and 1. Tree frogs may occasionally emit isolated calls from their hideouts under rocks or in bushes at times of the day or even periods of the year that leks are not formed in the water. Thus, the recordings with a calling index value of 1 (i.e., single vocalizations lacking continuity from one or two isolated individuals), which corresponds to tentative vocal activity outside of group displays, were included as absence of chorus occurrence. The response variable chorus duration was calculated as the number of recordings within the day (i.e., from sunrise to sunrise) with a value of the calling index between 2 and 5. In addition to these variables, a multinomial variable was also calculated as the daily maximum value of the calling index (hereafter, chorus size).
The temporal window of the daily time series of chorus occurrence was selected so that those series were composed of a similar number of presences and absences, i.e., a balanced design. Since only a low percentage of days within the calling season lacked choruses (mean <10 % of the nights), days before and after the period of calling activity were added in an amount equal to the duration of this period (i.e., half before and half after). We selected this temporal window to include environmental values to which tree frogs had been subjected just before and after the breeding season (between 3 and 8 weeks), and that might have influenced the onset and end of the group displays. Moreover, this balanced design with equal prevalence of presences and absences was used to avoid the statistical problems associated with unequal sample size between treatments in generalized linear models (Hosmer and Lemeshow 1989; Fielding and Bell 1997; Cramer 1999; Montgomery 2001; Manel et al. 2001; Gelman 2005). The temporal window of the daily time series of chorus duration was composed only of days with presence of chorus.
To determine the environmental and social factors influencing chorus occurrence and chorus duration in each study site, multiple binomial and linear regression analyses were computed, respectively. The set of independent variables entered into the multivariate analyses was composed of 17 variables for binomial regressions and of 16 variables for linear regression (Tables 3 and 4). These variables were selected as those that could presumably influence tree frogs or be assessed by tree frogs before the daily beginning of the chorus attendance (in the case of chorus occurrence) and before the daily ending of the chorus attendance (in the case of chorus duration). To examine potential predictors of chorus occurrence (Table 3), we used the daytime means, minima or maxima of the environmental variables monitored, as well as trend values from previous days and values 2 h before sunset (when choruses are typically close to be initiated). Daytime was considered from sunrise to 2 h before sunset, when no chorus occurs in the study sites, and it is expected that the decision making related to migration to the breeding points and chorus attendance takes place. To examine potential predictors of chorus duration (Table 4), we used the mean values of the environmental variables monitored during the chorus and values at the onset of the chorus. Values at the onset of the chorus were selected instead of values at the end of the chorus because the former were independent of the number of hours of chorusing activity unlike the latter (e.g., air temperature at the end of the chorus is consistently found to be colder in long choruses than in short ones since air temperature typically decreases along the night, and hence, a causal relationship between temperature at the end of the chorus and chorus duration would be found as a statistical artifact). Water temperature was added among the explanatory variables of chorus duration because most of tree frog males in the study sites typically called from the surface of the water. In addition, quadratic terms of temperature variables were included in both binomial and linear regressions to examine the presence of temperature optima.
Calling activity may diminish along the reproductive period due to mortality, decline in energy reserves of callers, or due to previous mating success in part of the population (e.g., Murphy 1994). Thus, the number of days elapsed since the onset of the study period was added to the regression analyses. Furthermore, the social structure of aggregations (e.g., the number of males within the group displays) in turn may influence chorus attendance and time invested by males in the chorus (Wells and Taigen 1986), as a result of male–male sexual competition and evoked vocal responses (e.g., Capranica 1966; Ryan and Rand 1998). Hence, chorus size the same night was also entered into regression models as independent variable to investigate whether nightly social conditions might affect chorus duration. In addition, to account for lagged effects of social factors in chorus occurrence and chorus duration, one lagged predictor variable was included in both binomial and linear regressions (Tables 3 and 4). Chorus size the previous night is expected to increase the likelihood of chorus occurrence as a proxy of the number of males in suitable conditions for sexual displays (hormonal levels, energetic reserves, etc.), so that larger proportion of males attending the chorus the previous night would presumably enable to more males maintain chorusing activity in subsequent nights. Moreover, chorus size may also increase the communication distance between conspecifics, and thus the range of social facilitation of calling activity. Interaction terms were excluded from the regression models because of the large number of candidate predictors.
Statistical analyses
Multimodel inference was applied as model selection approach (Burnham and Anderson 2002; Johnson and Omland 2004; Richards et al. 2011; Symonds and Moussalli 2011), using Akaike information criterion (AIC) corrected for small sample sizes (Akaike's second-order AIC, AICc; Burnham and Anderson 2004). This approach grounded on the K-L information theory is specially suited to test multiple competing hypotheses from complex systems, providing advantages over other methods, such as weighing of candidate models and model averaging (Johnson and Omland 2004). All possible regression models from every combination of the independent variables (i.e., full set of models) were computed and compared each other with AICc and Akaike weights (w i ). Regression models with ΔAICc < 2 were considered as equally plausible models and retained for model averaging. In model averaging, standardized regression coefficients (β) and relative importance of the predictor variables in the AIC multimodel inference (Σw i : sum of Akaike weights of the models where each variable was selected) were calculated to enable comparisons of the effect of the predictors on the response variable.
Regression diagnostics confirmed absence of multicollinearity, autocorrelation, overdispersion, and significant departures from normality in all final models. Multicollinearity was assessed with variance inflation factor (VIF; Montgomery and Peck 1992). When any VIF value ≥ 3 (Zuur et al. 2010), one of the predictor variables involved in multicollinearity was removed from the set of independent variables, and multimodel procedure was restarted from the onset. Univariate regression R 2 as well as standardized regression coefficients (β) and relative importance (Σw i ) of the predictor variables in the averaged model were used as criteria to select the variables excluded due to multicollinearity. Furthermore, model residuals showed lack of autocorrelation in all cases as tested by Durbin-Watson statistic (d = 1.93–2.38, P value > 0.39 in all cases). The ratio of residual deviance to degrees of freedom was below 1 in all binomial models, which indicates absence of overdispersion. Normality of the residuals was assessed with Shapiro-Wilk test and graphical methods.
Multiple ordered logistic regressions were also attempted with an alternative response variable (i.e., chorus size, the daily maximum of the calling index, with values between 0 and 5), but these regression analyses yielded poor goodness of fit in all cases (i.e., explained deviances <15 % and failures of the normality assumption). Thus, the daily presence of chorus was entered as binomial variable (chorus occurrence) instead of multinomial variable (chorus size), and the ordered logit analysis rejected.
Finally, to test whether species respond to the same environmental and social variables across populations at thermal extremes, we followed a standard method to contrast among regression equations (Neter et al. 1996). Two regression models were fitted and statistically compared: (1) a combined model for both conspecific populations by joining the datasets from the cold and hot populations; and (2) the same previous model, but including a categorical variable for population and an interaction term between this variable and each of the remaining predictors. Combined models showing higher information loss and smaller strength of evidence (i.e., ΔAICc > 2 and lower Akaike weights) than models with separate equations for populations would point out differences in the regression equations (intercepts, slopes, or both) for the conspecific populations (Neter et al. 1996; Murphy 2003), and hence, this would imply environmental and social determinants being population-specific, rather than showing a general species-specific pattern. This statistical procedure was used to compare between each pair of conspecific populations (CHmo vs. HHmo and CHme vs. HHme), for both chorus occurrence and chorus duration. Additional comparisons were also conducted between species at the sympatric site (HHmo vs. CHme) in order to test whether the two tree frog species respond to the same environmental and social variables when they are subjected to the same conditions.
All statistical analyses and figures were conducted with R 2.15.0 (R Development Core Team 2012).
Results
Weather conditions
In all study sites, temperature conditions during the study period were similar to those during the reference period of 1971–2000 (“typical meteorological year”; AEMET 2008). Annual rainfall was less abundant (20–40 % of its range in 1971–2000), except in the southernmost site (HHme) where rainfall reached similar rates to those of the 1971–2000 period. Within H. molleri sites, weather conditions were markedly warmer and drier in HHmo (mean ± SD; air temperature, 15.0 ± 7.2 °C; relative humidity, 62 ± 20 %) than in CHmo (9.2 ± 6.3 °C; 95 ± 13 %). For H. meridionalis, the hot site was also warmer, but less dry (HHme, mean ± SD; air temperature, 15.6 ± 5.8 °C; relative humidity, 74 ± 18 %) than the cold site (CHme; 13.0 ± 5.8 °C; 66 ± 18 %), although these differences in air temperature and relative humidity were less marked than in the pair of H. molleri sites. Rates of other weather factors measured in the study sites are summarized in Table 1.
Chorusing activity
Tree frog choruses were recorded between 38 and 118 nights per site (Table 2), and their presence was largely continuous across the breeding season. The percentage of nights lacking chorusing activity within the season averaged only 9.8 % (range, 5–21 %). In the majority of the calling nights (>61 %), chorus size was assigned to its maximum level (equals 5). In contrast, chorus duration was markedly more variable than chorus occurrence and chorus size, with larger coefficients of variation and a duration range of 1–13 h per calling night (Table 2). In all populations, most of the recordings containing tree frog choruses occurred between sunset and sunrise (>90 %).
The duration of chorusing activity, both seasonally (i.e., number of nights from the first to the last night of chorus occurrence) and nightly (i.e., number of hours calling per night or chorus duration), were strongly population-specific and longer in the hot sites than in the cold ones, as shown in Table 2. In HHme, tree frogs formed the most prolonged and larger choruses of the four populations, between 26 December and 21 May and with a mean duration of 7.2 ± 3.2 h (tracks) per calling night (n = 118). In sympatry, under the same environmental conditions, choruses of the two species lasted similarly per night (HHmo: 5.6 ± 1.9 h; CHme: 5.1 ± 2.5 h), but calling season was a month shorter for H. meridionalis (HHmo: n = 84 nights; CHme: n = 56 nights).
Predictive models of chorusing activity
From the AIC multimodel inference, six to 14 logistic regression models per population showed ΔAICc < 2 (ESM Table 1) and accounted for 51–79 % (weighted average of the explained deviance for all selected models per population) of the daily likelihood of presence/absence of tree frog chorus (chorus occurrence) by including a total of five to eight predictor variables (Table 5; ESM Table 1). In all populations, the predictor variable with the highest strength of evidence (Σw i = 1.00, i.e., included in all models with ΔAICc < 2) and the highest magnitude effects (β = 4.54–22.54) was prevSizeCHORUS (i.e., the maximum size of the chorus the previous night, a calling index with values between 0 and 5) that increased the likelihood of presence of chorusing activity. Moreover, other environmental factors were consistently included in models with ΔAICc < 2 (e.g., maxDayTEMP, chgTEMP, chgPRESS, dayRH, or prevRH), although with relative importance (Σw i = 0.10–1.00) and magnitude effects (β = −0.84–2.12) differing among populations (Table 5 and Fig. 2; ESM Table 1). In most of the study sites, the likelihood of presence of tree frog chorus increased on warmer and wetter days with a rise in air temperature and barometric pressure from the previous day.
Scatter plots and logistic functions showing the univariate relationships between chorus occurrence (probability of daily presence/absence of tree frog chorus) and its major exogenous determinants in each study site, as shown by AIC multimodel inference: (top row) prevSizeCHORUS, maximum size of the chorus the previous night (calling index with values between 0 and 5); (second row) chgTEMP, change in daytime mean of air temperature (degrees Celsius) from the previous day; (third row) dayRH, mean relative humidity (percent) during daytime; and (bottom row) chgPRESS, change in daytime mean of barometric pressure (hectopascal) from the previous day. CHmo and HHmo: cold and hot populations of Hyla molleri, respectively. CHme and HHme: cold and hot populations of Hyla meridionalis, respectively. HHmo and CHme are sympatric populations. Size of points is proportional to the square root of the number of observations
Similarly, two to 13 linear regression models per population showed ΔAICc < 2 (ESM Table 2) and accounted for 38–69 % (weighted average of R 2 for all selected models per population) of the variance of the nightly number of hours of chorusing activity of tree frogs (chorus duration) by including a total of six to seven predictor variables (Table 6; ESM Table 2). All predictor variables of these models showed standardized regression coefficients (β) below 1, which suggests that major predictors had similar magnitude effects on chorus duration in all populations. According to their strength of evidence and magnitude effects, four factors were the most prominent determinants of chorus duration: sizeCHORUS (Σw i = 0.84–1.00; β = 0.30–0.80), numDAY (Σw i = 0.05–1.00; β = −0.63–0.18), onsetAirTEMP (Σw i = 0.27–1.00; β = 0.16–0.78), and onsetRH (Σw i = 0.31–1.00; β = 0.17–0.49), which were incorporated as predictors in three to four of the populations. Overall, the number of hours in which tree frog choruses were active increased in nights earlier in the breeding season, with larger number of males attending the chorus and with warmer and wetter air at the onset of the calling activity (Table 6 and Fig. 3). Among populations, the influence of sizeCHORUS on chorus duration was positively associated with the population size and the seasonal mean of chorus duration. The sized-biggest population showed the largest rates of chorus duration as well as the largest magnitude effects of sizeCHORUS in regression models of chorus duration (HHme, 7.2 h per night; β = 0.8), while the sized-smallest population showed the shortest rates in these two indices (CHmo, 3.6 h per night; β = 0.0). Other predictor variables were nightCLOUD [i.e., mean barometric pressure (hectopascal) during the chorus] and prevCHORUS (i.e., the number of hours of chorusing activity the previous night), which were both positively associated with chorus duration in HHme (Σw i = 1.00). Thus, chorus duration exhibited similar number of associations with social and environmental factors than chorus occurrence, although with less strong effects and accounting for a lower portion of its variance.
Scatter plots and linear functions showing the univariate relationships between chorus duration (nightly duration of tree frog chorus) and its major exogenous determinants in each study site, as shown by AIC multimodel inference: (top row) sizeCHORUS, maximum size of the chorus that night (calling index with values between 0 and 5); (second row) numDAY, day number of the breeding season (from 1 onwards); (third row) onsetAirTEMP, air temperature (degrees Celsius) at the onset of the chorus; and (bottom row) onsetRH, relative humidity (percent) at the onset of the chorus. CHmo and HHmo: cold and hot populations of Hyla molleri, respectively. CHme and HHme: cold and hot populations of Hyla meridionalis, respectively. HHmo and CHme are sympatric populations. Size of points is proportional to the square root of the number of observations
Model comparisons
The statistical comparisons between regression models revealed that the intercepts and the slope coefficients of the environmental and social determinants of chorus occurrence were similar between conspecific populations at thermal extremes, whereas those of chorus duration differed markedly. Higher information loss was obtained in all the models of chorus occurrence including an indicator for population (i.e., models with separate equations for each population; H. molleri: ΔAICc = 22.2; H. meridionalis: ΔAICc = 10.5; in sympatry: ΔAICc = 21.9) than in the combined models with common equations for both populations, which showed a larger strength of evidence in all cases (H. molleri: w i = 1.00; H. meridionalis: w i = 0.99; in sympatry: w i = 1.00). In contrast with chorus occurrence, the coefficients for chorus duration exhibited marked differences between populations, as shown by higher Akaike weights in all the models with an indicator for population and interaction terms between this factor and each predictor (H. molleri: w i = 1.00; H. meridionalis: w i = 1.00; in sympatry: w i = 0.99). The combined models of chorus duration with common equations for both cold and hot populations resulted in models with higher AICc values (H. molleri: ΔAICc = 29.2; H. meridionalis: ΔAICc = 24.0; in sympatry: ΔAICc = 9.8). From the AIC multimodel inference in combined models of chorus duration, the indicator for population were incorporated in all models with ΔAICc < 2 (Σw i = 1.00 in all cases; H. molleri: β = 1.07; H. meridionalis: β = −0.60; in sympatry: β = 0.09), as well as several interaction terms (H. molleri: numDAY, onsetAirTEMP, meanWatTEMP, and onsetRH; Σw i = 0.04–0.58; β = −0.80–0.50; H. meridionalis: onsetRH, onsetAirTEMP, prevCHORUS, numDAY, and sizeCHORUS; Σw i = 0.39–1.00; β = −0.17–0.65; in sympatry: numDAY; Σw i = 1.00; β = −0.38).
Discussion
Determinants of chorus occurrence
Multimodel inference supports the effect of the daytime weather conditions on the daily lek formation in tree frogs (chorus occurrence), suggesting that decision making related to chorus attendance (e.g., migration and calling behavior) is environment-dependent and takes place at time between lekking events. As shown in previous works, air temperature (Heinzmann 1970; Schneider 1971; Blankenhorn 1972; Obert 1975; Salvador and Carrascal 1990; Fukuyama and Kusano 1992; Brooke et al. 2000; Oseen and Wassersug 2002; Steelman and Dorcas 2010), relative humidity (Oseen and Wassersug 2002; Steelman and Dorcas 2010), and barometric pressure (Blankenhorn 1972; Henzi et al. 1995; Brooke et al. 2000; Oseen and Wassersug 2002; Steelman and Dorcas 2010) markedly affected the temporal patterns of chorusing activity (β > 1), although their magnitude effects differed among populations and species. This implies that environmental factors may act as cues for lekking species to determine the optimal time to lek, as suggested by Brooke et al. (2000). The prominent costs associated with lekking behavior (e.g., Ryan et al. 1981; Tuttle and Ryan 1981; Taigen and Wells 1985; Prestwich 1994; Grafe and Thein 2001) would promote that males attending leks or choruses restrict their presence in arenas or breeding points according to environmental conditions.
Such behavioral strategy is expected to be specially marked in species with prolonged breeding patterns (Wells 1977; Oseen and Wassersug 2002), as both species of this study, in which the arrival of females at the breeding points occurs asynchronously along the season. Our results were in agreement with the hypothesis proposed by Wells (1977) that predicts that prolonged breeders displaying in lek or chorus would respond intensely to environmental conditions as a means of regulating their energy budget throughout long reproductive periods and of increasing the probability to encounter receptive females. Moreover, Murphy (2003) found a similar response to environmental variables in both females and males of Hyla gratiosa, suggesting that males could assess the same variables to predict female abundance and thereby the payoff of attending the chorus, which might explain the widespread correlations between females and males attending leks or choruses (e.g., Henzi et al. 1995; Höglund and Alatalo 1995).
Among the daytime environmental variables, those recorded along the entire day (i.e., mean and maximum) or calculated as changes from previous days (i.e., trends) were selected over variables measuring conditions at a short time before chorus formation (i.e., 2 h before sunset). This indicates that males may be influenced by weather factors across a large temporal window: more than 2 h before the onset of chorusing activity. In contrast, Blankenhorn (1972) found temperature at 19 h as the major predictor factor of chorusing in a German population of H. arborea.
In most populations, maximum or minimum daytime of air temperature was positively associated with chorus occurrence, suggesting that warmer daytime favor chorusing activity in populations of tree frogs located at both cold and hot thermal extremes. Ectothermy conditions multiple physiological and behavior processes in amphibians, including those related to acoustic communication (reviewed in Narins 2001). In other temperate zone anurans, temperature has been found to be the most common weather factor influencing calling activity (e.g., Heinzmann 1970; Fukuyama and Kusano 1992; Oseen and Wassersug 2002; Steelman and Dorcas 2010). Within particular thresholds of temperature, higher body temperatures favor calling activity because they enhance some physiological processes involved in sound production, such as metabolic rate, heart rate, circulating sexual hormones, or mechanical properties of muscles (Rome et al. 1992; Prestwich 1994; Navas and Bevier 2001). Furthermore, as a consequence, acoustical features of emitted signals at higher temperature become shorter in duration and higher in rate (e.g., Schneider and Eichelberg 1974; Crespo et al. 1989; Prestwich 1994). Such acoustical features are often preferably selected by females in many species (e.g., Gerhardt and Huber 2002), and also in H. arborea and H. meridionalis (Gerhardt and Schneider 1980; Schneider 1982). Thus, the effect of temperature on anuran activity has been proposed to explain the phenological differences in the onset of breeding season across a latitude gradient, as a reflection of the successive rise of temperature from southernmost to northernmost sites (Schneider 1971).
However, regression analysis revealed that the major exogenous determinant of the daily occurrence of lekking behavior in all populations was the estimated number of males attending the lek the previous night (positive association), which showed a greater predictive ability than a combination of variables measuring the weather conditions before lek attendance. When the size of the group display is restricted or nil the previous night, the probability of chorus formation decreases. This was probably due to a twofold reason. First, chorus size the previous night may increase the probability of chorus formation because (1) chorusing activity may induce other males to migrate and aggregate to the chorus in the consecutive nights (e.g., Brooke et al. 2000; Gerhardt and Huber 2002), and it may expect that the bigger the chorus size, the further the communication distance and the social facilitation between conspecifics; (2) levels of circulating sexual hormones related to the triggering of calling behavior may remain high in males during a few days (for review, see Walkowiak 2007; Fritzsch et al. 1988), enabling a male to be active for more than a night, and hence, the larger the chorus size, the larger the number of males with hormonal conditions to attend the chorus in subsequent days; (3) factors constraining chorus attendance (e.g., Murphy 1994) are expected to have a more restricted effect, in absolute terms, on larger choruses than in small ones, so that the larger the chorus size the previous night, the smaller the number of males affected by mortality, depletion of energy reserves, or mating success that abandon the chorus in the next nights. Second, the timing of lek formation followed a clumped pattern across the season. Once it began, chorusing activity occurred almost daily within the breeding season, with a mean of only 10 % of the days lacking of choruses. Thus, it is not surprising that chorus size the previous night showed a markedly association with the daily occurrence of tree frog chorus. This explanatory variable functions as a first-order autoregressive factor in the time series of chorus occurrence (Chatfield 2003).
It should be noted that in this study, we investigate the responses of the lek or chorus (i.e., male aggregation) as a whole, instead of individual responses of the males within the lek or chorus that cannot be discriminated by using ARS. Chorus tenure (i.e., number of nights males attended a chorus along the season) seems to be abbreviated in hylids (e.g., Grafe and Meuche 2005), which may be determined by multiple factors, such as energetic constraints, mortality, and hormonal levels fluctuations (Murphy 1994; Emerson 2001). However, as shown in this and other studies (Grafe and Meuche 2005), chorusing activity of tree frogs occurs nearly all nights within the breeding season, owing to an asynchronous presence of males in the chorus. Therefore, factors constraining chorus attendance in a given individual male only can be partly accounted for results from acoustic monitoring as those reported here.
Determinants of chorus duration
Similarly to what was observed for timing, the nightly duration of chorusing activity (chorus duration) was influenced by a large set of exogenous factors, although with lower magnitude effects (β < 1). As major predictor variables of chorus duration, the AIC multimodel inference selected chorus size, number of day, air temperature, and relative humidity at the onset of the chorus. The negative relationship between number of day and the nightly duration of tree frog chorus consistently found in all populations, except the population with the shortest breeding season (CHmo), indicates that chorusing activity was more intense in the early season. At this time, the peak activity tends to take place (Brooke et al. 2000; Grafe and Meuche 2005), with longer and larger choruses. This was supported by our results, which showed that also the estimated number of males in the chorus (chorus size) was positively associated with the number of hours of chorusing (chorus duration). The larger the size of the chorus, the longer it lasted nightly. Previous studies have found such correlation in other hylids (Wells and Taigen 1986; Murphy 1999), which may probably be owing to a twofold reason.
First, the probability of late female arrival to the chorus is environment-dependent, as has been shown in H. gratiosa (Murphy 1999), and presumably increases in larger choruses because the number of males and females attending leks or choruses is often largely correlated (e.g., Henzi et al. 1995; Höglund and Alatalo 1995). Thus, it might be expected that males use exogenous variables in decision making related to the time invested in chorusing, and they prolong their calling activity in larger choruses to increase the probability of encountering late-arriving females.
Second, the positive association between chorus size and chorus duration may be interpreted as a feedback process caused by intrasexual competition and acoustic social facilitation. It has been well established that social facilitation markedly influences on acoustic displays. Playback experiments that have explored acoustic male–male interaction (e.g., Alexander 1961; Lopez et al. 1988; Weary et al. 1991; Jehle and Arak 1998; Bosch and Márquez 2001), as well as physiological studies on the neural basis of vocal production (for review, see Gerhardt and Huber 2002; Walkowiak 2007), have emphasized that acoustic displays results from a complex integration of auditory and motor systems. Calling behavior can be acoustically stimulated by the presence of conspecific calls, which may promote hormonal secretion related to sexual displays (Wilczynski and Chu 2001), and evoke vocal responses from both active and silent males (Capranica 1966; Ryan and Rand 1998). Thus, calling activity from conspecifics affects the timing of group acoustic displays (Brooke et al. 2000; Wells and Taigen 1986). Thereby, it is reasonable to assume that, in a feedback process, the number of calling males in a chorus influences in turn the time invested by these males in calling activity or even the recruitment of more males into the chorus. Sexual competition has been suggested as the ultimate cause of this type of social facilitation (e.g., Gerhardt and Huber 2002).
Intraspecific variation at thermal extremes
The contrast of determinants of timing and duration of sexual displays under different climatic conditions indicates that the factors constraining lekking behavior show both a general species-specific pattern (for timing) and a population-specific pattern (for duration). This suggests that the exogenous determinants of the daily occurrence of tree frog chorus may be stable across species ranges, despite the large differences on climatic conditions and altitude among their thermal extremes. However, intraspecific variation in factors influencing lekking behavior may also be found, such as in chorus duration, similarly to the intraspecific variation in other aspects of mating systems (territoriality or display patterns; e.g., Lott 1991; Höglund and Alatalo 1995). Under different selection pressures, e.g., those undergone by populations in divergent climatic conditions, it is expected that different behavioral strategies evolve to achieve optimal mating results. The finding of intraspecific variation in exogenous determinants of lekking behavior implies the occurrence of mechanisms providing lekking species with means to cope with environmental heterogeneity and changing environments. Since Iberian and Mediterranean tree frogs show both low intraspecific and interspecific genetic variation (Rosa and Oliveira 1994; Stöck et al. 2008), plasticity mechanisms might presumably underlie this intraspecific variation in exogenous determinants of chorus duration.
Previous works based on regression analyses of multiple weather variables have found that Pseudacris crucifer show similar environmental determinants of chorusing activity between distant populations, namely those located at New Brunswick (Canada; Oseen and Wassersug 2002) and North Carolina (USA; Steelman and Dorcas 2010). Moreover, major predictors of chorus occurrence in this study were also consistent with those found for a Central European population of H. arborea (Schneider 1971). Although more work should be done, these results suggest that the species-specific factors influencing the timing of group acoustic displays might be nearly homogeneous throughout the distribution range of anuran species. However, few studies have examined determinants of chorus duration under contrasted environmental conditions. Differences in methods used between studies prevent us to draw conclusions for other species, e.g., Lithobates catesbeianus, monitored in Texas (USA; Blair 1961) and New Brunswick (Canada; Oseen and Wassersug 2002). Furthermore, Navas (1996) found neither intraspecific difference in body temperature of calling anurans nor in daily pattern of sexual displays between populations at 2,900 and 3,500 m.a.s.l. of three tropical Andean species. Ritke et al. (1992) and Brooke et al. (2000) examined the relationships between weather and chorusing behavior at several breeding sites located at less than 2 km from each other. They found slight variations on daily and seasonal pattern of chorusing among sites due to site-specific factors (e.g., water availability) as well as common factors (temperature, rainfall, etc.).
Steelman and Dorcas (2010) proposed the use of predictive models of chorusing activity based on environmental variables in calling surveys to increase the likelihood of detection and to assist in the interpretation of data. To extend the application of this proposed method, one must assume first that species-specific predictive models obtained from a single location are extrapolated to other populations of the same species but under different climatic conditions. Our results suggest that such extrapolation is possible. At least for H. molleri and H. meridionalis, but probably for other species displaying in leks or choruses, one model for each species may be enough to predict accurately the daily or seasonal pattern of acoustic displays of populations across their distribution range. However, further studies should confirm this suggestion.
The comparison between sympatric populations showed that both H. molleri and H. meridionalis responded to the same exogenous factors determining chorus occurrence. This similarity in behavioral responses found between species might be due to low interspecific genetic variation. Although they show a clear genetic differentiation in Iberia (Rosa and Oliveira 1994; Stöck et al. 2008), pre-zygotic isolation mechanisms exceptionally fail between these species, and hybrids have been reported from Iberian sympatric populations (Oliveira et al. 1991; Rosa and Oliveira 1994; Barbadillo and Lapena 2003). Therefore, one may hypothesize that genetic divergence is still low. Consequently, it is not surprising that the ecological constraints influencing the initiation and maintenance of lekking behavior be very similar in Iberian and Mediterranean tree frogs, and thus the probability of occurrence of group acoustic displays be affected by the same exogenous factors.
The results of this intraspecific comparison between populations at thermal extremes might be interpreted in terms of anuran adaptation to global warming. In the last decades, it has become pivotal for current biology assessing the influence of temperature on animal communities to better predict and comprehend the biological consequences of the recent climate change (Schwenk et al. 2009). Temperature is the most pervasive factor that affects amphibian physiology, behavior (e.g., Rome et al. 1992; Wells 2007), and that influences multiple stages of anuran acoustic communication, such as calling behavior, acoustic signal features, signal reception, and post-reception behavior (Fritzsch et al. 1988; Ryan 2001; Gerhardt and Huber 2002; Narins et al. 2007). Anuran populations from the southern limit of the temperate zone are particularly vulnerable to climate change. Global and regional scenarios for the Mediterranean Basin suggest that this region might be one of the most climatically altered in the next decades (Solomon et al. 2007; Giorgi and Lionello 2008). For the Iberian Peninsula, climate projections for 2041–2100 predict a sharp warming and pronounced decrease in rainfall, mainly in spring and summer (Trigo and Palutikof 2001; Paredes et al. 2006; Solomon et al. 2007). In light of our results, chorusing activity in Iberian and Mediterranean tree frogs may not be greatly affected by the recent global warming, other than to show an earlier phenology. As a result of the global rise of temperatures, the lower calling threshold will probably be met at an earlier date, and consequently, chorusing activity and breeding would also start earlier in these species. A general trend toward early breeding as an effect of global warming has already been determined on amphibian populations (e.g., Beebee 1995; Blaustein et al. 2001; Tryjanowski et al. 2003). However, further studies and long-term acoustic monitoring will be necessary to clarify the impact of these changes on population dynamics of temperate zone anurans.
References
Acevedo MA, Villanueva-Rivera LJ (2006) Using automated digital recording systems as effective tools for the monitoring of birds and amphibians. Wildlife Soc B 34:211–214
AEMET (2008) Resumen anual climatológico 2007. Agencia Estatal de Meteorología. Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid
Alexander RD (1961) Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17:130–223
Baines D (1996) Seasonal variation in lek attendance and lekking behaviour by male Black Grouse Tetrao tetrix. Ibis 138:177–180
Barbadillo LJ, Lapena M (2003) Hibridación natural de Hyla arborea (Linnaeus, 1758) e Hyla meridionalis (Boettger, 1874) en la Península Ibérica. Munibe 16:140–145
Beebee TJC (1995) Amphibian breeding and climate. Nature 374:219–220
Berg KS, Brumfield RT, Apanius V (2006) Phylogenetic and ecological determinants of the neotropical dawn chorus. Proc R Soc Lond B 273:999–1005
Blair WF (1961) Calling and spawning seasons in a mixed population of anurans. Ecology 42:99–110
Blankenhorn HJ (1972) Meteorological variables affecting onset and duration of calling in Hyla arborea L. and Bufo calamita calamita Laur. Oecologia 9:223–234
Blaustein AR, Belden LK, Olson DH, Green DM, Root TL, Kiesecker JM (2001) Amphibian breeding and climate change. Conserv Biol 15:1804–1809
Bosch J, Márquez R (2001) Call timing in male-male acoustical interactions and female choice in the midwife toad Alytes obstetricans. Copeia 2001:169–177
Bradbury JW (1981) The evolution of leks. In: Alexander RD, Tinkle DW (eds) Natural selection and social behavior. Chiron, New York, pp 138–169
Bridges AS, Dorcas ME (2000) Temporal variation in anuran calling behavior: implications for surveys and monitoring programs. Copeia 2000:587–592
Brooke PN, Alford RA, Schwarzkopf L (2000) Environmental and social factors influence chorusing behaviour in a tropical frog: examining various temporal and spatial scales. Behav Ecol Sociobiol 49:79–87
Broquet T, Jaquiéry J, Perrin N (2009) Opportunity for sexual selection and effective population size in the lek-breeding European treefrog (Hyla arborea). Evolution 63:674–683
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burnham KP, Anderson DR (2004) Multimodel inference. Understanding AIC and BIC in model selection. Sociol Meth Res 33:261–304
Cambron ME, Bowker RG (2006) An automated digital sound recording system: the Amphibulator. Proceeding of the Eighth IEEE International Symposium on Multimedia, San Diego, pp 592–600
Capranica RR (1966) Vocal response of the bullfrog to natural and synthetic mating calls. J Acoust Soc Am 40:1131–1139
Castellano S, Zanollo V, Marconi V, Berto G (2009) The mechanisms of sexual selection in a lek-breeding anuran, Hyla intermedia. Anim Behav 77:213–224
Chatfield C (2003) The analysis of time series: an introduction, 6th edn. Chapman & Hall, London
Clutton-Brock TH, Deutsch JC, Nefdt RJC (1993) The evolution of ungulate leks. Anim Behav 46:1121–1138
Cramer JS (1999) Predictive performance of the binary logit model in unbalanced samples. J R Stat Soc 48:85–89
Cree A (1989) Relationship between environmental conditions and nocturnal activity of the terrestrial frog, Leiopelma archeyi. J Herpetol 23:61–68
Crespo EG, Oliveira ME, Rosa HD, Paillette M (1989) Mating calls of the Iberian midwife toads Alytes obstetricans boscai and Alytes cisternasii. Bioacoustics 2:1–9
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. ISBN: 3-900051-07-0
Dorcas ME, Price SJ, Walls SC, Barichivich WJ (2009) Auditory monitoring of anuran populations. In: Dodd CK (ed) Amphibian ecology and conservation. A handbook of techniques. Oxford University Press, Oxford, pp 281–298
Downes JA (1969) The swarming and mating flight of Diptera. Annu Rev Entomol 14:271–298
Emerson SB (2001) Male advertisement calls: behavioural variation and physiological processes. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, Washington, pp 36–44
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49
FitzGerald GJ, Bider JR (1974) Seasonal activity of the toad Bufo americanus in southern Quebec as revealed by a sand-transect technique. Can J Zool 52:1–5
Friedl TWP, Klump GM (2005) Sexual selection in the lek-breeding European treefrog: body size, chorus attendance, random mating and good genes. Anim Behav 70:1141–1154
Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (1988) The evolution of the amphibian auditory system. Wiley, New York
Frommolt KH, Bardeli R, Clausen M (2008) Computational bioacoustics for assessing biodiversity. BfN-Skripten, Vol. 234, Bonn
Fukuyama K, Kusano T (1992) Factors affecting breeding activity in a stream-breeding frog, Buergeria buergeri. J Herpetol 26:88–91
García-París M, Montori A, Herrero P (2004) Fauna Ibérica, vol 24., Amphibia, Lissamphibia. Museo Nacional de Ciencias Naturales-CSIC, Madrid
Gelman A (2005) Analysis of variance? Why it is more important than ever. Ann Stat 33:1–53
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. The University of Chicago Press, Chicago
Gerhardt HC, Schneider H (1980) Mating call discrimination by females of the treefrog Hyla meridionalis on Tenerife. Behav Process 5:143–149
Giorgi F, Lionello P (2008) Climate change projections for the Mediterranean region. Global Planet Change 63:90–104
Grafe TU (1997) Costs and benefits of mate choice in the lek-breeding reed frog, Hyperolius marmoratus. Anim Behav 53:1103–1117
Grafe TU, Meuche I (2005) Chorus tenure and estimates of population size of male European tree frogs Hyla arborea: implications for conservation. Amphibia-Reptilia 26:437–444
Grafe TU, Thein J (2001) Energetics of calling and metabolic substrate use during prolonged exercise in the European treefrog Hyla arborea. J Comp Physiol B 171:69–76
Grant RA, Chadwick EA, Halliday T (2009) The lunar cycle: a cue for amphibian reproductive phenology? Anim Behav 78:349–357
Han EN, Gatehouse AG (1991) Effect of temperature and photoperiod on the calling behaviour of a migratory insect, the oriental armyworm Mythimna separata. Physiol Entomol 16:419–427
Hatano FH, Rocha CFD, Sluys MV (2002) Environmental factors affecting calling activity of a tropical diurnal frog (Hylodes phyllodes: Leptodactylidae). J Herpetol 36:314–318
Heinzmann U (1970) Untersuchungen zur bio-akustik und ökologie der geburtshelferkröte, Alytes o. obstertricans (Laur.). Oecologia 5:19–55
Henzi SP, Dyson ML, Piper SE, Passmore NE, Bishop P (1995) Chorus attendance by male and female painted reed frogs (Hyperolius marmoratus): environmental factors and selection pressures. Funct Ecol 9:485–491
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Höglund J, Alatalo RV (1995) Leks. Princeton University Press, Princeton
Hosmer DW, Lemeshow S (1989) Applied logistic regression. Wiley, New York
Jaquiéry J, Broquet T, Aguilar C, Evanno G, Perrin N (2009) Good genes drive female choice for mating partners in the lek-breeding European treefrog. Evolution 64:108–115
Jehle R, Arak A (1998) Graded call variation in male Asian cricket frogs (Rana nicobariensis). Bioacoustics 9:35–48
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Knopp T, Heimovirta M, Kokko H, Merilä J (2008) Do male moor frogs (Rana arvalis) lek with kin? Mol Ecol 17:2522–2530
Kokko H, Sutherland WJ, Lindstöm J, Reynolds JD, Mackenzie A (1998) Individual mating success, lek stability, and the neglected limitations of statistical power. Anim Behav 56:755–762
Lloyd L (1867) The game birds and wild fowl of Sweden and Norway. Frederick Warne, London
Llusia D, Márquez R, Bowker RG (2011) Terrestrial sound monitoring systems, a methodology for quantitative calibration. Bioacoustics 20:277–286
Lopez PT, Narins PM, Lewis ER, Moore SW (1988) Acoustically induced call modification in the white-lipped frog, Leptodactylus albilabris. Anim Behav 36:1295–1308
Lott DF (1991) Intraspecific variation in the social systems of wild vertebrates. Cambridge University Press, Cambridge
Loureiro A, Ferrand de Almeida N, Carretero MA, Paulo OS (2008) Atlas dos anfíbios e répteis de Portugal. Instituto da Conservação da Natureza e da Biodiversidade, Lisboa
Mackenzie A, Reyolds JD, Brown VJ, Sutherland WJ (1995) Variation in male mating success on leks. Am Nat 145:633–652
Manel S, Williams HC, Ormerod SJ (2001) Evaluating presence-absence models in ecology: the need to account for prevalence. J Appl Ecol 38:921–931
Márquez R, Tejedo M (1990) Size-based mating pattern in the tree frog Hyla arborea. Herpetologica 46:176–182
Márquez R, Moreira C, Do Amaral JPS, Pargana JM, Crespo EG (2005) Sound pressure level of advertisement calls of Hyla meridionalis and Hyla arborea. Amphibia-Reptilia 26:391–395
Montgomery DC (2001) Design and analysis of experiments, 5th edn. Wiley, New York
Montgomery DC, Peck EA (1992) Introduction to linear regression analysis. Wiley, New York
Moreira G, Barreto L (1997) Seasonal variation in nocturnal calling activity of a savanna anuran community in central Brazil. Amphibia-Reptilia 18:49–57
Murphy CG (1994) Determinants of chorus tenure in barking treefrogs (Hyla gratiosa). Behav Ecol Sociobiol 34:285–294
Murphy CG (1999) Nightly timing of chorusing by male barking treefrogs (Hyla gratiosa): the influence of female arrival and energy. Copeia 1999:333–347
Murphy CG (2003) The cause of correlations between nightly numbers of male and female barking treefrogs (Hyla gratiosa) attending choruses. Behav Ecol 14:274–281
Narins PM (2001) Ectothermy's last stand. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, Washington, pp 61–70
Narins PM, Feng AS, Fay RR, Popper AN (2007) Hearing and sound communication in amphibians. Springer, New York
Navas CA (1996) The effect of temperature on the vocal activity of tropical anurans: a comparison of high and low-elevation species. J Herpetol 30:488–497
Navas CA, Bevier CR (2001) Thermal dependency of calling performance in the eurythermic frog Colostethus subpunctatus. Herpetologica 57:384–395
Neter J, Kutner MH, Nachtsheim C, Wasserman W (1996) Applied linear statistical models, 3rd edn. Irwin, Chicago
Obert HJ (1975) The dependence of calling activity in Rana esculenta Linné 1758 and Rana ridibunda Pallas 1771 upon exogenous factors (Ranidae, Anura). Oecologia 18:317–328
Obrist MK, Pavan G, Sueur J, Riede K, Llusia D, Márquez R (2010) Bioacoustics approaches in biodiversity inventories. Abc Taxa 8:68–99
Oliveira ME, Paillette M, Rosa HD, Crespo EG (1991) A natural hybrid between Hyla arborea and Hyla meridionalis detected by mating calls. Amphibia-Reptilia 12:15–20
Oseen KL, Wassersug RJ (2002) Environmental factors influencing calling in sympatric anurans. Oecologia 133:616–625
Paillette M (1967) Rhythm of acoustic activity of Hyla arborea (Linné) and Hyla meridionalis Boettger anourous amphibians. CR Soc Biol 161:986–992
Paredes D, Trigo RM, Garcia-Herrera R, Trigo IF (2006) Understanding precipitation changes in Iberia in early spring: weather typing and storm-tracking approaches. J Hydrometeorol 7:101–113
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sc 11:1633–1644
Pemberton JM, Balmford AP (1987) Lekking in fallow deer. J Zool 213:762–765
Peterson CR, Dorcas ME (1994) Automated data acquisition. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS (eds) Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institution Press, Washington, pp 47–57
Pleguezuelos JM, Márquez R, Lizana M (2002) Atlas y Libro Rojo de los anfibios y reptiles de España. Dirección General de Conservación de la Naturaleza-Asociación Herpetológica. Española, Madrid
Prestwich KN (1994) The energetics of acoustic signalling in anuran and insects. Am Zool 34:625–643
Richards SA, Whittingham MJ, Stephens PA (2011) Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behav Ecol Sociobiol 65:77–89
Ritke ME, Babb JG, Ritke MK (1992) Temporal patterns of reproductive activity in the gray treefrog (Hyla chrysoscelis). J Herpetol 26:107–111
Rome LC, Stevens ED, John-Alder HB (1992) The influence of temperature and thermal acclimation on physiological function. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians. The University of Chicago Press, Chicago, pp 183–205
Rosa HD, Oliveira ME (1994) Genetic differentiation of the Iberian tree frogs Hyla arborea molleri and Hyla meridionalis (Amphibia, Anura). J Zool Syst Evol Res 32:117–128
Ryan MJ (2001) Anuran communication. Smithsonian Institution Press, Washington
Ryan MJ, Rand AS (1998) Evoked vocal response in male Tungara frogs: pre-existing biases in male responses? Anim Behav 56:1509–1516
Ryan MJ, Tuttle MD, Taft LK (1981) The costs and benefits of frog chorusing behaviour. Behav Ecol Sociobiol 8:273–278
Salvador A, Carrascal LM (1990) Reproductive phenology and temporal patterns of mate access in Mediterranean anurans. J Herpetol 24:438–441
Schneider H (1971) Die steuerung des täglichen rufbeginns beim Laubfrosch, Hyla arborea arborea (L.). Oecologia 8:310–320
Schneider H (1974) Structure of the mating calls and relationships of the European tree frogs (Hylidae, Anura). Oecologia 14:99–110
Schneider H (1982) Phonotaxis bei weibchen des kanarischen Laubfrosches, Hyla meridionalis. Zool Anz 208:161–174
Schneider H, Eichelberg H (1974) The mating call of hybrids of the fire-bellied toad and yellow-bellied toad (Bombina bombina (L.), Bombina v. variegata (L.), Discoglossidae, Anura). Oecologia 16:61–71
Schwartz JJ, Ressel SJ, Bevier CR (1995) Carbohydrate and calling: depletion of muscle glycogen and the chorusing dynamics of the neotropical treefrog Hyla microcephala. Behav Ecol Sociobiol 37:125–135
Schwenk K, Padilla DK, Bakken GS, Full RJ (2009) Grand challenges in organismal biology. Integr Comp Biol 49:7–14
Shirose LJ, Bishop CA, Green DM, MacDonald CJ, Brooks RJ, Helferty NJ (1997) Validation tests of an amphibian call count survey technique in Ontario, Canada. Herpetologica 53:312–320
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freedman, New York
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Steelman CK, Dorcas ME (2010) Anuran calling survey optimization: developing and testing predictive models of anuran calling activity. J Herpetol 44:61–68
Stöck M, Dubey S, Klütsch C, Litvinchuk SN, Scheidt U, Perrin N (2008) Mitochondrial and nuclear phylogeny of circum-Mediterranean tree frogs from the Hyla arborea group. Mol Phylogenet Evol 49:1019–1024
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav Ecol Sociobiol 65:13–21
Taigen TL, Wells KD (1985) Energetics of vocalization by an anuran amphibian (Hyla versicolor). J Comp Physiol B 155:163–170
Trigo RM, Palutikof JP (2001) Precipitation scenarios over Iberia: a comparison between direct GCM output and different downscaling techniques. J Climate 14:4422–4446
Tryjanowski P, Mariusz R, Sparks TH (2003) Changes in spawning dates of common frogs and common toads in western Poland in 1978–2002. Ann Zool Fenn 40:459–464
Tuttle MD, Ryan MJ (1981) Bat predation and the evolution of frog vocalizations in the Neotropics. Science 214:677–678
Walkowiak W (2007) Call production and neural basis of vocalization. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. Springer, New York, pp 87–112
Weary DM, Lambrechts MM, Krebs JR (1991) Does singing exhaust male great tits? Anim Behav 41:540–542
Weir LA, Mossman MJ (2005) North American amphibian monitoring program (NAAMP). In: Lannoo M (ed) Amphibian declines: the conservation status of United States species. University of California Press, Berkeley, pp 307–313
Wells KD (1977) The social behaviour of anuran amphibians. Anim Behav 25:666–693
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wells KD, Taigen TL (1986) The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19:9–18
Widemo F, Owens IPF (1995) Lek size, male mating skew and the evolution of lekking. Nature 373:148–151
Wilczynski W, Chu J (2001) Acoustic communication, endocrine control, and the neurochemical systems of the brain. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, Washington, pp 23–35
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Meth Ecol Evol 1:3–14
Acknowledgments
We want to thank José Manuel Mendes for granting us access to the pond in his property in Castelo de Vide. Permits to work and use of facilities in protected areas were granted by Somiedo Natural Park (Principado de Asturias), ICTS Doñana (CSIC), and Consejerías de Medio Ambiente (Principado de Asturias and Andalucía). We are also grateful to Richard G. Bowker and Mark E. Cambron of Western Kentucky University for designing and building the Amphibulator (timer-controller for ARS). Meteorological data were kindly provided by AEMET (Agencia Estatal de Meteorología. Ministerio de Medio Ambiente y Medio Rural y Marino) and Guillermo Ballester (OGIMET Weather Information Service). David Sánchez provided information about bioclimatic variables for the Iberian distribution range of tree frogs. Xavier Eekhout and Antón Arias helped in the field and with the data processing. José Miguel Oliveira, Maribel Benítez, Manolo Chirosa, and Hélder Duarte also helped in the field. We appreciate suggestions from anonymous reviewers that greatly improved this research report. The first author was supported by an FPI predoctoral grant from Ministerio de Ciencia e Innovación (BES-2006-13104, Spain). The last author was in receipt of a fellowship from the Fundação para a Ciência e a Tecnologia (SFRH/BPD/3514/2000, Portugal) and a SYNTHESYS project (ES-TAF-1251, Spain). Research was funded by projects TEMPURA (CGL2005-00092/BOS), ACOURA (CGL2008-04814-C02), and project TATANKA (CGL2011-25062), Ministerio de Ciencia e Innovación, Spain (PI. R. Márquez).
Ethical standards
All experiments reported in this article comply with the current laws of Spain and Portugal, the countries in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Gibbons
Rights and permissions
About this article
Cite this article
Llusia, D., Márquez, R., Beltrán, J.F. et al. Environmental and social determinants of anuran lekking behavior: intraspecific variation in populations at thermal extremes. Behav Ecol Sociobiol 67, 493–511 (2013). https://doi.org/10.1007/s00265-012-1469-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1469-2